Abstract
As nanoparticles (NPs) are cleared via phagocytes of the mononuclear phagocyte system (MPS), we hypothesized that the function of circulating monocytes and dendritic cells (MO/DC) in blood can predict NP clearance (CL). We measured MO/DC phagocytosis and reactive oxygen species (ROS) production in mice, rats, dogs, and patients with refractory solid tumors. Pharmacokinetic studies of polyethylene glycol (PEG)-encapsulated liposomal doxorubicin (PEGylated liposomal doxirubicin [PLD]), CKD-602 (S-CKD602), and cisplatin (SPI-077) were performed at the maximum tolerated dose. MO/DC function was also evaluated in patients with recurrent epithelial ovarian cancer (EOC) administered PLD. Across species, a positive association was observed between cell function and CL of PEGylated liposomes. In patients with EOC, associations were observed between PLD CL and phagocytosis (R2 = 0.43, P = 0.04) and ROS production (R2 = 0.61, P = 0.008) in blood MO/DC. These findings suggest that probes of MPS function may help predict PEGylated liposome CL across species and PLD CL in patients with EOC.
Introduction
Nanoparticles (NPs), which include polyethylene glycol (PEG)-encapsulated (PEGylated) liposomes, are novel drug-delivery platforms that have the potential to improve tumor drug exposure and reduce accumulation in normal tissues more so than their small-molecule counterparts (Zamboni and Tonda, 2000; Zamboni, 2005, 2008). The pharmacokinetics (PK) of NPs are dependent upon the carrier and not the drug encapsulated within the carrier until the drug gets released from the carrier (Papahadjopoulos et al., 1991; Park et al., 2004; Zamboni, 2005, 2008). The drug that remains encapsulated within NPs is an inactive prodrug, and thus the drug must be released from the carrier to be active. After the drug is released from the carrier, the PK disposition of the drug will be the same as that following administration of the noncarrier form of the drug (Zamboni and Tonda, 2000; Zamboni, 2005, 2008).
The PK disposition of PEGylated liposomal formulations of CKD602 (S-CKD602), doxorubicin (Doxil; Janssen (of J&J), Titusville, NJ), and cisplatin (SPI-077), have been evaluated in preclinical models and patients (Lee et al., 2000; Gabizon et al., 2003; Zamboni et al., 2007). The ability to extrapolate animal data to predict PK parameters in humans is an essential step in drug development (Gabizon et al., 2008). We have previously explored the use of allometric scaling to predict the PK of PEGylated liposomal agents across species (Caron et al., 2011). Our study indicated that while a relationship exists between species body weight and clearance (CL), there is considerable variability in PK among species, particularly when scaled by conventional and nonconventional parameters. Thus, the development of new methods of scaling and/or measures of NP interaction at the biologic level are warranted to further explore the variability observed in NP PK.
Studies suggest that the significantly high and clinically relevant interpatient variability in the PK and pharmacodynamic (PD) disposition of NP anticancer agents is related to the function of monocytes and dendritic cells (MO/DC) of the mononuclear phagocyte system (MPS) (Zamboni et al., 2011a; Zamboni et al., 2011b). PEGylated and non-PEGylated nanoparticle agents are cleared via the MPS, but PEGylated nanoparticle agents are cleared at a slower rate because of delayed and/or reduced recognition by the MPS (Dobrovolskaia et al., 2008; Caron et al., 2012). The MPS is defined as a group of cells having the ability to ingest large numbers of particles (Hume et al., 2002). These cells (comprising MO/DC circulating in the blood, fixed macrophages of various connective tissues, Kupffer cells in the liver, and macrophages in the lymph nodes, bone marrow, and spleen) serve as a potential CL pathway for NPs (Lichanska et al., 1999; Hume et al., 2002; Dobrovolskaia et al., 2008). We have previously reported a significant relationship between the PK and PD of S-CKD602 and changes in circulating monocyte numbers and absolute neutrophil count (Zamboni et al., 2011b). The results of our previous study suggest that monocytes are more sensitive to toxic effects of S-CKD602 compared with neutrophils and that the increased sensitivity appears to be related to the liposomal formulation and not the small molecule drug, CKD-602, encapsulated inside the liposome. Thus, blood monocytes may play a key role or be a surrogate marker for NP CL in patients.
Epithelial ovarian cancer (EOC) is a disease characterized by large numbers of peritoneal MO and macrophages, the primary cells of the MPS (Bookman, 2005). As a result of high relapse rates several chemotherapeutic strategies have been developed for this patient population. PEGylated liposomal doxorubicin (PLD) is frequently used second- and third-line for the treatment of recurrent EOC and is one of the few U.S. Food and Drug Administration–approved NPs currently available (Caron et al., 2012). However, in the second-line treatment of platinum-refractory EOC, PLD achieves overall response rates of only 14 to 20% (Ozols et al., 1997; Bookman, 2005). Moreover, there is significant variability in the PK and PD associated with PLD. Therefore, some patients are much more likely to receive a nonefficacious or toxic, particularly palmar-plantar erythrodysethesia (PPE), response (La-Beck et al., 2011). Thus, there is a compelling need to guide PLD dosing to improve the response rate and quality of life for women with EOC (Amantea et al., 1997; Gabizon et al., 1994, 2008; Sidone et al., 2007; Uziely et al., 1995).
One approach to improve the treatment of patients is to identify and use a phenotypic probe to individualize therapy (van der Bol et al., 2010). A phenotypic probe is a test or agent than can be administered to a patient as an indicator of the PK and/or PD of a drug, which can then be used to individualize therapy. Phenotypic probes measuring cellular function in blood could be used to evaluate the relationship between activity of the MPS and the effect on NP PK and PD in a relatively noninvasive fashion. The objective of this study was to evaluate phenotypic probes of MPS function in blood as predictors of PK and PD of PEGylated liposomal agents in animal models and patients. The function of MO/DC and polymorphonuclear leukocytes (PMNs) of the MPS in blood was evaluated using phagocytosis and reactive oxygen species (ROS) production.
