Abstract
Inhibitors of dopamine β-hydroxylase (DBH), the enzyme that converts dopamine (DA) to norepinephrine (NE) in noradrenergic cells, have shown promise for the treatment of cocaine abuse disorders. However, the mechanisms underlying the beneficial effects of these compounds have not been fully elucidated. We used the drug discrimination paradigm to determine the impact of DBH inhibitors on the interoceptive stimulus properties of cocaine. Sprague-Dawley rats were trained to discriminate cocaine (5.6 mg/kg) from saline using a multicomponent, food-reinforced discrimination procedure. On test days, subjects were pretreated with the nonselective DBH inhibitor disulfiram (0–100.0 mg/kg i.p.) or the selective DBH inhibitor nepicastat (0–56.0 mg/kg i.p.) 2 hours prior to a test session either alone or in combination with cumulatively administered cocaine (0–5.6 mg/kg i.p.). Neither disulfiram nor nepicastat substituted for the cocaine stimulus when tested up to doses that nonspecifically reduced responding. However, in combination studies, pretreatment with either disulfiram or nepicastat produced leftward shifts in the cocaine dose-response function and also conferred cocaine-like stimulus effects to the selective NE transporter inhibitor, reboxetine (0.3–5.6 mg/kg i.p.). These results indicate that pharmacological inhibition of DBH does not produce cocaine-like interoceptive stimulus effects alone, but functionally enhances the interoceptive stimulus effects of cocaine, possibly due to facilitated increases in DA released from noradrenergic terminals. These findings suggest that DBH inhibitors have low abuse liability and provide support to clinical reports that some subjective effects produced by cocaine, particularly aversive effects, are enhanced after DBH inhibition.
Introduction
Disulfiram has been used for the treatment of alcoholism for several decades (Fuller et al., 1986). The mechanism of action underlying this therapeutic effect is inhibition of aldehyde dehydrogenase, which after alcohol intake causes accumulation of the toxic, intermediate alcohol metabolite acetaldehyde and a consequent aversive physiologic reaction that deters further alcohol use (Hald and Jacobsen, 1948; Johansson, 1992). Interestingly, several human laboratory and clinical studies subsequently demonstrated that disulfiram also reduces cocaine craving and promotes cocaine abstinence independent of alcohol intake, effects that could not be explained via aldehyde dehydrogenase inhibition (George et al., 2000; Petrakis et al., 2000; Carroll et al., 2004; Oliveto et al., 2011; Kosten et al., 2013). Disulfiram has since emerged as a promising candidate medication for the treatment of cocaine abuse disorders (Weinshenker and Schroeder, 2007; Gaval-Cruz and Weinshenker, 2009).
Although cocaine functionally increases extracellular levels of dopamine (DA), norepinephrine (NE), and serotonin via equipotent inhibition of the three monoamine transporters (Ritz et al., 1987, 1990), its primary reinforcing effects have been attributed to blockade of DA reuptake (Ritz et al., 1987), with NE and serotonin playing modulatory roles. The primary metabolite of disulfiram, diethyldithiocarbamate, chelates copper and therefore inhibits the function of any enzymes that require copper as a cofactor (Hald and Jacobsen, 1948; Johnston, 1953). One such enzyme is dopamine β-hydroxylase (DBH), which converts DA to NE in the final step of NE biosynthesis within noradrenergic cells. As such, disulfiram reduces NE and increases DA and DA-metabolite levels in rodents (Goldstein, 1966; Musacchio et al., 1966; Bourdélat-Parks et al., 2005) and humans (Rogers et al., 1979; Rosen and Lobo, 1987; Paradisi et al., 1991). Consequently, DBH inhibition has been proposed to mediate the pharmacotherapeutic benefits of disulfiram observed in clinical cocaine-abusing populations (Weinshenker and Schroeder, 2007; Gaval-Cruz and Weinshenker, 2009). In support of this hypothesis, the effects of disulfiram on cocaine-induced neurochemical and behavioral phenotypes in rodents are mimicked by DBH knockout and/or the selective DBH inhibitor, nepicastat (Schank et al., 2008; Gaval-Cruz et al., 2008, 2012; Devoto et al., 2013; Schroeder et al., 2013). However, the impact of disulfiram on the positive subjective effects of cocaine in humans has produced mixed results, with studies reporting facilitation (McCance-Katz et al., 1998a), attenuation (Baker et al., 2007; Grassi et al., 2007), or no change (McCance-Katz et al., 1998b; Petrakis et al., 2000). By contrast, most studies agree that some aversive effects of cocaine (e.g., anxiety, paranoia) are enhanced after pharmacological or genetic reduction of DBH activity (Hameedi et al., 1995; Cubells et al., 2000; Kalayasiri et al., 2007; Mutschler et al., 2009). In considering DBH inhibitors as a potential pharmacotherapy for cocaine dependence, it is critical to understand whether DBH inhibition has abuse potential on its own and how it alters the subjective effects of cocaine.
Drug discrimination procedures in animals have been frequently used to assess the interoceptive stimulus effects of drugs, which are believed to be related to their subjective effects in humans (Schuster and Johanson, 1988). Discrimination studies have consistently shown a predominant role for DA systems in mediating the interoceptive stimulus effects of cocaine and related psychostimulants (Woolverton, 1991; Callahan et al., 1997). Recent microdialysis experiments in rats indicate that short-term pretreatment with disulfiram blunts basal and cocaine-induced increases in NE overflow but enhances basal and cocaine-induced increases in DA overflow (Devoto et al., 2012). Those studies also determined that the excess DA detected after disulfiram treatment originated from noradrenergic neurons, which were unable to convert DA to NE due to DBH inhibition. Based on the observations that disulfiram increases basal extracellular DA levels and facilitates cocaine-induced DA increases, one might predict that DBH inhibition would produce an interoceptive stimulus that is qualitatively similar to that produced by cocaine and/or enhance the interoceptive stimulus effects of cocaine itself.
