Abstract
The feasibility of a malaria vaccine is supported by the fact that children in endemic areas develop naturally acquired immunity to disease. Development of disease immunity is characterized by a decrease in the frequency and severity of disease episodes over several years despite almost continuous infection1, suggesting that immunity may develop through the acquisition of a repertoire of specific, protective antibodies directed against polymorphic target antigens1-3. Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) is a potentially important family of target antigens, because these proteins are inserted into the red cell surface and are prominently exposed4-6 and because they are highly polymorphic and undergo clonal antigenic variation7,8,18, a mechanism of immune evasion maintained by a large family of var genes9-11. In a large prospective study of Kenyan children, we have used the fact that anti-PfEMP1 antibodies agglutinate infected erythrocytes in a variant-specific manner10,12-16, to show that the PfEMP1 variants expressed during episodes of clinical malaria were less likely to be recognized by the corresponding child’s own preexisting antibody response than by that of children of the same age from the same community. In contrast, a heterologous parasite isolate was just as likely to be recognized. The apparent selective pressure exerted by established anti-PfEMP1 antibodies on infecting parasites supports the idea that such responses provide variant-specific protection against disease.
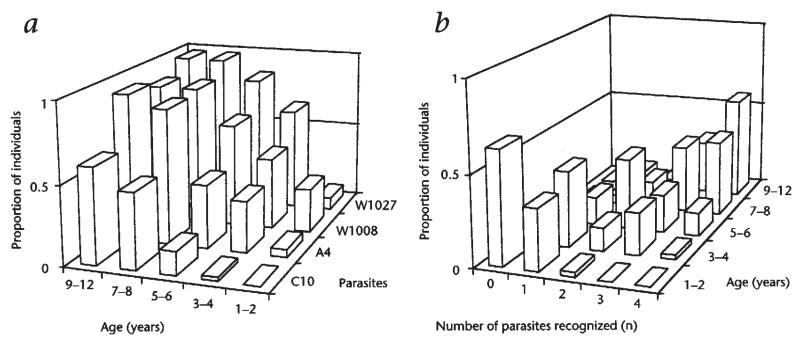
To study protection by anti-PfEMP1 antibodies it was necessary to focus on a group of children who were at various stages of developing a full repertoire of anti-PfEMP1 responses. As the period over which the antibody repertoire develops will vary with local rate of exposure, it was important first to determine the age range to study. To this end, 200 serum samples (40 from each of five age classes: 1–2, 3–4, 5–6, 7–8 and 9–12 years) were taken from children in March 1993. Each was tested for its ability to agglutinate four parasites (C10, A4, W1008 and W1027). As shown in Fig. 1a, the prevalence of antibody responses to all isolates rose between 1 and 5 years of age. This agrees closely with the age range over which naturally acquired immunity to malaria develops in this part of Kenya (data not shown). As expected, responses to the different isolates were independent and specific, since the number of individuals in each age group that recognized between zero and four isolates (Fig. 1b) has a diagonal structure that fits very closely that predicted by a binomial distribution (not shown).
Fig. 1.
Age-specific recognition of four P. falciparum isolates by children in the Kilifi area. C10 and A4 are subclones derived from laboratory line IT04 (ref. 18); W1008 and W1027 are wild isolates from Kilifi. a, The proportion of each age class with agglutinating antibodies to each isolate, b, The proportion of each age class with antibodies to n isolates.