Materials and Methods
The preclinical studies were approved by the University of North Carolina’s Institutional Animal Care and Use Committee. The clinical studies were approved by the Committee for the Rights of Human Subjects (Institutional Review Board) at the University of North Carolina at Chapel Hill. All patients were advised of the purpose, procedures, and associated risks and gave written informed consent.
Phenotypic and PK Studies in Preclinical Animal Models and Patients
Phenotypic studies of MPS function and PK of PEGylated liposomal agents were performed in mice, rats, dogs and patients with refractory solid tumors as part of clinical phase I studies (Meerum Terwogt et al., 2000, 2002; Gabizon et al., 2003, 2008; Zamboni et al., 2007). A blood sample was obtained using a sodium heparinized tube from each of the species to assess MO/DC phagocytosis and ROS production using flow cytometry (methods are detailed in EOC clinical study). Blood samples were obtained prior to administration of liposomal agents in triplicate in each species.
S-CKD602, PLD, and SPI-077 were administered to SCID mice, Sprague-Dawley rats, beagle dogs, and as part of phase I clinical studies as described previously (Lee et al., 2000; Meerum Terwogt et al., 2000, 2002; Gabizon et al., 2003, 2008; Zamboni et al., 2007). Serial blood sampling times and analytical methods used to determine sum total nanoparticle concentrations are also provided in these previously published studies. The concentration versus time data were imported into Phoenix WinNonlin Version 6.1 (Pharsight Corp., Mountain View, CA) and a noncompartmental analysis was used to determine CL in each species.
PLD PK and PD Studies in EOC
Inclusion Criteria.
Women receiving PLD as part of their standard-of-care treatment of recurrent EOC were eligible for enrollment in this study. Doxil, doxorubicin, encapsulated in STEALTH liposomes, was purchased from Janssen and used in all patients. Patients had to be ≥18 years of age and have a documented hysterectomy or negative pregnancy test.
Clinical Study Design.
Baseline characteristics and treatment regimens of the 10 women enrolled are listed in Table 1. Patients were administered standard premedications, including 10 mg of dexamethasone, 25 mg of diphenhydramine, 20 mg of famotidine, and 8 mg of ondansetron, all intravenous × 1, 30 minutes prior to PLD. Patients were administered PLD at 40 mg/m2 alone or PLD at 30 mg/m2 i.v. × 1, over approximately 1 hour in combination with carboplatin infused intravenously × 1 over 30 minutes at a dose to achieve area under the curve (AUC) = 5 (Calvert equation). Serial blood PK samples were obtained at baseline prior to the administration of PLD or PLD with carboplatin; at the end of infusion; and 1, 3, 24, 48, 72, 96, 192, and 672 hours after the administration of PLD. Plasma was processed immediately, and the encapsulated and released components of PLD were separated using solid phase separation methods as described previously (Zamboni and Tonda, 2000; Zamboni et al., 2007, 2009). Noncompartmental analysis was performed using Phoenix WinNonlin Version 6.1 to calculate PK parameters (Table 2).
TABLE 1.
Baseline characteristics of patients enrolled in the study
| Patient | Age | Race | Weight | BSA | Chemotherapy |
|---|---|---|---|---|---|
| years | kg | ||||
| 1 | 63 | C | 54 | 1.63 | PLD |
| 2 | 51 | C | 73 | 1.9 | PLD |
| 3 | 57 | C | 71 | 1.78 | PLD |
| 4 | 67 | C | 89 | 1.96 | PLD + carboplatin |
| 5 | 76 | C | 91 | 1.91 | PLD |
| 6 | 51 | C | 72 | 1.84 | PLD + carboplatin |
| 7 | 53 | C | 94 | 1.94 | PLD + carboplatin |
| 8 | 75 | C | 48 | 1.46 | PLD |
| 9 | 44 | C | 77 | 1.91 | PLD + carboplatin |
| 10 | 52 | AA | 116 | 2 | PLD |
| Mean ± S.D. or Total | 59 ± 10.9 | 9 C, 1AA | 78 ± 20 | 1.8 ± 0.2 | 6 PLD alone 4 PLD + carboplatin |
AA, African American; BSA, body surface area; C, Caucasian.
TABLE 2.
PEGylated liposomal doxorubicin plasma PK parameters in patients with recurrent EOC
| Parameter |
Encapsulated |
Released |
Ratio of AUCreleased to AUCencapsulated |
||||||
|---|---|---|---|---|---|---|---|---|---|
| CL |
AUC |
t1/2 |
Vd |
CL |
AUC |
t1/2 |
Vd |
||
| ml/h | ng/ml*h | h | ml | ml/h | ng/ml*h | h | ml | ng/ml*h | |
| Mean ± S.D. | 25.7 ± 12.1 | 2,898,782 ± 974,518 | 79.6 ± 25.3 | 2,569 ± 502 | 253.0 ± 175.8 | 243,541 ± 171,578 | 189.7 ± 252.6 | 40,166 ± 34,838 | 0.10 ± 0.05 |
Blood (3 ml) was obtained at baseline, 48, 72, and 96 hours to test the function of MO/DC. At each visit, vital signs were obtained, physical examinations and blood work was performed at the discretion of the individual physician, and patients were asked about any adverse symptoms they experienced, including but not limited to nausea/vomiting, PPE, neuropathy, and stomatitis. Grade of toxicity was determined by the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI CTCAE, version 4.03). Patients were followed until disease progression or toxicity necessitated discontinuation of PLD. Progression-free survival (PFS) was determined by Response Evaluation Criteria in Solid Tumors (version 1.1).
Phenotypic Probes.