The overall goals of the present studies were 2-fold. First, we determined whether disulfiram substitutes for cocaine in rats trained to discriminate 5.6 mg/kg cocaine from saline. Second, we assessed whether pretreatment with disulfiram would alter the discriminative stimulus effects of cocaine. To confirm that any observed effects with disulfiram were mediated exclusively by actions on DBH, we examined the effects of nepicastat, a highly selective DBH inhibitor that acts via competitive antagonism at the active site on the DBH protein and also increases basal and cocaine-induced increases in DA overflow (Stanley et al., 1997; Kapoor et al., 2011; Devoto et al., 2013). Finally, to determine whether the modulatory effects of disulfiram and nepicastat were mediated by excess DA originating from noradrenergic neurons, we assessed their impact on the cocaine-like stimulus effects of the selective NE transporter inhibitor reboxetine.
Materials and Methods
Subjects
Eight adult male Sprague-Dawley rats (Charles River Laboratories Inc., Wilmington, MA) weighing approximately 300–450 g for the duration of studies served as subjects. Rats were individually housed in a climate-controlled room under a reverse 12-hour light/dark cycle (lights on 8:00 PM to 8:00 AM) and maintained at approximately 85–90% free-feeding weight by providing 16–18 g of standard rodent chow daily. Water was available ad libitum except during behavioral sessions. Behavioral experiments were conducted 5 days per week in operant chambers located within the vivarium between the hours of 2:00 PM and 6:00 PM. All studies were conducted in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Emory University.
Apparatus
Behavioral sessions were conducted in standard rodent operant chambers located within light- and sound-attenuating enclosures (Med Associates Inc., St. Albans, VT). Each chamber contained an operant panel equipped with two retractable levers (right and left), a white stimulus light located above each lever, and a receptacle for food-pellet delivery centered between the two operant levers. A house light was mounted to the chamber wall opposite of the operant panel, and a food hopper was mounted behind the operant panel that delivered 45 mg of food pellets to the food receptacle via a plastic tubing connection. A fan was secured to the enclosure wall to provide constant airflow throughout behavioral sessions and to further reduce the influence of ambient noise. Med-PC IV software (Med Associates Inc.) was interfaced with each chamber to allow for automated output control and lever-press recording.
Procedure
Discrimination Training.
Subjects were first trained 5 days per week (Monday through Friday) to lever-press using a fixed ratio (FR) schedule of reinforcement. The session began with extension of both levers into the operant chamber and the illumination of a stimulus light above each lever to signal reinforcer availability. A single lever-press on either lever (FR1) resulted in delivery of a 45-mg food pellet (F0165; Bio-Serv, Frenchtown, NJ), retraction of the lever on which the response was emitted, termination of both stimulus lights, and illumination of the house light for 5 seconds, during which responses on the other lever had no scheduled consequences. After this 5-second timeout (TO), the house light was extinguished, both stimulus lights were illuminated, and a single response on the remaining lever resulted in reinforcement and TO as described above. Both levers were then re-extended and sessions continued in this manner until 1 hour elapsed or 60 reinforcers were earned, whichever occurred first. The FR requirement was increased by 1 every 20th reinforcer and carried over across sessions. When the FR was ≥2, responses on one lever reset the ratio of the opposite lever to 0; thus, consecutive responses were required to satisfy an FR requirement and rats were forced to emit equivalent operant responses across both levers. This phase of training continued until animals performed stably across three consecutive sessions under an FR10 schedule of reinforcement.
Next, rats were trained in single-component sessions 5 days per week (Monday through Friday) to discriminate cocaine (5.6 mg/kg i.p.) from saline (1.0 ml/kg i.p.). This training dose of cocaine has been shown in several previous studies to produce a reliable and effective discriminative stimulus in rats (Craft and Stratmann, 1996; Lamas et al., 1998; Caine et al., 2000). Each rat was injected with either cocaine or saline and immediately placed into the operant chamber for a presession start delay of 10 minutes, during which all lights were off and levers were retracted. After the 10-minute start delay elapsed, both levers were extended and stimulus lights above each lever were illuminated to signal reinforcer availability. Completion of an FR10 on the injection-appropriate lever resulted in delivery of a food pellet, termination of both stimulus lights, and illumination of the house light for 5 seconds. During this TO, responses on either lever had no scheduled consequences. For half of the animals, the left lever was designated the “cocaine-appropriate” lever, and the right lever was designated the “saline-appropriate” lever. For the other half of subjects, these designations were reversed. Responses on the injection-inappropriate lever reset the ratio on the injection-appropriate lever, but otherwise had no scheduled consequences. Sessions were terminated if 10 minutes elapsed or 15 reinforcers were earned, whichever occurred first. If 15 reinforcers were earned, the animals remained in the operant chamber for the remainder of the 10-minute period, but all lights were extinguished and levers were retracted. Each rat was administered cocaine in consecutive sessions until the following criteria were satisfied in a single session: 1) ≥80% of responses emitted on the injection-appropriate lever prior to first reinforcer, 2) ≥80% of responses emitted on the injection-appropriate lever across the entire session, and 3) ≥10 reinforcers earned. Next, rats were administered saline in consecutive sessions until these criteria were again satisfied, and then switched back to cocaine injections. This manner of alteration between saline and cocaine testing continued until animals had reliably satisfied the above-mentioned criteria in three consecutive, single-alternation sessions.
Next, the number of components was gradually increased over a period of several weeks until sessions consisted of a maximum of four components per day. Each daily session consisted of one to four components, with cocaine always being administered prior to the final component, preceded by zero to three saline components. Some sessions also consisted of one to four saline components without administration of cocaine. On days when more than one saline component was scheduled, the first saline component was preceded by intraperitoneal administration of saline, and subsequent saline-appropriate components were preceded by sham injections in which the animal was restrained for injection normally but a capped syringe was gently pressed against the intraperitoneal injection site surface. Between components, subjects were removed from the operant chamber, injected with either saline or cocaine (or sham injection), and immediately replaced into the operant chamber for the 10-minute start delay. The schedule of cocaine/saline administration was pseudo-randomized for each individual subject to prevent order effects and maintain the unpredictable nature of cocaine administration across sessions. This phase of training continued until animals satisfied the following criteria across five consecutive sessions: 1) ≥80% of responses emitted on the injection-appropriate lever prior to the first reinforcer of each component, 2) ≥80% of responses emitted on the injection-appropriate lever across the entire session, and 3) ≥10 reinforcers earned in each component. Once these criteria were met, discrimination testing commenced.