Defining the role of parasite antigens in naturally acquired immunity has been problematic. The ubiquity of asymptomatic infection in those age groups susceptible to disease combined with the difficulty of determining the immune status of any individual means that relating any particular immune response to clinical immunity is only possible in large, prospective studies17. With this in mind we recruited a cohort of 4783 children aged between 1 and 5 years old. Blood samples were taken from the children in a cross-sectional survey at the beginning of the long rainy season during May 1995. Over the following 8 months (which included two periods of malaria transmission), 65 parasite isolates were collected from children of the cohort who returned to the hospital with clinical malaria. Parasites were collected from patients with both severe and mild malaria; 34 were outpatients and 31 were admitted to the hospital. Since only immature parasites that do not express PfEMP1 are present in peripheral blood, parasite isolates were grown in vitro until they were mature trophozoites. Because the repertoire of PfEMP1 variants that can be expressed by a single genotype is large and the rate of antigenic switching is high, parasites sampled at any point in time may constitute complex mixtures of different variant types18. Thus, to assess the antibody response to these proteins, we used agglutination assays to measure response to the whole parasite population present at the time of sampling. Assays were performed to test for recognition of each isolate by the corresponding infected child’s own serum collected in the initial cross-sectional survey (index) and plasma collected at the time of acute illness (acute). To determine the expected prevalence of antibodies in the population, each parasite was also assayed against sera from 20 age-matched controls also drawn from the samples collected in May 1995. Data from the 65 parasite isolates were pooled.
A marked difference was seen in the frequency with which parasites causing disease were recognized by index sera compared with sera from age-matched controls from the same community (Table 1). This difference cannot be accounted for by differences in prior exposure, as the parasite rates in the two groups were almost identical at the time of sampling (Table 1). Since the parasite rate was found to be rising with age within the controls and has therefore not reached saturation (data not shown), this measurement is likely to provide a reasonable estimate of exposure. We further tested the possibility that there were, nonetheless, differences in exposure on a more local scale by categorizing the controls into 54 residential zones, defined previously in the 1991 national census, each containing approximately 100 homesteads and 850 people. The overall rates of positive responses in controls drawn from the zones of children who presented as cases and those living in zones from which no case presented were compared. Although one might expect higher overall exposure in the zones from which cases were drawn, no difference was detected (Table 1).
Table 1.
Agglutination of parasite infected red cells by sera from clinical isolates and controls
| n | Agglutination frequency (%) |
Odds ratio (confidence interval) |
P valuea | Parasite rate (%)B |
|
| Controls in zones producing cases |
861 | 20.2 | 1.07 (0.79-1.45)c | 0.70 | 35.0 |
| Controls in zones without cases |
434 | 19.1 | 33.2 | ||
| Homologous parasites | |||||
| Control d | 1295 | 19.8 | 34.4 | ||
| Indexe | 65 | 4.6 | 0.20 (0.04-0.60)f | 0.004 | 32.3 |
| Acuteg | 65 | 35.4 | 2.21 (1.26-3.85)h | 0.004 | |
| Parasite 1759i | |||||
| Controls | 156 | 34.0 | 39.7 | ||
| Index | 52 | 30.8 | 0.86 (0.42-1.78)f | 0.80 | 28.9 |
| Acute | 52 | 34.6 | 1.03 (0.50-2.09)h | 0.93 |
P value from a Yates corrected chi-square test. P values agreed well with those calculated using logistic regression (not shown).
Percentage of samples in which one or more parasites were observed in 200 fields of a thick blood film.
Comparison, using the pooled data from 65 assays, between agglutination frequency in controls from zones producing malaria cases and controls from zones that did not.
For each index case, 20 control sera tested, except for five assays in which only 19 controls were used; a total of 1295 controls were tested against 65 isolates.
Each parasite tested against corresponding serum collected in May 1995.
Comparison between agglutination frequency in index sera and control sera.
Each parasite was tested against the corresponding acute plasma sampled at the time of presentation with malaria.
Comparison between agglutination frequency in acute plasma and control sera.
Sera from 52 index cases and 156 controls tested against heterologous parasite 1759.