Innate immune function (phagocytosis and ROS production) of peripheral blood monocytes and PMNs was assessed by flow cytometry. Initially, data were used to determine whether there was a relationship between cellular function and PK among species. A single 1-ml blood sample was taken from each species (n = 3) used in the PK studies to test cellular function. Data were then used to assess possible correlations between cellular function (phagocytosis and ROS production) and PK (CL of PLD) and PD (PFS and PPE) of PLD in women with recurrent EOC. Studies of MPS function prior to the administration of PLD in each patient were used to predict PLD PK and PD. Additionally, the changes in cellular phagocytosis and ROS over time were assessed within and between all patients.
Flow cytometry was performed in the University of North Carolina Flow Cytometry Core Facility using a DakoCyan flow cytometer, and data were analyzed using FlowJo software (version 7.6.5.). For both the phagocytosis and ROS assays, MO/DC and PMN populations were gated based on light scatter properties (forward scatter versus side scatter) and subsequently plotted for histogram analysis (Supplemental Fig. 1). The proportion of positive cells (i.e., cells that exhibit fluorescence) was determined as those events, which shifted to the right out of the “negative” region on the fluorescence intensity scale. Mean fluorescent intensity (MFI) of the positive cell population served as an index of phagocytic or ROS activity.
Phagocytosis Assay.
Twenty microliters of fluorescein isothiocyanate–labeled opsonized Eschericia coli bacteria bioparticles (1 × 108 particles/ml) (Orpegen Pharma, San Diego, CA) were added to 100 µl of whole blood and incubated for 10 minutes at 37°C. Additional samples kept on ice (0°C) served as a negative control. After incubation, 100 µl of Trypan blue was added to quench extracellular fluorescence. Phagocytic activity (number of bacteria internalized per cell) was quantified as the MFI of the “positive” cells.
ROS Production Assay.
ROS was assessed in MO/DC in response to no stimuli and to a variety of stimulants, including opsonized nonfluorescent E. coli as a phagocytic stimulus, N-formyl-methionine-leucine-phenylalanine as a physiologic peptide, phorbol 12-myristate 13-acetate (PMA) a synthetic ester, and phosphate-buffered saline as a control (no stimulus; baseline measurement). Following a 10-minute exposure to the stimulus, nonfluorescent dihydrorhodamine 123 (Orpegen Pharma, San Diego, CA) was added to the samples as a fluorogenic substrate, which, following intracellular oxidation was converted to fluorescent rhodamine 123. MFI of rhodamine 123 fluorescence served as a quantitative measure of intracellular oxidative activity.
Statistics.
All statistical analyses were performed using SAS version 9.2 (Cary, NC) software. Simple linear regression was used to explore the linear relationship between two continuous variables, including the relationship between MO/DC or PMN cellular function and PK (CL) or PD (PPE grade and PFS). The coefficient of determination, R2 was used to measure the linear association between PK/PD outcomes and cellular function. The relationship between CL and phagocytosis was evaluated using multiple linear regression, including a term for treatment type (PLD versus PLD + carboplatin). A Kruskal-Wallis test was used to test for differences in median MFI between patients and within patients over the course of cycle 1. A Cox proportional hazards model, using progression-free survival as the outcome variable and phagocytosis as a covariate, was used to estimate predicted progression-free survival at differing levels of phagocytosis. The value of α was set at 0.05 for all statistical tests, and all P values are two-sided.
Results
Relationship between Cellular Function and PEGylated Liposome PK in Preclinical Models and Patients with Refractory Solid Tumors.
The relationship between phenotypic probes of MPS function and PK of PEGylated liposomal agents was evaluated in preclinical tumor models and in patients with refractory solid tumors as part of phase I studies of PEGylated liposomal doxorubicin (Doxil; PLD), CKD-602 (S-CKD602), and cisplatin (SPI-077). There was a direct linear relationship between MPS activity and the CL of PEGylated liposomes across mice, rats, dogs, and humans. The average mean fluorescence intensity in the MO/DC population following the phagocytosis assay in the four species evaluated was correlated with CL of PLD (R2 = 0.95), S-CKD602 (R2 = 0.99), and SPI-077 (R2 = 0.73) as shown in Fig. 1A. There was a similar trend observed when comparing the production of ROS across species without any stimulus (baseline) with CL of PLD (R2 = 0.77), S-CKD602 (R2 = 0.77), and SPI-077 (R2 = 0.66) (Fig. 1B). The relationship was also seen between production of ROS when stimulated with PMA and CL of PLD (R2 = 0.83), S-CKD602 (R2 = 0.84), and SPI-077 (R2 = 0.69).
Fig. 1.
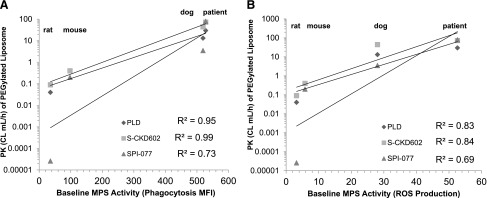
Relationship between phagocytosis (A) and production of ROS (B) in MO/DC from blood and CL of PEGylated liposomal agents in mice, rats, dogs, and patients. The ability to translate results of NP preclinical data to human patients may require measuring cellular function of the cells responsible for NP uptake and CL. The mean values for three species are represented by individual symbols, with diamonds as PLD, squares as S-CKD602, and triangles as SPI-077. The species data are in vertical columns from left to right: rats, mice, dogs, and patients. The best fit line for each group is represented by the solid lines. Across species, a positive association was observed between cell function and CL of PEGylated liposomes.
Phenotypic Probes Predict PLD PK in Patients with EOC.
The relationship between phenotypic probes of MPS function and PLD PK was evaluated in patients with EOC. On day 1 of the study, phagocytosis and ROS production were assessed in MO/DC prior to the start of the PLD infusion in patients with EOC (n = 10). A linear relationship (R2 = 0.43, P = 0.04) was found between MFI of the phagocytic cells and PLD CL for all patients, shown in Fig. 2A. A relationship between MFI of ROS production without stimulus at baseline and PLD CL for all patients was also observed, as shown in Fig. 2B (R2 = 0.61, P = 0.008). The relationship between the MPS function is more significant in patients treated with PLD alone (R2 = 0.57 and 0.61 for phagocytosis and production of ROS without stimulus, respectively) (Fig. 2, C and D).