Discrimination Testing.
Test sessions were conducted on Tuesdays and Fridays, with training sessions conducted on intervening weekdays. Test sessions occurred only if the following criteria were satisfied for at least four of five previous training sessions and on the day immediately prior to a test session: 1) ≥80% of responses emitted on the injection-appropriate lever prior to the first reinforcer of each component, 2) ≥80% of responses emitted on the injection-appropriate lever across the entire session, and 3) ≥10 reinforcers earned in each component. If animals failed to meet these criteria prior to a test day, a training session was conducted instead. Each test session consisted of a maximum of four components, with each component preceded by increasing doses of a test drug according to well established cumulative-dosing procedures (Wenger, 1980; Schechter, 1997). Drug doses were increased by either 0.25 or 0.5 log units across components. Test sessions were identical to training sessions with the following exceptions. First, completion of an FR10 on either lever resulted in food reinforcement. Responses on one lever did not reset the ratio on the other lever, and thus consecutive responses were not required for reinforcer delivery. Second, each component was terminated if 3 minutes elapsed or 10 reinforcers were earned, whichever occurred first. If an animal earned 10 reinforcers, they remained in the operant chamber for the remainder of the 3-minute component with all lights extinguished and levers retracted. Third, if an animal failed to earn any reinforcers in a given component, nonspecific rate suppression was assumed and the session was terminated.
For generalization studies, disulfiram (0–100 mg/kg i.p.) or nepicastat (0–56 mg/kg i.p.) were administered 2 hours prior to onset of a test session in which saline was administered prior to each component. The percentage of cocaine-appropriate responding, as well as response rate, was averaged across all four components for individual animals and then a group average was determined. The pretreatment time and dose ranges for disulfiram and nepicastat were chosen based on previous studies indicating effective reductions of NE content using these parameters (Schroeder et al., 2010). For combination studies, disulfiram (0–100 mg/kg i.p.) or nepicastat (0–56 mg/kg i.p.) were administered 2 hours prior to onset of a test session in which cumulatively increasing doses of cocaine (0.56–5.6 mg/kg i.p.) or reboxetine (0.3–5.6 mg/kg i.p.) were administered prior to each component. In cocaine combination studies, multiple test sessions were conducted using partially overlapping cocaine doses to study a complete cocaine dose-response function.
Drugs.
Disulfiram (tetraethylthiuram disulfide) was purchased from Sigma-Aldrich (St. Louis, MO) and injected as a suspension in sterile 0.9% saline. Nepicastat (SYN-117) was generously provided by Synosia Therapeutics (South San Francisco, CA) and injected as a suspension in sterile 0.9% saline containing 1.5% dimethylsulfoxide (Sigma-Aldrich) and 1.5% Cremophor EL (Sigma-Aldrich). Cocaine HCl was generously provided by the National Institute on Drug Abuse (Bethesda, MD) and dissolved in sterile 0.9% saline. Reboxetine mesylate was generously provided by Pfizer Inc. (New York, NY) and dissolved in sterile 0.9% saline. Drug doses were calculated from the salt weights.
Data Analyses
For all experiments, primary dependent measures were the percentage of cocaine-appropriate lever responding (calculated as the number of lever presses on the cocaine-appropriate lever divided by the total number of responses across both levers, multiplied by 100) and response rate (calculated as the number of total responses across both levers divided by the total run time in seconds). Only responses made during periods of reinforcer availability were used for calculations (i.e., responses during TO periods were omitted). Percentage of cocaine-appropriate lever responding and response rates were determined for each component in individual animals, and mean and S.E.M. values were then calculated across the group for each data point and plotted graphically. If an animal failed to earn at least one reinforcer in a given component, the percentage of cocaine-appropriate lever responding data from that component was not included in the group mean calculation, although response rate data were included. If fewer than three animals satisfied this criterion for any particular dose of drug or drug combination, then no mean values were calculated. When nonspecific rate suppression occurred prior to the final component of a test session, then a response rate value of 0 was included in the group mean calculation for remaining doses that would have been administered in subsequent components.
For generalization studies (disulfiram alone, nepicastat alone, reboxetine alone, reboxetine + disulfiram, reboxetine + nepicastat), partial generalization of the training drug to the test drug(s) was considered present if the resulting mean percentage of cocaine-lever responding was between 40 and 80%. Full generalization of the training drug to the test drug(s) was considered present if the resulting percentage of cocaine-lever responding was ≥80%. A lack of generalization was considered present if the test drug(s) engendered <40% cocaine-appropriate responding.
For studies in which disulfiram or nepicastat was administered prior to cumulatively administered cocaine, the dose of cocaine required to engender 50% responding on the cocaine-appropriate lever (ED50) was calculated for individual rats after each pretreatment dose. ED50 values were estimated by fitting straight lines to the linear portion of the dose-response function that spanned the 50% cocaine-appropriate lever responding value and included not more than one dose that engendered responding below 25% and not more than one dose that engendered responding above 75%. When the linear portion of the curve was defined by more than two doses, a linear regression analysis was used, whereas linear interpolation was used if the linear portion was defined by two data points. ED50 values were then averaged across the group and 95% confidence limits were determined. ED50 values were considered to be significantly different from one another if their 95% confidence limits did not overlap.
For each individual rat, the highest dose of disulfiram and nepicastat that did not nonspecifically suppress responding when administered in combination with cocaine was identified. Cocaine discrimination data based on these individually identified effective doses of disulfiram or nepicastat were then averaged together, and ED50 values were again calculated and compared as described above. This analysis corrected for differential, individual sensitivities to the rate-suppressant effects of the DBH inhibitors by identifying and analyzing effects produced only by the highest pretreatment doses that failed to significantly disrupt lever-pressing. These same individually determined doses of disulfiram and nepicastat were also used for reanalysis of data acquired from reboxetine-combination studies.