We considered the possibility that the lower prevalence of variant-specific antibodies in index sera was the result of a generalized lower response to the infected erythrocyte surface rather than the specific absence of a response to the infecting isolate. To test this possibility, 52 of the index sera were tested for their ability to agglutinate a single, heterologous wild isolate, 1759. To maximize the chances of detecting a reduced prevalence of antibody, each index serum was compared with three controls matched both by age and zone of residence within the study area. There was no significant difference between the two groups (Table 1), suggesting that the index sera have a specific reduction in the prevalence of antibodies directed against the parasites subsequently causing clinical disease in these children. The almost exclusive appearance during clinical disease of parasite variants that correspond to gaps in each child’s developing repertoire of anti-PfEMP1 antibodies suggests that preexisting antibodies provide protection against parasite variants to which they are directed.
Previous studies have described the development of strong, variant-specific anti-PfEMP1 responses over the weeks following a clinical episode12,15. As shown in Table 1, such responses apparently evolve rapidly. Although there was no increase in the prevalence of antibodies to parasite 1759 at the time of disease, there was already a significant increase in the prevalence of antibodies specific to the infecting isolates. Since the acute plasma and parasites were sampled several hours before the parasites would express PfEMP1 in vivo, the coexistence of parasite variants and corresponding antibody in vitro reflects a situation that will occur in vivo as the parasites mature, immediately before immune selection. Rapid production of antibody to the infecting isolate, together with a high rate of PfEMP1 antigen switching as previously observed in vitro18, could result in a close dynamic relationship between the emergence of new parasite variants and their removal by the immune system.
Taken together, the variant-specific protection provided by anti-PfEMP1 antibodies, their rapid induction in response to infection and the expansion of the antibody repertoire during the period in life when disease immunity is known to be developing support the idea that these antibodies play an important role in the acquisition of immunity to malaria in children. It is possible that antibodies to nonvariant epitopes on the red cell surface are also involved in immunity, particularly in adults19. However, although such antibodies have been isolated from immune adult serum12, the agglutinating antibody response in adults remains predominantly variant specific14, suggesting that variant-specific antibodies may play a continuing role in the maintenance of immunity throughout life.
Methods
Study area
The study was carried out at Kilifi district hospital, situated 60 km north of Mombasa on the Kenyan coast. An area immediately surrounding the administrative town of Kilifi was defined in 1991 for surveillance20. More than 10% of the children under the age of 5 years who reside within the study area are admitted to the hospital each year. The area has prolonged seasonal P. falciparum transmission following the long and short rains, transmitted by the Anopheles gambiae s.l. complex21.
Collection of sera before disease occurrence
Information about the study was given out in the local language to people living in the study area, and parents then came to hospital with their children in May 1995 and gave written consent at this time. Blood samples were collected from finger pricks into 1-ml Serum Separator Microtainer tubes (Becton Dickinson, San Jose, CA). Tubes were centrifuged then stored refrigerated at −70 °C. Thick blood films were prepared, and each of the samples was examined for parasites. Each child was given a personal (EZ) number, which included information on zone of residence within the study area, and a sticker on his or her health card to show participation in the study. The age, EZ number, name and blood group of each child was stored on a Foxpro database.
Collection of parasites
During the next 8 months, every child who came to the hospital for care carrying a health card and who had fever and a parasitemia above 100 trophozoites/100 white cells (regardless of the severity of disease) was recruited to the study. Blood (5 ml) was taken into a tube containing heparin sulfate, cells were centrifuged at 1800 r.p.m. for 5 min, and acute plasma was decanted and stored. Mononuclear cells were removed by centrifugation through Lymphoprep (Nycomed, Oslo, Norway). Granulocytes were removed by flotation on Plasmagel (Bellon, Neully Sur Seine, France). Packed infected red cells (100 μl) were cultured in RPMI 1640 (Gibco/BRL, Gaithersburg, MD) in the presence of 10% European plasma22 until they matured into pigmented trophozoites. To synchronize growth, 1.5 μg/ml aphidicolin (Sigma) was included in the culture23. The presence of aphidicolin was found to have no effect on agglutination phenotype (data not shown). In total, 202 children of the cohort met the inclusion criteria. Following culture, parasitemia was determined, and those cultured with a parasitemia below 1 trophozoite/100 uninfected red cells (1%) were excluded. In total, 105 isolates had a high enough parasitemia for inclusion.