Fig. 2.
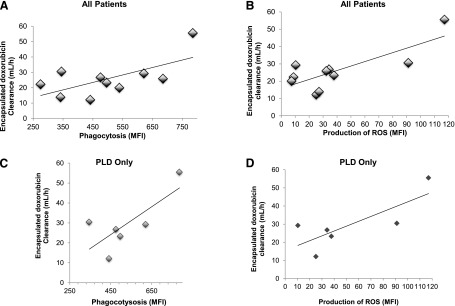
Relationship between MO/DC function and encapsulated doxorubicin CL in patients presented in a linear regression model. Measuring phagocytosis and production of ROS of MO/DC from patient blood samples at baseline (prior to the start of chemotherapy) was used as a phenotypic probe of MPS function and encapsulated doxorubicin CL. Each open circle represents an individual patient, and the solid line is the regression line. (A) Phagocytic activity (MFI) is significantly correlated with CL of encapsulated doxorubicin in 10 patients (R2 = 0.43, P = 0.04). (B) Production of reactive oxygen species: MFI is significantly correlated with CL of encapsulated doxorubicin in 10 patients (R2 = 0.61, P = 0.008). (C) Phagocytic activity: MFI is significantly correlated with CL of encapsulated doxorubicin in six patients receiving PLD alone (R2 = 0.57, P = 0.03). (D) Production of reactive oxygen species: MFI is significantly correlated with CL of encapsulated doxorubicin in six patients receiving PLD alone (R2 = 0.61, P = 0.001).
There was also a relationship between the ROS probe with the addition of a stimulant, and PLD CL in all patients at baseline (Table 3). The only stimulant that did not show a strong relationship in all patients was PMA (R2 = 0.23). PMA was also the only probe that had lower association in the PLD alone versus PLD + carboplatin group. However, the other oxidative burst stimulants performed similarly to the phagocytosis probe and also demonstrated stronger relationships in the cohort of patients which received PLD alone versus PLD + carboplatin.
TABLE 3.
Ability of an oxidative burst phenotypic probe to predict PLD clearance in patients
Linear regression of the oxidative burst probes and PLD CL in the 10 patients enrolled in the study. Values are reported for patients who received PLD (only) or PLD + carboplatin as their standard therapy for recurrent EOC.
| Stimulant | All Patients (R2, rs) | PLD Only (R2, rs) | PLD + Carboplatin (R2, rs) |
|---|---|---|---|
| No stimulant (baseline cell ROS production) | 0.61, 0.64,‡ | 0.61, 0.6,* | 0.0005, 0.4 |
| E. coli (particulate) | 0.46, 0.22,* | 0.44, 0.2 | 0.26, 0.4 |
| fMLP (physiologic) | 0.54, 0.57,* | 0.48, 0.7 | 0.14, 0.4,‡ |
| PMA (synthetic) | 0.23, 0.72 | 0.21, 0.8 | 0.59, 0.8 |
fMLP, N-formyl-methionine-leucine-phenylalanine.
P ≤ 0.001; *P ≤ 0.05.
A multiple linear regression model was also used to examine the relationship between phagocytosis and doxorubicin clearance, adjusting for treatment. The model had doxorubicin clearance as the dependent variable and phagocytosis and treatment (an indicator variable for PLD or PLD plus carboplatin) as the independent variables. This model, which results in two intercepts (intercept for PLD alone = β0 and intercept for PLD plus carboplatin = β0 + βtreatment) and a common slope (βphagocytosis), suggests a positive linear association between phagocytosis and CL of PLD (βphagocytosis = 0.04, P = 0.07) where patients with higher MPS function have a higher CL of PLD (Fig. 3). Patients on PLD plus carboplatin (dotted regression line) had somewhat lower doxorubicin clearance compared with patients on PLD only (solid regression line); however, the treatment effect was not significant (βtreatment = 6.06, P = 0.38).
Fig. 3.
The multiple linear regression model with doxorubicin clearance as the dependent variable and phagocytosis and treatment (an indicator variable for PLD or PLD plus carboplatin) as independent variables. Individual data points are represented as the symbols. The results suggest a positive linear association between phagocytosis and CL of PLD (βphagocytosis = 0.04, P = 0.07). The observations for the two treatment types are denoted by x = PLD + carboplatin and open circle = PLD only. The regression lines are displayed for the two treatment types (dotted line = PLD + carboplatin, and solid line = PLD only). This model, which results in two intercepts (intercept for PLD alone = β0 and intercept for PLD plus carboplatin = β0 + βtreatment) and a common slope (βphagocytosis), suggests that patients with higher MPS function have a higher CL of PLD. Patients on PLD + carboplatin (dotted regression line) had somewhat lower doxorubicin clearance compared with patients on PLD only (solid regression line); however, the treatment effect was not significant (βtreatment = 6.06, P = 0.38).
The correlation between either phagocytosis or ROS production in PMNs and PLD CL failed to reach statistical significance in either the total patient population or subpopulations, suggesting that PMN are not involved in the PK of PLD. In the study, 87.4 ± 10.9% of gated MO/DC in patients tested positive to the phagocytosis or ROS probe. Therefore, differences in MFI between patients were due to cellular function variability and not the ability of the assay to detect positive events.
Cellular Function over Time in Patients with EOC.
The cellular function of MO/DC and PMNs was also assessed over time in the first cycle of PLD with or without carboplatin. Phagocytosis measured in both MO/DC (P = 0.85) and PMNs (P = 0.66) was not significantly different in patients over the course of measurement (days 1, 3, 5, 28). The same held for ROS (no stimulus) in both MO/DC (P = 0.37) and PMNs (P = 0.25) over cycle 1. On day 1, just prior to PLD administration, the MFI of ROS (no stimulus) in all patients ranged from 7.4 to 117.05. The mean ± S.D. MFI of ROS was 39.1 ± 36.4 on day 1. The MFI for ROS in patient 3 was 117.05. Without patient 3, the mean ± S.D. MFI for ROS of the other nine patients was 30.5 ± 25.5.