Data were graphically plotted and analyzed using Prism (version 6.0; GraphPad Software Inc., La Jolla, CA). For all statistical analyses, significance was accepted at the 95% level of confidence (α = 0.05).
Results
Cocaine Discrimination
All subjects learned to reliably discriminate 5.6 mg/kg cocaine from saline within a range of 62–100 training sessions (mean 74.25). Thereafter, cocaine discrimination was maintained throughout the duration of experiments (approximately 12 months) in all rats, although one animal was euthanized prior to reboxetine-combination studies due to health issues unrelated to experimental protocols. No changes in cocaine potency or response rates were noted over the course of the studies (data not shown). In training sessions, cocaine typically produced >90% cocaine-appropriate responding, whereas saline typically produced <10% cocaine-appropriate responding.
Substitution Studies
Disulfiram and Nepicastat.
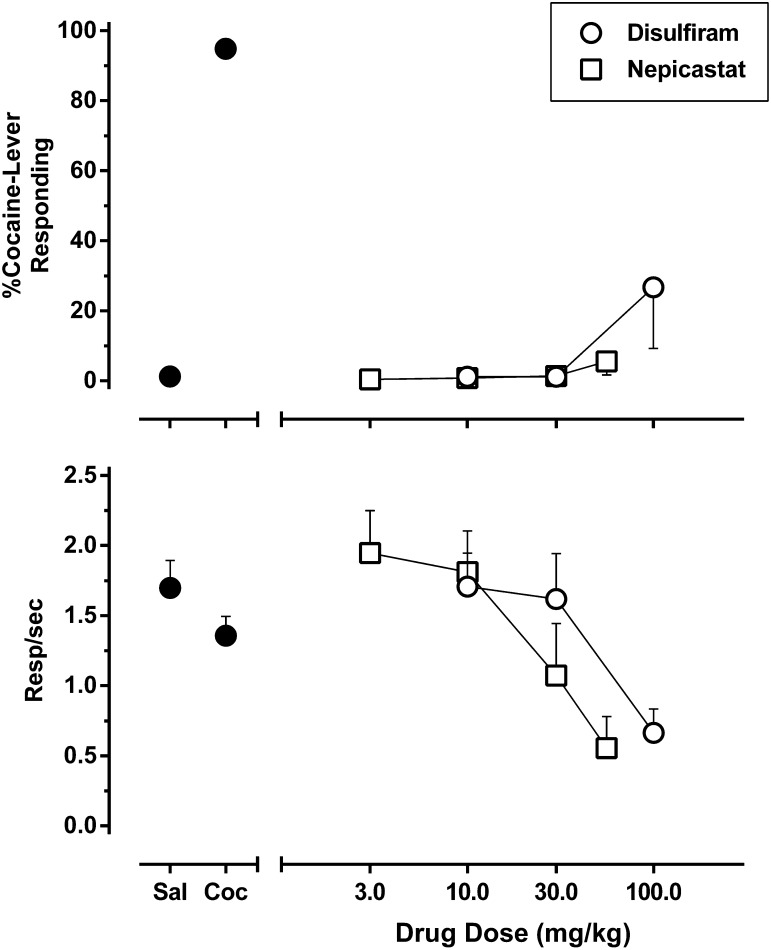
The results of disulfiram and nepicastat substitution tests are shown in Fig. 1. During training sessions, administration of saline produced approximately 1% cocaine-appropriate responding, whereas administration of the training dose of cocaine produced approximately 95% cocaine-appropriate responding. Pretreatment with disulfiram engendered primarily saline-appropriate responding across all tested doses, with maximal cocaine-appropriate responding of approximately 27% after the 100 mg/kg dose. This same pretreatment dose also reduced response rates to approximately 39% of baseline values, with only six of eight subjects meeting the response criterion, and higher doses were therefore not tested. Pretreatment with nepicastat produced similar results, with animals exhibiting a maximal level of approximately 6% cocaine-appropriate responding after pretreatment with a dose (56 mg/kg) that reduced response rates to approximately 32% baseline values, with only five of eight rats meeting the response criterion at this highest dose. Nepicastat was approximately 2-fold more potent than disulfiram at suppressing response rates.
Fig. 1.
Effect of DBH inhibitors in rats trained to discriminate 5.6 mg/kg cocaine from saline. Disulfiram (open circles) or nepicastat (open squares) was administered 2 hours prior to the onset of a test session in which all four components were preceded by saline injection. Shown is the mean ± S.E.M. of percentage cocaine-appropriate responding (top panel) and response rate (bottom panel). Data points above “Sal” and “Coc” (filled circles) depict averaged data acquired after administration of saline or the training dose of 5.6 mg/kg cocaine during training sessions, respectively. n = 8.
Combination Studies
Disulfiram/Nepicastat and Cocaine.
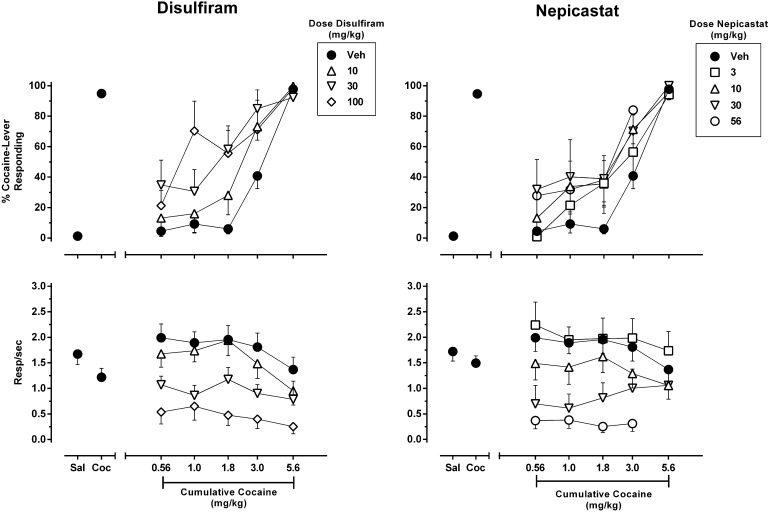
The effects of disulfiram or nepicastat pretreatment on cocaine discrimination are shown in Fig. 2. During training sessions, administration of saline produced approximately 1% cocaine-appropriate responding, whereas administration of the training dose of cocaine produced approximately 95% cocaine-appropriate responding. After pretreatment with the vehicle of either disulfiram or nepicastat, cocaine dose-dependently substituted for the training dose with an estimated ED50 value (±S.E.M.) of 3.54 ± 0.21 mg/kg, and full substitution was achieved by administration of the 5.6 mg/kg training dose. Pretreatment with disulfiram produced a dose-dependent leftward shift of the cocaine dose-response function, with the highest doses of disulfiram tested (30 and 100 mg/kg) producing estimated cocaine ED50 values of 1.68 ± 0.32 and 1.32 ± 0.36 mg/kg, respectively. Similar to disulfiram, pretreatment with nepicastat also produced a leftward shift of the cocaine dose-response function, with the maximum change achieved with the 30 mg/kg dose (cocaine ED50 of 1.99 ± 0.60 mg/kg), although there was little evidence for dose dependence. Estimated ED50 values for cocaine after pretreatment with each dose of disulfiram and nepicastat are shown in Table 1. Both disulfiram and nepicastat also produced prominent rate-decreasing effects that were largely independent of cocaine dose (Fig. 2, bottom).
