Abstract
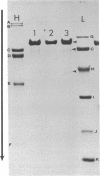
Citrate-soluble extracts of chicken sciatic nerve were fractionated biochemically and added to cultures of embryonic chicken skeletal muscle in order to identify the component that exerted trophic influences on the muscle. A protein fraction that expressed trophic activity was obtained by ion-exchange chromatography on DEAE-cellulose followed by gel filtration on Sephadex G-100 superfine. This fraction enhanced the rate and degree of morphological maturation and the level of protein synthesis in embryonic muscle cells. Muscle fibers treated with this fraction after maturation in culture survived for longer periods in vitro than did comparable controls. Characterization of the active protein fraction by sodium dodecyl sulfate/polyacrylamide gel electrophoresis revealed the presence of one major protein (molecular weight 84,000) and three minor proteins. Electrophoretic analysis of biologically inactive gel filtration fractions indicated that the three minor proteins were contaminants and that biological activity was associated with the protein of molecular weight 84,000. Analytical isoelectric focusing revealed that the active protein was acidic and focused as four species with isoelectric points (pI) of 5.74, 5.77, 5.92, and 6.15. Maximal incorporation of [14C]-leucine into muscle cell protein was elicited by 20 microgram of active protein per culture dish. These data suggest that an acidic protein having trophic influences upon muscle has been identified and partially purified.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Warnick J. E., Tasse J. R., Sansone F. M. Effects of vinblastine and colchicine on neural regulation of the fast and slow skeletal muscles of the rat. Exp Neurol. 1972 Dec;37(3):607–634. doi: 10.1016/0014-4886(72)90103-3. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Deshpande S. S., Albuquerque E. X., Guth L. Neurotrophic regulation of prejunctional and postjunctional membrane at the mammalian motor endplate. Exp Neurol. 1976 Oct;53(1):151–165. doi: 10.1016/0014-4886(76)90289-2. [DOI] [PubMed] [Google Scholar]
- Drachman D. B. Trophic functions of the neuron. 3. Mechanisms of neurotrophic interactions. The role of acetylcholine as a neurotropic transmitter. Ann N Y Acad Sci. 1974 Mar 22;228(0):160–176. doi: 10.1111/j.1749-6632.1974.tb20508.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Robbins N. Effect of chronic disuse of rat soleus neuromuscular junctions on postsynaptic membrane. J Neurophysiol. 1971 Jul;34(4):562–569. doi: 10.1152/jn.1971.34.4.562. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J. S. Growth factors in mammalian cell culture. Annu Rev Biochem. 1976;45:531–558. doi: 10.1146/annurev.bi.45.070176.002531. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D. Purification of a fibroblast growth factor from bovine pituitary. J Biol Chem. 1975 Apr 10;250(7):2515–2520. [PubMed] [Google Scholar]
- Gospodarowicz D., Weseman J., Moran J. Presence in brain of a mitogenic agent promoting proliferation of myoblasts in low density culture. Nature. 1975 Jul 17;256(5514):216–219. doi: 10.1038/256216a0. [DOI] [PubMed] [Google Scholar]
- Guth L. "Trophic" influences of nerve on muscle. Physiol Rev. 1968 Oct;48(4):645–687. doi: 10.1152/physrev.1968.48.4.645. [DOI] [PubMed] [Google Scholar]
- Guth L., Albuquerque E. X. The neurotrophic regulation of resting membrane potential and extrajunctional acetylcholine sensitivity in mammalian skeletal muscle. Physiol Bohemoslov. 1978;27(5):401–414. [PubMed] [Google Scholar]
- Gutmann E. Neurotrophic relations. Annu Rev Physiol. 1976;38:177–216. doi: 10.1146/annurev.ph.38.030176.001141. [DOI] [PubMed] [Google Scholar]
- Hasegawa S., Kuromi H. Effects of spinal cord and other tissue extracts on resting and action potentials of organ-cultured mouse skeletal muscle. Brain Res. 1977 Jan 1;119(1):133–140. doi: 10.1016/0006-8993(77)90095-6. [DOI] [PubMed] [Google Scholar]
- Jabaily J., Singer M. Neutrophic and hepatotrophic stimulation of proliferation of embryonic chick muscle cells in vitro: assay and partial characterization of mitogenic activity in chick embryonic organ and tissue extracts. Dev Biol. 1978 Jun;64(2):189–202. doi: 10.1016/0012-1606(78)90071-4. [DOI] [PubMed] [Google Scholar]