Phenotypic Probes Predict PLD PD in Patients with EOC.
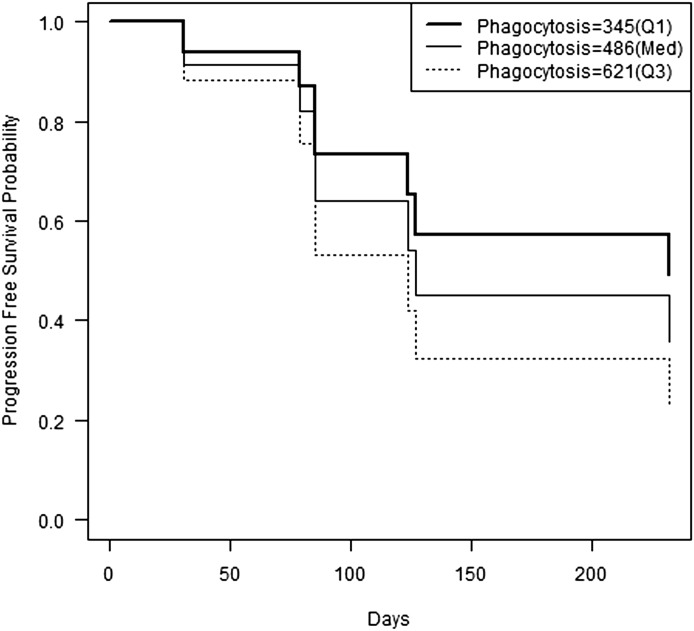
All patients enrolled in the study were followed until disease progression and/or PLD-related adverse events required discontinuation of PLD treatment. PLD could be stopped for grade 3/4 myelosuppression, stomatitis, PPE, or treating physician discretion. Patient 3 had rapidly progressive disease and died prior to the start of cycle 2 of PLD. Three additional patients (1, 8, and 10) had progressive disease while on PLD. For these four patients, the phenotypic probes of phagocytosis (R2 = 0.77, P = 0.02) and ROS (R2 = 0.67, P < 0.0001) prior to PLD administration were predictive of PFS in days (data not shown). A Cox proportional hazard model with phagocytosis as the independent variable was fit, and we determined the predicted probability of progression-free survival based on the level of MO/DC phagocytosis (Fig. 4) using the three quartiles of blood phagocytosis: Q1 = 345 (MFI), med = 486 (MFI), and Q3 = 621 (MFI).
Fig. 4.
Progression-free survival probability over time in days for 10 patients with recurrent EOC receiving PLD. Using the three quartiles of blood phagocytosis [Q1 = 345 (MFI), med = 486 (MFI), and Q3 = 621 (MFI)], a Cox proportional hazard model with phagocytosis as the independent variable can be fit to the data. The model suggests lower progression-free survival probabilities for higher levels of phagocytosis in MO/DC in blood.
PLD PK Predicts PD in Patients with EOC.
The relationship between PLD PK and PD progression-free survival and PPE was evaluated. There was a significant association observed between encapsulated PLD exposure (AUC) and PFS (days) in the four patients receiving PLD alone who progressed while on PLD treatment (R2 = 0.88, P < 0.0001) (data not shown). For the five patients who experienced PPE during the course of the study, there was a nonstatistically significant relationship between their exposure to PLD and the highest grade of PPE reported (R2 = 0.08, P = 0.6) (data not shown)
Discussion
We have previously reported a relationship between physiologic parameters such as body weight, organ blood flow, and monocyte count and the PK of PEGylated liposomes in animal models and in patients with refractory solid tumors (Caron et al., 2011). In this prior study, variability in the PK, particularly CL and exposure as measured by area under the concentration versus time profile was noted across species. However, this current study looks at a plausible biologic explanation for the variability in PK of NP across species and in a clinically relevant patient population. We found that the phagocytic capacity and level of ROS production in MO/DC in blood of mice, rats, dogs, and humans is correlated with the CL of PEGylated liposomal agents across all species. This finding, in addition to our prior clinical studies of PEGylated liposomal CKD-602 (S-CKD602), prompted the development of a second clinical study that used the same phenotypic probes of MPS function to predict PLD PK and PD in patients with recurrent EOC (Zamboni et al., 2009). For the first time, we have demonstrated that a fast and inexpensive blood test of MPS function obtained prior to the administration of PLD can be used to predict PK, efficacy, and toxicity and can be used to individualize therapy. These probes may also predict PK and PD of other NP, conjugates, monoclonal antibodies, and antibody drug conjugates in animal models and in patients (Caron et al., 2012).
We observed a linear relationship between MPS activity and the CL of PEGylated liposomes across species. The phagocytic capacity and production of ROS of MO/DC was correlated with CL of the PEGylated liposomes PLD, S-CKD602, and SPI-077. The relationship was particularly noteworthy in MO/DC phagocytosis with the CL of PLD (R2 = 0.92), S-CKD602 (R2 = 0.92), and SPI-077 (R2 = 0.77). This was the first study reporting a relationship between MPS function in blood and CL of an NP across species, including patients with cancer. The phenotypic probes developed in this study can be used to profile various types of NP agents in preclinical models and patients. In addition, the probes of MPS function can be used to determine which animal model(s) predict MPS function and PK and PD of NP in patients.
To evaluate the interaction of NPs with the MPS in a clinically relevant patient population and building upon our previous findings, we performed a clinical study using circulating MO/DC in blood as a surrogate measure of the MPS function to predict PLD PK and PD (PFS and PPE toxicity). Results of the study reported here demonstrate that probes of MPS function predict PLD PK and PD. There was a linear relationship between encapsulated doxorubicin CL and both phagocytosis (R2 = 0.43, P = 0.04) and ROS activity (R2 = 0.61, P = 0.008) in blood MO/DC.