Fig. 2.
Effect of DBH inhibitors on the discriminative stimulus effects of cocaine in rats trained to discriminate 5.6 mg/kg cocaine from saline. Disulfiram (left panels) or nepicastat (right panels) was administered 2 hours prior to the onset of a test session in which cocaine was cumulatively administered across multiple components. Shown is the mean ± S.E.M. of percentage cocaine-appropriate responding (top panels) and response rate (bottom panels). Data points above “Sal” and “Coc” (filled circles) depict averaged data acquired after administration of saline or the training dose of 5.6 mg/kg cocaine during training sessions, respectively. n = 8.
TABLE 1.
ED50 values for cocaine and number of animals meeting response criteria after pretreatment with disulfiram or nepicastat
Data are presented as ED50 values with 95% confidence limits and the number of animals responding.
| Pretreatment | Cocaine ED50 | Animals Responding |
|---|---|---|
| Disulfiram | ||
| Vehicle | 3.54 (3.04–4.04) | 8 |
| 10 mg/kg | 2.69 (1.93–3.41) | 8 |
| 30 mg/kg | 1.68 (0.91–2.46)a | 8 |
| 100 mg/kg | 1.32 (0.40–2.24)a | 7 |
| Nepicastat | ||
| Vehicle | 3.54 (3.04–4.04) | 8 |
| 3 mg/kg | 2.77 (1.55–3.99) | 8 |
| 10 mg/kg | 2.73 (1.69–3.78) | 8 |
| 30 mg/kg | 1.99 (0.32–3.65) | 5 |
| 56 mg/kg | 2.05 (0.50–3.59) | 4 |
P < 0.05 compared with vehicle.
Based on the rate suppression observed after disulfiram or nepicastat administration, a careful analysis of the number of subjects meeting the response criterion revealed that nepicastat disrupted responding in a larger proportion of subjects and with greater potency compared with disulfiram (Table 1). Specifically, all subjects responded after 10 and 30 mg/kg disulfiram, and seven of eight subjects responded after 100 mg/kg disulfiram. In contrast, subject attrition was apparent at lower doses of nepicastat (e.g., 30 mg/kg) and half of the rats failed to respond after the highest dose of nepicastat (56 mg/kg). We therefore speculated that the lack of dose dependence evidenced by nepicastat-induced leftward shifts of the cocaine dose-response function may have been the result of differential sensitivities to nepicastat across subjects, which was only revealed by close inspection of individual responding.
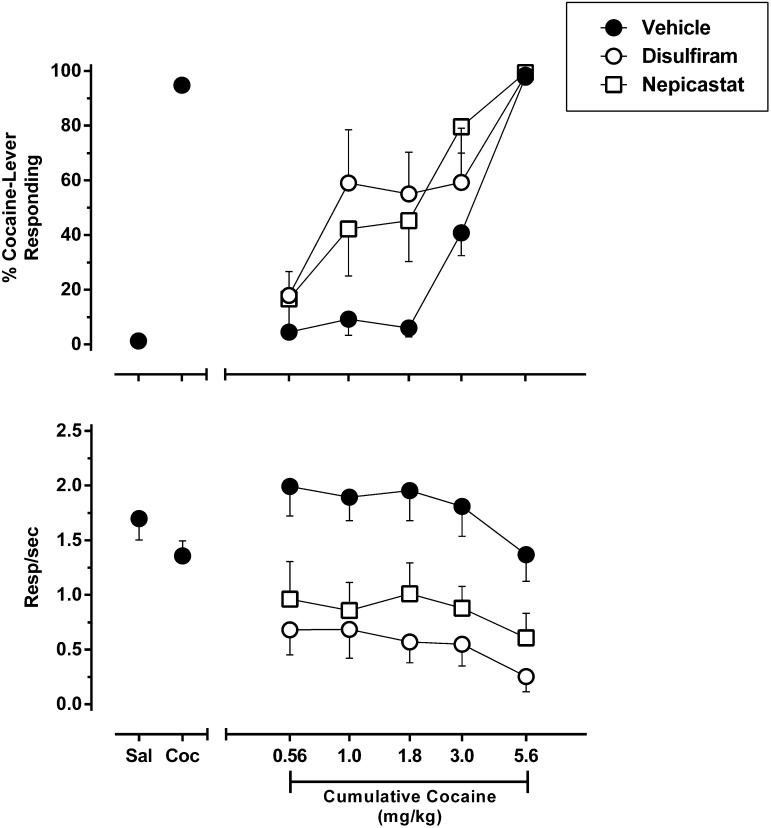
To address this concern, we identified for each individual rat the highest dose of disulfiram or nepicastat that could be administered without nonspecifically suppressing responding according to the pre-established response criteria. These doses were identified as follows: disulfiram: 30 mg/kg (n = 1) and 100 mg/kg (n = 7); and nepicastat: 10 mg/kg (n = 3), 30 mg/kg (n = 1), and 56 mg/kg (n = 4). The cocaine dose-response curves were then redetermined for each individual rat using these predetermined doses of disulfiram and nepicastat (Fig. 3). This reanalysis that controlled for individual difference in rate suppression revealed that disulfiram and nepicastat produced nearly identical leftward shifts of the cocaine dose-response function, with changes in ED50 cocaine values being significantly different from vehicle pretreatment (Table 2). It is noteworthy that both DBH inhibitors also produced similar reductions in response rate that were again independent of cocaine dose.