Retrieval of index serum and selection of controls
The index serum from the 1995 cross-sectional survey corresponding to each parasite was retrieved from the database together with 20 control sera, matched to the index by blood group and to within 2 months in age. Control selection was carried out by extracting all samples from the database that met the matching criteria and selecting 20 samples manually from this list. The number of times that each sample had been thawed was recorded on the database, and controls were only selected if they had been thawed at least as many times as the corresponding index serum. Since the list from which controls were selected varied for each index serum and contained no information related to exposure, the chances of systematic bias were minimized.
Agglutination
Cells were washed once in RPMI 1640, and fresh uninfected erythrocytes were added, if necessary, to ensure that the parasitemia was between 1% and 5%. The agglutination method was modified from one reported12. Cells were diluted to a 5% hematocrit in RPMI 1640, and ethidium bromide (Sigma) was added to a final concentration of 10 μg/ml. Agglutination was performed (with these 21 sera together with the acute plasma and serum samples from a hyperimmune adult and a nonimmune European) in 25-μl (for the first 29 assays) or 12.5-μl reaction volumes (for the latter 36 assays) by using a final test serum dilution of 1:5. Cells were rotated in a U-bottomed microtiter plate at 60 r.p.m. on a vertical rotator for 60 min at room temperature. The 25-μl reaction volumes were split into two and gently pipetted onto two glass slides and covered with a 22-mm coverslip. The 12.5-μl sample reactions were pipetted onto one slide and covered with an 18-mm coverslip. Following blind labeling, parasites were examined with a UV microscope. Reactions were scored positive or negative on the basis of the observation of two or more agglutinates of at least five cells within a search of 5% of the entire reaction volume, or the observation of clusters of agglutinates during a subsequent search of at least a further 20% of the reaction volume. Of the total, 40 assays were excluded at this stage because the observer was unable to determine variant-specific agglutination. Among those excluded, 19 parasites exhibited an autoagglutinating phenotype18 or formed large clumps containing both infected and uninfected cells, so that the infected cells were scored positive for agglutination in nonimmune serum. The remainder (21 parasites) were not agglutinated by age-matched controls. Of these, 11 also failed to be agglutinated by the hyperimmune adult serum.
Parasite 1759
This frozen parasite isolate from Kilifi was chosen because it had previously been shown to be commonly recognized by children’s sera. This isolate showed no tendency to agglutinate in nonimmune serum, since 30 agglutination reactions containing nonimmune serum were included in the blinded assay, and none were scored positive.
Acknowledgments
This paper is published with the permission of the director of KEMRl. We thank the head of the KEMRl unit at Kilifi, N. Peshu and the staff. We thank the following people in particular for their contribution to this work: G. Baya, A. juma, N. Kaleli, B. Kitsau, L. New, C. Ngetsa, F. Njuga, E. Obiero, J. Obiero, A. Scott, D. Smith. This work was supported by KEMRl and The Wellcome Trust. KM is a Wellcome Trust Senior Research Fellow in clinical sciences (631342).