Consistent with the association between MPS probes and PLD PK, there was an association between phagocytosis (R2 = 0.77, P = 0.02) and ROS (R2 = 0.67, P = 0.06) probes with PFS in the four patients who progressed while on PLD alone at the time of manuscript preparation. These results suggest that patients with higher MPS activity have a faster CL of PLD and a lower plasma exposure, which may be associated with less drug being available for delivery to the tumor and lower response. This relationship is further demonstrated by a Cox proportional hazard model that includes all 10 patients and assesses the relationship of the phagocytosis probe and its influence on the outcome of progression-free survival.
Our results suggest that the phenotypic probes may potentially provide valuable information toward dose individualization. Probes could be used to measure MPS function in each patient before administration of PLD, and then the dose of PLD may be adjusted based on MPS function and target plasma exposure (AUC). This is a similar process to that used to individualize carboplatin dose based on renal function and target plasma AUC (Calvert et al., 1989; Egorin et al., 1994). One MPS probe also was predictive of PPE toxicity in patients, as the ROS production at baseline was correlated with PPE grade on a scale of 0–5 (R2 = 0.56); however, this will need to be validated in a larger cohort of patients. If probes may be used to determine efficacy, such as PFS, they could also indicate early in the treatment plan whether PLD is a worthwhile option for the particular patient.
When comparing the association between phagocytosis or ROS phenotypic probes and encapsulated doxorubicin CL, patient 3 consistently had the highest value in both measures. All data points were included in this study of 10 patients; however, patient 3 noticeably improves the relationship using simple linear regression. Interestingly, patient 3 had the most extensive disease burden of all patients enrolled. A magnetic resonance imaging scan taken just prior to her start on the study indicated moderate volume ascites and multiple tumor masses abutting the liver, the largest measuring 5.2 × 3.7 cm. She was the only patient enrolled on our study with detectable tumors in her liver. Thus, the higher MPS activity and CL of PLD in this patient may be explained in part by the reported relationship between tumor metastases in liver and the CL of NP (Wu et al., 2011). Our group has previously reported that patients with primary or metastatic tumors in their liver (n = 21) had a significantly (P = 0.02) higher CL of the NP S-CKD602 compared with individuals without tumors in their liver (n = 8) (22). This suggests that patients with tumors in their liver may require a higher dose of NP compared with patients without tumors in their liver. This is a paradigm shift from what is normally seen with small-molecule agents where patients with tumors in their livers have a reduced CL of drugs that are metabolized by phase I and II enzymes (Stewart et al., 1990).
One potential reason for the difference in the relationship between encapsulated doxorubicin CL and cellular functional assays between the PLD only and PLD in combination with carboplatin could be secondary to platinum effects on the cellular function of the MPS cells. The effect of platinum agents on monocytes has been explored in vitro (Nielsen, 1984). In this study, a 1 µM exposure of cisplatin for 60 minutes was shown to selectively inhibit chemotaxis, which can then also inhibit phagocytosis, in monocytes isolated from venous blood of healthy volunteers (Nielsen, 1984). Fumarulo et al. (1980) also reported an in vitro chemotaxis inhibition by cisplatin using peritoneal macrophages of the rat. In addition, an in vivo study has reported impaired blood monocyte chemotaxis in cancer patients ≥20 hours after receiving cisplatin at 20 mg/m2 i.v. × 1 (Nielsen et al., 1985). On the basis of these studies and our results, the quick onset of chemotaxis and phagocytosis inhibition by cisplatin could explain a lack of functioning monocytes in the area of drug uptake and subsequently a lower NP CL.
We are aware that, due to the relatively small number of subjects in this study of PLD in patients with EOC, some of our statistical comparisons are likely underpowered, which may have affected our ability to detect significant relationships. Nevertheless, we were able to observe suggestive associations between monocyte function and PLD CL, PFS, and PPE toxicity in these exploratory, rather than confirmatory analyses. Moreover, the data show that the patient with the highest probe activity had a different pathophysiology than the other nine patients enrolled. Not only did this patient have the most extensive disease burden, but the highest ROS measurement. These results may indicate an environment of oxidative stress and imbalanced redox systems (Elbim and Lizard, 2009).
This study shows that the CL of PEGylated liposomes across four different species is related to the cellular function of MO/DC. MPS function can be easily measured using flow cytometry and serve as a phenotypic probe to relatively noninvasively predict the PK and PD for PLD in women with recurrent EOC. Phenotypic probes are reproducible, highly translatable, and readily transferable to clinical practice, as both the cell-based assays and flow cytometry analyses are available in hospitals and are straightforward to perform. We demonstrated that phenotypic probes can predict PLD PK, the PLD PK predicts PD, and ultimately that the phenotypic probes can predict PLD PD. The ability to employ a clinical test that is fast, inexpensive, and can be used to individualize PLD therapy and potentially treatment with other NP agents in patients is of great potential value. A randomized clinical trial comparing response and toxicity of PLD in patients with EOC treated with standard PLD based on body surface area compared with the dose of PLD individualized based on our MPS probes is planned.
Supplementary Material
Acknowledgments
The authors thank Nancy Fisher and the UNC Flow Cytometry Core Facility for use of their instruments and Charlene Santos and the UNC Animal Studies Facility for assisting with the animal studies.
Abbreviations
- AUC
area under the curve
- CL
clearance
- EOC
epithelial ovarian cancer
- MFI
mean fluorescent intensity
- MO/DC
monocytes and dendritic cells
- MPS
mononuclear phagocyte system
- NP
nanoparticle
- PD
pharmacodynamic
- PEG
polyethylene glycol
- PFS
progression-free survival
- PK
pharmacokinetic
- PLD
PEGylated liposomal doxorubicin
- PMA
phorbol 12-myristate 13-acetate
- PMN
polymorphonuclear leukocyte
- PPE
palmar-plantar erythrodysethesia
- ROS
reactive oxygen species
Author Contributions
Participated in research design: Caron, Lay, La-Beck, Clarke-Pearson, Brewster, Van Le, Bae-Jump, Gehrig, Zamboni.