Fig. 3.
Effect of DBH inhibitors on the discriminative stimulus effects of cocaine in rats trained to discriminate 5.6 mg/kg cocaine from saline. Shown is a reanalysis of the data shown in Figure 2 in which the highest dose of disulfiram and nepicastat that failed to nonspecifically suppress response rates based on criteria described in detail in Materials and Methods were identified for each individual subject. n = 8.
TABLE 2.
ED50 values for cocaine after pretreatment with the highest doses of disulfiram and nepicastat that failed to nonspecifically suppress responding (identified for each individual subject)
Data are presented as ED50 values with 95% confidence limits.
| Pretreatment | Cocaine ED50 |
|---|---|
| Vehicle | 3.54 (3.04–4.04) |
| Disulfiram | 1.39 (0.63–2.14)a |
| Nepicastat | 1.95 (0.86–3.03)a |
P < 0.05 compared with vehicle.
Disulfiram/Nepicastat and Reboxetine.
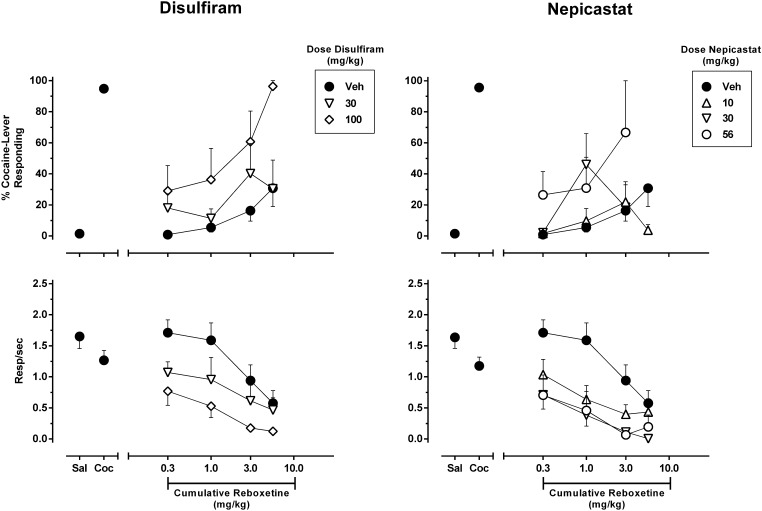
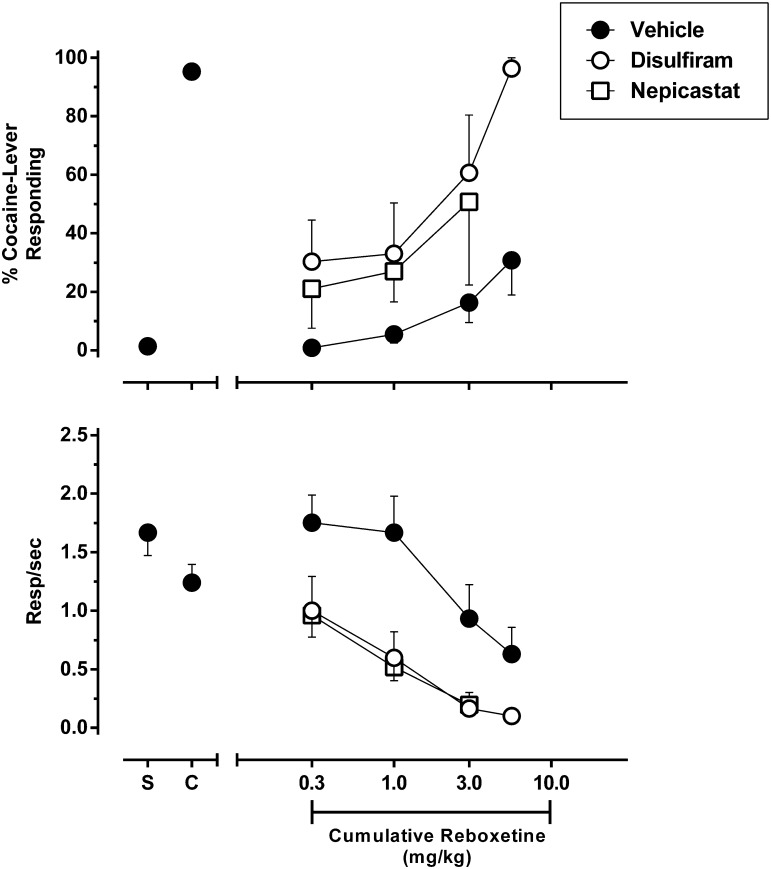
To determine the role of catecholamine release originating from noradrenergic neurons in mediating the modulatory effect of DBH inhibition on cocaine discrimination, we assessed the cocaine-like stimulus effects of the selective NE transporter (NET) inhibitor reboxetine alone and after pretreatment with disulfiram or nepicastat. The results are shown in Fig. 4. During training sessions, administration of saline produced approximately 1% cocaine-appropriate responding, whereas administration of the training dose of cocaine produced approximately 95% responding. After pretreatment with the vehicle of disulfiram or nepicastat, reboxetine failed to substitute for the training dose of cocaine but dose-dependently increased responding on the cocaine-appropriate lever with a maximal effect of approximately 31% after a cumulative dose of 5.6 mg/kg. This dose of reboxetine also reduced response rates to approximately 38% baseline levels, and therefore higher doses were not tested. Pretreatment with disulfiram produced a leftward and upward shift of the reboxetine dose-response function. After the highest pretreatment dose of disulfiram (100 mg/kg), an intermediate dose of reboxetine (3.0 mg/kg) partially substituted for the cocaine stimulus (approximately 61%), whereas administration of 5.6 mg/kg reboxetine produced full substitution (approximately 97%). Administration of 30 mg/kg disulfiram did not significantly alter the discriminative stimulus effects of reboxetine, because no dose of reboxetine achieved partial substitution. Pretreatment with 10 mg/kg nepicastat likewise failed to alter the discriminative stimulus properties of reboxetine. However, 56 mg/kg nepicastat produced an upward shift of the reboxetine dose-response function similar to that produced by disulfiram, as 3.0 mg/kg reboxetine partially substituted for the cocaine stimulus (approximately 67% cocaine-appropriate responding). Whether the higher cumulative dose of 5.6 mg/kg reboxetine would produce full substitution for the cocaine stimulus after 56 mg/kg nepicastat pretreatment could not be assessed because too few animals responded to satisfy criteria for inclusion of these data.