- Jakob A., Hauri C., Froesch E. R. Nonsuppressible insulin-like activity in human serum. 3. Differentiation of two distinct molecules with nonsuppressible ILA. J Clin Invest. 1968 Dec;47(12):2678–2688. doi: 10.1172/JCI105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Kuromi H., Hasegawa S. Neurotrophic effect of spinal cord extract on membrane potentials of organ-cultured mouse skeletal muscle. Brain Res. 1975 Dec 12;100(1):178–181. doi: 10.1016/0006-8993(75)90256-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lentz T. L. Nerve trophic function: in vitro assay of effects of nerve tissue on muscle cholinesterase ctivity. Science. 1971 Jan 15;171(3967):187–189. doi: 10.1126/science.171.3967.187. [DOI] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Further studies on the control of ACh sensitivity by muscle activity in the rat. J Physiol. 1975 Nov;252(3):603–626. doi: 10.1113/jphysiol.1975.sp011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markelonis G. J., Oh T. H. A protein fraction from peripheral nerve having neurotrophic effects on skeletal muscle cells in culture. Exp Neurol. 1978 Jan 15;58(2):285–295. doi: 10.1016/0014-4886(78)90141-3. [DOI] [PubMed] [Google Scholar]
- Oh T. H., Johnson D. D. Effects of acetyl- -methylcholine on development of acetylcholinesterase and butyrylcholinesterase activities in cultured chick embryonic skeletal muscle. Exp Neurol. 1972 Nov;37(2):360–370. doi: 10.1016/0014-4886(72)90080-5. [DOI] [PubMed] [Google Scholar]
- Oh T. H., Johnson D. D., Kim S. U. Neurotrophic effect on isolated chick embryo muscle in culture. Science. 1972 Dec 22;178(4067):1298–1300. doi: 10.1126/science.178.4067.1298. [DOI] [PubMed] [Google Scholar]
- Oh T. H., Markelonis G. J. Neurotrophic protein regulates muscle acetylcholinesterase in culture. Science. 1978 Apr 21;200(4339):337–339. doi: 10.1126/science.635593. [DOI] [PubMed] [Google Scholar]
- Oh T. H. Neurotrophic effects: characterization of the nerve extract that stimulates muscle development in culture. Exp Neurol. 1975 Feb;46(2):432–438. doi: 10.1016/0014-4886(75)90147-8. [DOI] [PubMed] [Google Scholar]
- Oh T. H. Neurotropic effects of sciatic nerve extracts on muscle development in culture. Exp Neurol. 1976 Feb;50(2):376–386. doi: 10.1016/0014-4886(76)90012-1. [DOI] [PubMed] [Google Scholar]
- Peterson E. R., Crain S. M. Regeneration and innervation in cultures of adult mammalian skeletal muscle coupled with fetal rodent spinal cord. Exp Neurol. 1972 Jul;36(1):136–159. doi: 10.1016/0014-4886(72)90142-2. [DOI] [PubMed] [Google Scholar]
- Podleski T. R., Axelrod D., Ravdin P., Greenberg I., Johnson M. M., Salpeter M. M. Nerve extract induces increase and redistribution of acetylcholine receptors on cloned muscle cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2035–2039. doi: 10.1073/pnas.75.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politoff A. L., Blitz A. L. Neurotrophic control of RNA synthesis in amphibian striated muscle. Brain Res. 1978 Aug 11;151(3):561–570. doi: 10.1016/0006-8993(78)91087-9. [DOI] [PubMed] [Google Scholar]
- Richler C., Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970 Sep;23(1):1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Inagami T., Cohen S. The primary structure of epidermal growth factor. J Biol Chem. 1972 Dec 10;247(23):7612–7621. [PubMed] [Google Scholar]
- Schlaepfer W. W., Micko S. Chemical and structural changes of neurofilaments in transected rat sciatic nerve. J Cell Biol. 1978 Aug;78(2):369–378. doi: 10.1083/jcb.78.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Caston J. D. Neurotrophic dependence of macromolecular synthesis in the early limb regenerate of the newt, Triturus. J Embryol Exp Morphol. 1972 Aug;28(1):1–11. [PubMed] [Google Scholar]
- Singer M., Maier C. E., McNutt W. S. Neurotrophic activity of brain extracts in forelimb regeneration of the urodele, Triturus. J Exp Zool. 1976 May;196(2):131–150. doi: 10.1002/jez.1401960202. [DOI] [PubMed] [Google Scholar]
- Steiner D. F. The Banting Memorial Lecture 1976. Insulin today. Diabetes. 1977 Apr;26(4):322–340. doi: 10.2337/diab.26.4.322. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. Supersensitivity of skeletal muscle produced by botulinum toxin. J Physiol. 1960 Jun;151:598–607. doi: 10.1113/jphysiol.1960.sp006463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon S., Normura J., Shooter E. M. Reversible dissociation of the mouse nerve growth factor protein into different subunits. Biochemistry. 1968 Apr;7(4):1296–1303. doi: 10.1021/bi00844a008. [DOI] [PubMed] [Google Scholar]
- Warnick J. E., Albuquerque E. X., Guth L. The demonstration of neurotrophic function by application of colchicine or vinblastine to the peripheral nerve. Exp Neurol. 1977 Nov;57(2):622–636. doi: 10.1016/0014-4886(77)90094-2. [DOI] [PubMed] [Google Scholar]
- Westgaard R. H. Influence of activity on the passive electrical properties of denervated soleus muscle fibres in the rat. J Physiol. 1975 Oct;251(3):683–697. doi: 10.1113/jphysiol.1975.sp011116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younkin S. G., Brett R. S., Davey B., Younkin L. Substances moved by axonal transport and released by nerve stimulation have an innervation-like effect on muscle. Science. 1978 Jun 16;200(4347):1292–1295. doi: 10.1126/science.78522. [DOI] [PubMed] [Google Scholar]