Conducted experiments: Caron, Newman, La-Beck.
Contributed new reagents or analytic tools: Caron, Lay.
Performed data analysis: Caron, Fong, Lay, La-Beck, Kumar, Zhou, Monaco.
Wrote or contributed to the writing of the manuscript: Caron, Gehrig, Zamboni.
Footnotes
This work was supported by the National Institutes of Health National Cancer Institute [Grants 1U54-CA151652-01 and P01-CA142538] (to Carolina Center for Cancer Nanotechnology Excellence); the Nationals Institutes of Health National Institute of Environmental Health Sciences [Grant R01-ES0201900]; the University Cancer Research Fund Grant from UNC Lineberger Comprehensive Cancer Center; and the North Carolina Translational and Clinical Sciences Institute, NC TraC$ Award [Grant 10KR61005].This work was previously presented as follows: Caron WP, Lay JC, Fong AM, La-Beck NM, Newman SE, Clarke-Pearson DL, Brewster WR, Van Le L, Bae-Jump VL, Gehrig PA, et al. (2012) Cellular function of the mononuclear phagocyte system (MPS) as a phenotypic probe for pharmacokinetics (PK) and pharmacodynamics (PD) of PEGylated liposomal doxorubicin (PLD) in patients with recurrent ovarian cancer, in Proceedings of the 2012 Annual Meeting of the American Society of Clinical Oncology; 2012 June 1–5; Chicago, IL.
Caron WP, Lay JC, Fong AM, La-Beck NM, Newman SE, Clarke-Pearson DL, Brewster WR, Van Le L, Bae-Jump VL, Gehrig PA, et al. (2011) Cellular function of the mononuclear phagocyte system (MPS) as a phenotypic probe for pegylated liposomal doxorubicin (PLD) pharmacokinetics (PK) in patients with recurrent ovarian cancer. Proceedings of the 2011 Annual Meeting of the American Society of Clinical Oncology; 2011 June 4–8; Chicago, IL.
Conflicts of Interest/Patents Filed: Caron WP and Zamboni WC (2010), inventor; University of North Carolina at Chapel Hill, assignee. Predictors of the pharmacokinetic and pharmacodynamic disposition of carrier-mediated agents. U.S. Patent Application 61/325,698. 2010 April 19.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Amantea MA, Forrest A, Northfelt DW, Mamelok R. (1997) Population pharmacokinetics and pharmacodynamics of pegylated-liposomal doxorubicin in patients with AIDS-related Kaposi’s sarcoma. Clin Pharmacol Ther 61:301–311 [DOI] [PubMed] [Google Scholar]
- Bookman MA. (2005) Standard treatment in advanced ovarian cancer in 2005: the state of the art. Int J Gynecol Cancer 15 (Suppl 3):212–220 [DOI] [PubMed] [Google Scholar]
- Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E. (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7:1748–1756 [DOI] [PubMed] [Google Scholar]
- Caron WP, Clewell H, Dedrick R, Ramanathan RK, Davis WL, Yu N, Tonda M, Schellens JH, Beijnen JH, Zamboni WC. (2011) Allometric scaling of pegylated liposomal anticancer drugs. J Pharmacokinet Pharmacodyn 38:653–669 [DOI] [PubMed] [Google Scholar]
- Caron WP, Song G, Kumar P, Rawal S, Zamboni WC. (2012) Interpatient pharmacokinetic and pharmacodynamic variability of carrier-mediated anticancer agents. Clin Pharmacol Ther 91:802–812 [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. (2008) Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm 5:487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorin MJ, Reyno LM, Canetta RM, Jodrell DI, Swenerton KD, Pater JL, Burroughs JN, Novak MJ, Sridhara R. (1994) Modeling toxicity and response in carboplatin-based combination chemotherapy. Semin Oncol 21(5, Suppl 12)7–19 [PubMed] [Google Scholar]
- Elbim C, Lizard G. (2009) Flow cytometric investigation of neutrophil oxidative burst and apoptosis in physiological and pathological situations. Cytometry A 75:475–481 [DOI] [PubMed] [Google Scholar]
- Fumarulo R, Giordano D, Riccardi S, Aresta M. (1980) Modification of macrophages chemotaxis caused by cis-Pt(NH3)2Cl2. Proc Soc Exp Biol Med 164:164–166 [DOI] [PubMed] [Google Scholar]
- Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, Martin F, Huang A, Barenholz Y. (1994) Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res 54:987–992 [PubMed] [Google Scholar]
- Gabizon A, Shmeeda H, Barenholz Y. (2003) Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet 42:419–436 [DOI] [PubMed] [Google Scholar]
- Gabizon A, Isacson R, Rosengarten O, Tzemach D, Shmeeda H, Sapir R. (2008) An open-label study to evaluate dose and cycle dependence of the pharmacokinetics of pegylated liposomal doxorubicin. Cancer Chemother Pharmacol 61:695–702 [DOI] [PubMed] [Google Scholar]
- Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. (2002) The mononuclear phagocyte system revisited. J Leukoc Biol 72:621–627 [PubMed] [Google Scholar]
- La-Beck NM, Zamboni BA, Gabizon A, Schmeeda H, Amantea M, Gehrig PA, Zamboni WC. (2011) Factors affecting the pharmacokinetics of pegylated liposomal doxorubicin in patients. Cancer Chemother Pharmacol 69:43–50 [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee JM, Lim KH, Kim JK, Ahn SK, Bang YJ, Hong CI. (2000) Preclinical and phase I clinical studies with Ckd-602, a novel camptothecin derivative. Ann N Y Acad Sci 922:324–325 [DOI] [PubMed] [Google Scholar]