Fig. 4.
Effect of DBH inhibitors on the discriminative stimulus effects of the selective NET inhibitor reboxetine in rats trained to discriminate 5.6 mg/kg cocaine from saline. Disulfiram (left panels) or nepicastat (right panels) was administered 2 hours prior to the onset of a test session in which reboxetine was cumulatively administered across multiple components. Shown is the mean ± S.E.M. of percentage cocaine-appropriate responding (top panels) and response rate (bottom panels). Data points above “Sal” and “Coc” (filled circles) depict averaged data acquired after administration of saline or the training dose of 5.6 mg/kg cocaine during training sessions, respectively. n = 7–8.
To more directly compare the effects produced by disulfiram and nepicastat, we again reanalyzed the discrimination data using individually determined maximal doses of each pretreatment that failed to nonspecifically suppress rates of responding. As shown in Fig. 5, and similar to what was found earlier in combination with cocaine, disulfiram and nepicastat produced nearly identical shifts of the reboxetine dose-response function. These compounds also produced equivalent rate-decreasing effects that were independent of reboxetine dose, evidenced by a parallel downward shift of the reboxetine dose-response function (Figs. 4 and 5, bottom). This additive effect on response rates was similar to that observed after combined administration of disulfiram or nepicastat with cocaine.
Fig. 5.
Effect of DBH inhibitors on the discriminative stimulus effects of the selective NET inhibitor reboxetine in rats trained to discriminate 5.6 mg/kg cocaine from saline. Disulfiram (open circles) or nepicastat (open squares) was administered 2 hours prior to the onset of a test session in which reboxetine was cumulatively administered across multiple components. Shown is a reanalysis of the data shown in Fig. 4 in which the highest dose of disulfiram and nepicastat that failed to nonspecifically suppress response rates based on criteria described in detail in Materials and Methods were identified for each individual subject. Shown is the mean ± S.E.M. of percentage cocaine-appropriate responding (top panel) and response rate (bottom panel). n = 7.
Discussion
DBH inhibitors are being considered as novel pharmacotherapeutics for the treatment of cocaine abuse disorders, but clinical studies investigating their impact on the subjective effects of cocaine have produced mixed results. Because preclinical drug discrimination procedures have been used to predict drug-induced subjective responses in humans, the present studies were undertaken to clarify the clinical data by systematically evaluating whether DBH inhibition produces cocaine-like effects and/or modulates the interoceptive stimulus effects of cocaine in rats. The major findings of this work are that both nonselective and selective DBH inhibitors fail to substitute for, but do potentiate, the discriminative stimulus effects of cocaine.
To our knowledge, these studies are the first to examine whether disulfiram or nepicastat engender cocaine-like stimulus effects in experimental animals. Recent in vivo microdialysis studies in rats found that disulfiram or nepicastat alone produced robust increases in extracellular DA levels (approximately 300% above baseline) within the prefrontal cortex (PFC) (Devoto et al., 2012, 2013), a terminal region of DAergic mesocorticolimbic projections known to play a prominent role in the abuse-related effects of cocaine (Wise, 2009). In contrast, neither DBH inhibitor altered basal DA levels within the nucleus accumbens (NAc). Because cocaine-induced increases in DA within the NAc rather than the PFC predominantly mediate the discriminative stimulus effects of cocaine (Callahan et al., 1997), it is not surprising that in the present studies, cocaine failed to generalize to either disulfiram or nepicastat. Importantly, our dose range (up to 100 mg/kg disulfiram and 56 mg/kg nepicastat) and pretreatment time (2 hours) were chosen based on our own previous work and the aforementioned microdialysis studies, which reported maximal increases in DA levels within the PFC and reductions of NE levels across multiple brain regions using these parameters (Schroeder et al., 2010; Devoto et al., 2012, 2013). Thus, the experiments were optimized to detect the presence of a cocaine-like interoceptive stimulus. At the highest dose tested, disulfiram produced some cocaine-appropriate lever responding (approximately 27%), although the effect failed to meet a priori criteria for classification as partial substitution (a minimum of 40% cocaine-appropriate lever responding). Nepicastat produced almost no cocaine-appropriate lever responding (maximal effect of approximately 6%). Because disulfiram is a nonselective DBH inhibitor and affects the function of numerous other enzymes, this small difference between disulfiram and nepicastat may be due to disulfiram-mediated off-target effects. Nevertheless, the results indicate that DBH inhibition fails to engender cocaine-like interoceptive stimulus effects.