References
- 1.Marsh K. Malaria—a neglected disease? Parasitology. 1992;104:S53–s69. doi: 10.1017/s0031182000075247. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Trenholme K, Anderson RM, Day KP. Antigenic diversity and the transmission dynamics of Plasmodium falciparum. Science. 1993;263:961–963. doi: 10.1126/science.8310293. [DOI] [PubMed] [Google Scholar]
- 3.Molineaux L. Plasmodium falciparum malaria: Some epidemiological implications of parasite and host diversity. Ann. Trop. Med. Parasitol. 1996;90:379–393. doi: 10.1080/00034983.1996.11813067. [DOI] [PubMed] [Google Scholar]
- 4.Magowan C, Wollish W, Anderson L, Leech J. Cytoadherence by Plasmodium falciparum-infected erythrocytes is correlated with the expression of a family of variable proteins on infected erythrocytes. J. Exp. Med. 1988;168:1307–1320. doi: 10.1084/jem.168.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baruch DI, Gormley JA, Ma C, Howard RJ, Pasloske BL. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner JP, Pinches RA, Roberts DJ, Newbold CI. Variant antigens and endothelial receptor adhesion in Plasmodium falciparum. Proc. Notl, Acad. Sci. USA. 1996;93:3503–3508. doi: 10.1073/pnas.93.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biggs B-A, et al. Adherence of infected erythrocytes to venular endothelium selects for antigenic variants of Plasmodium falciparum. J. Immunol. 1992;149:2047–2054. [PubMed] [Google Scholar]
- 8.Roberts DJ, Biggs B-A,, Brown G, Newbold CI. Protection, pathogenesis and phenotypic plasticity in Plasmodium falciparum malaria. Parasitol. Today. 1993;9:281–286. doi: 10.1016/0169-4758(93)90121-u. [DOI] [PubMed] [Google Scholar]
- 9.Baruch DI, et al. Cloning the P. falciparum gene encoding PfEMP1, a maiarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 10.Smith JD, et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su X, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 12.Marsh K, Howard RJ. Antigens induced on erythrocytes by P. falciparum: Expression of diverse and conserved determinants. Science. 1986;231:150–153. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- 13.Forsyth KP, et al. Diversity of antigens expressed on the surface of erythrocytes infected with mature Plasmodium falciparum parasites in Papua New Guinea. Am. J. Trop. Med. Hyg. 1989;41:259–265. [PubMed] [Google Scholar]
- 14.Newbold CI, Pinches R, Roberts DJ, Marsh K. Plasmodium falciparum: The human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp. Parasitol. 1992;75:281–292. doi: 10.1016/0014-4894(92)90213-t. [DOI] [PubMed] [Google Scholar]
- 15.Iqbal J, Perlmann P, Berzins K. Serological diversity of antigens expressed on the surface of erythrocytes infected with Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 1993;87:583–588. doi: 10.1016/0035-9203(93)90097-a. [DOI] [PubMed] [Google Scholar]
- 16.Reeder JC, et al. Diversity of agglutinating phenotype, cytoadherence, and rosette-forming characteristics of Plasmodium falciparum isolates from Papua New Guinean children. Am. J. Trop. Med. Hyg. 1994;51:45–55. doi: 10.4269/ajtmh.1994.51.45. [DOI] [PubMed] [Google Scholar]
- 17.Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. Antibodies to blood stage antigens of Plasmodium faiciparum in rural Gambians and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 18.Roberts DJ, et al. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anita R, Nowak MA, Anderson RM. Antigenic variation and the within-host dynamics of parasites. Proc Natl. Acad. Sci. USA. 1996;93:985–989. doi: 10.1073/pnas.93.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snow RW, et al. Periodicity and space-time clustering of severe childhood malaria on the coast of Kenya. Trans. R. soc. Trop. Med. Hyg. 1993;87:386–390. doi: 10.1016/0035-9203(93)90007-d. [DOI] [PubMed] [Google Scholar]
- 21.Mbogo CNM, et al. Low-level Plasmodium faiciparum transmission and the incidence of severe malaria infections on the Kenyan coast. Am. J. Trop. Med. Hyg. 1993;49:245–253. doi: 10.4269/ajtmh.1993.49.245. [DOI] [PubMed] [Google Scholar]
- 22.Trager W, jensen JB, Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 23.Inselburg J, Banyal HS. Plasmodium falciparum; Synchronization of asexual development with aphidicolin, a DNA synthesis inhibitor. Exp. Parasitol. 1984;57:48–54. doi: 10.1016/0014-4894(84)90061-4. [DOI] [PubMed] [Google Scholar]