- Lichanska AM, Browne CM, Henkel GW, Murphy KM, Ostrowski MC, McKercher SR, Maki RA, Hume DA. (1999) Differentiation of the mononuclear phagocyte system during mouse embryogenesis: the role of transcription factor PU.1. Blood 94:127–138 [PubMed] [Google Scholar]
- Meerum Terwogt JM, Groenewegen G, Pluim D, Maliepaard M, Tibben MM, Huisman A, ten Bokkel Huinink WW, Schot M, Welbank H, Voest EE, et al. (2002) Phase I and pharmacokinetic study of SPI-77, a liposomal encapsulated dosage form of cisplatin. Cancer Chemother Pharmacol 49:201–210 [DOI] [PubMed] [Google Scholar]
- Meerum Terwogt JM, Tibben MM, Welbank H, Schellens JH, Beijnen JH. (2000) Validated method for the determination of platinum from a liposomal source (SPI-77) in human plasma using graphite furnace Zeeman atomic absorption spectrometry. Fresenius J Anal Chem 366:298–302 [DOI] [PubMed] [Google Scholar]
- Nielsen H. (1984) Effect of cis-platinum on human blood monocyte function in vitro. Cancer Immunol Immunother 18:223–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H, Rørth M, Bennedsen J. (1985) Monocyte chemotaxis in patients with nonseminomatous testicular carcinoma. Effect of chemotherapy. Cancer Immunol Immunother 19:68–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols RF, Schwartz PE, Eifel PJ. (1997) Ovarian cancer, fallopian tube carcinoma, and peritoneal carcinoma, in Cancer: Principles and practice of oncology (DeVita VT, Hellman S, Rosenberg SA, eds) pp 1502–1534, Lippincott-Raven, Philadelphia [Google Scholar]
- Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C, et al. (1991) Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci USA 88:11460–11464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Benz CC, Martin FJ. (2004) Future directions of liposome- and immunoliposome-based cancer therapeutics. Semin Oncol 31(6, Suppl 13)196–205 [DOI] [PubMed] [Google Scholar]
- Sidone B, Edwards R, Zamboni B, Strychor S, Maruca L, Zamboni W (2007) Evalution of body surface area (BSA) based dosing, age, and body composition as factors affecting the pharmacokinetic (PK) variability of STEALTH liposomal doxorubicin (Doxil). AACR annual meeting abstracts C107.
- Stewart CF, Arbuck SG, Fleming RA, Evans WE. (1990) Changes in the clearance of total and unbound etoposide in patients with liver dysfunction. J Clin Oncol 8:1874–1879 [DOI] [PubMed] [Google Scholar]
- Uziely B, Jeffers S, Isacson R, Kutsch K, Wei-Tsao D, Yehoshua Z, Libson E, Muggia FM, Gabizon A. (1995) Liposomal doxorubicin: antitumor activity and unique toxicities during two complementary phase I studies. J Clin Oncol 13:1777–1785 [DOI] [PubMed] [Google Scholar]
- van der Bol JM, Mathijssen RH, Creemers GJ, Planting AS, Loos WJ, Wiemer EA, Friberg LE, Verweij J, Sparreboom A, de Jong FA. (2010) A CYP3A4 phenotype-based dosing algorithm for individualized treatment of irinotecan. Clin Cancer Res 16:736–742 [DOI] [PubMed] [Google Scholar]
- Wu H, Ramanathan RK, Zamboni BA, Strychor S, Ramalingam S, Edwards RP, Friedland DM, Stoller RG, Belani CP, Maruca LJ, et al. (2011) Population pharmacokinetics of pegylated liposomal CKD-602 (S-CKD602) in patients with advanced malignancies. J Clin Pharmacol 52:180–194 [DOI] [PubMed] [Google Scholar]
- Zamboni WC, Tonda ME. (2000) New designs of clinical trials. Highlights in Oncol Practice 18:2–7 [Google Scholar]
- Zamboni WC. (2008) Concept and clinical evaluation of carrier-mediated anticancer agents. Oncologist 13:248–260 [DOI] [PubMed] [Google Scholar]
- Zamboni WC. (2005) Liposomal, nanoparticle, and conjugated formulations of anticancer agents. Clin Cancer Res 11:8230–8234 [DOI] [PubMed] [Google Scholar]
- Zamboni WC, Eiseman JL, Strychor S, Rice PM, Joseph E, Zamboni BA, Donnelly MK, Shurer J, Parise RA, Tonda ME, et al. (2011a) Tumor disposition of pegylated liposomal CKD-602 and the reticuloendothelial system in preclinical tumor models. J Liposome Res 21:70–80 [DOI] [PubMed] [Google Scholar]
- Zamboni WC, Maruca LJ, Strychor S, Zamboni BA, Ramalingam S, Edwards RP, Kim J, Bang Y, Lee H, Friedland DM, et al. (2011b) Bidirectional pharmacodynamic interaction between pegylated liposomal CKD-602 (S-CKD602) and monocytes in patients with refractory solid tumors. J Liposome Res 21:158–165 [DOI] [PubMed] [Google Scholar]
- Zamboni WC, Ramalingam S, Friedland DM, Edwards RP, Stoller RG, Strychor S, Maruca L, Zamboni BA, Belani CP, Ramanathan RK. (2009) Phase I and pharmacokinetic study of pegylated liposomal CKD-602 in patients with advanced malignancies. Clin Cancer Res 15:1466–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni WC, Strychor S, Joseph E, Walsh DR, Zamboni BA, Parise RA, Tonda ME, Yu NY, Engbers C, Eiseman JL. (2007) Plasma, tumor, and tissue disposition of STEALTH liposomal CKD-602 (S-CKD602) and nonliposomal CKD-602 in mice bearing A375 human melanoma xenografts. Clin Cancer Res 13:7217–7223 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.