It has been previously suggested that noradrenergic signaling more prominently contributes to the discriminative stimulus effects produced by low (e.g., 3.0 mg/kg) compared with higher (e.g., 10.0 mg/kg) doses of cocaine, based predominantly on the observation that selective NET inhibitors substituted for, or enhanced the discriminative stimulus effects of, a low dose of cocaine (Terry et al., 1994; Kleven and Koek, 1998). However, it seems unlikely that a noradrenergic component contributes to the stimulus effects produced by the 5.6 mg/kg training dose of cocaine used in the present studies for several reasons. First, and most importantly, administration of the selective NET inhibitor reboxetine alone in the present study failed to substitute for the training dose of 5.6 mg/kg cocaine (Fig. 4). Second, the discriminative stimulus effects produced by 5.6 mg/kg cocaine in rats are fully blocked by pretreatment with the DA receptor antagonist flupenthixol (Lamas et al., 1998). Third, in rats trained to discriminate low (2.5 mg/kg) versus high (10.0 mg/kg) doses of cocaine, administration of 5.0 mg/kg produced full substitution for the high cocaine dose (Kleven and Koek, 1998). Finally, if DBH inhibition was suspected to functionally antagonize the noradrenergic component of the cocaine interoceptive stimulus via reductions of cocaine-induced increases of NE levels (Devoto et al., 2012, 2013), pretreatment with disulfiram or nepicastat would be predicted to produce a rightward shift of the cocaine dose-response function; however, the exact opposite effect was observed (Fig. 2). Collectively, these findings indicate that the discriminative stimulus properties of 5.6 mg/kg cocaine in the present experiments do not involve noradrenergic mechanisms and suggest that the enhancement of the discriminative stimulus effects of cocaine and the conferment of cocaine-like stimulus properties to a NET inhibitor after DBH inhibition would likely be reproduced utilizing a higher cocaine training dose than that presently employed.
Despite their failure to substitute for a cocaine stimulus when administered alone, pretreatment with either disulfiram or nepicastat enhanced the discriminative stimulus effects of cocaine in combination studies, as indicated by a significant leftward shift of the cocaine dose-response function. Given recent evidence that cocaine-induced increases in DA overflow are dramatically enhanced after pretreatment with either disulfiram or nepicastat (Devoto et al., 2012, 2013), the observed potentiation of cocaine discriminative stimulus effects was not unexpected. The cocaine discriminative stimulus is believed to be largely dependent on cocaine-induced increases of extracellular DA within the NAc, but DA increases within the PFC also contribute to this effect (Wood and Emmett-Oglesby, 1989; Callahan et al., 1997). The results of our drug discrimination data are consistent with this notion. For example, administration of disulfiram or nepicastat alone, which selectively but modestly increases DA levels within the PFC, failed to substitute for the cocaine stimulus. However, both DBH inhibitors greatly facilitate cocaine-induced DA increases within the PFC, an effect that we now show coincides with an augmentation of cocaine discriminative stimulus effects. Therefore, it seems plausible that the modest increase in DA levels within PFC resulting from DBH inhibition alone is insufficient to produce a cocaine-like interoceptive stimulus, whereas the robust potentiation of cocaine-induced DA increases within PFC after DBH inhibition enhances the discriminative stimulus effects of low doses of cocaine.
It has been suggested that the enhancement of cocaine-mediated increases in DA overflow by DBH inhibitors can be attributed to “ectopic” DA release from noradrenergic neurons (Devoto et al., 2012). DBH inhibition prevents the conversion of DA to NE within noradrenergic neurons, yet DA synthesis in both noradrenergic and dopaminergic neurons remains unaffected. Consequently, DBH inhibition transiently “transforms” noradrenergic neurons into DA-releasing neurons, although they retain other features typical of noradrenergic cells such as the presence of α2-adrenergic autoreceptors and NET (Weinshenker et al., 2002; Sanders et al., 2006). DA overflow from these cells may therefore be enhanced after DBH inhibition due to a loss of NE tone on inhibitory autoreceptors, and/or after NET blockade by cocaine (Paladini et al., 2007; Devoto et al., 2012, 2013). In particular, the NET has a more prominent role in clearing synaptic DA within the PFC than does the DAT (Carboni et al., 1990; Tanda et al., 1997; Yamamoto and Novotney, 1998; Morón et al., 2002), which may explain why cocaine-induced DA increases are more effectively facilitated by DBH inhibitors in this brain region compared with other regions (e.g., NAc, caudate nucleus), which contain more DAT and less NET (Schroeter et al., 2000). To determine whether this putative mechanism mediated the enhancement of the cocaine discriminative stimulus after DBH inhibition, we assessed whether the cocaine-like effects of a selective NET inhibitor would be altered after pretreatment with disulfiram or nepicastat. Consistent with previous experiments in rodents and nonhuman primates (Kleven et al., 1990; Cunningham and Callahan, 1991; Spealman, 1995; Filip and Papla, 2001; Tella and Goldberg, 2001), administration of a selective NET inhibitor alone (reboxetine) failed to substitute for the cocaine stimulus in the present study. However, pretreatment with disulfiram or nepicastat shifted the reboxetine dose-response curve upward and leftward, with reboxetine producing partial-to-full substitution for the cocaine stimulus in the presence of either DBH inhibitor. This result is consistent with the hypothesis that the facilitation of cocaine-induced DA overflow after DBH inhibition arises via ectopic DA release from noradrenergic neurons and NET blockade. Importantly, neither DBH inhibition nor NET blockade alone is capable of engendering cocaine-like interoceptive stimulus effects; only their combined administration is sufficient, suggesting that a threshold of DA increases within the PFC must be surpassed in order for cocaine-like interoceptive stimulus effects to emerge.
The behavioral profile of DBH inhibition has proven complex; some effects appear to be mediated by reductions in NE, whereas others may be attributed to increases in DA. For example, whereas administration of disulfiram or nepicastat enhances the discriminative stimulus effects of cocaine (present results), cocaine-induced locomotor sensitization (Haite et al., 2003; Gaval-Cruz et al., 2012), and cocaine-induced seizures (Gaval-Cruz et al., 2008), their administration reduces progressive ratio responding for cocaine and the reinstatement of previously extinguished cocaine-seeking behavior induced by a cocaine prime, cocaine-associated cues, or stress (Schroeder et al., 2010, 2013). How can we reconcile both enhancements and suppressions of cocaine-induced behaviors with the ability of DBH inhibitors to reduce cocaine use in clinical populations? We speculate that DBH inhibition preferentially enhances the aversive subjective effects of cocaine, which deters further use and relapse, and accumulating evidence supports this idea. For example, genetic or pharmacological DBH inhibition produces a conditioned place aversion to cocaine in rodents at doses that normally support a place preference (Schank et al., 2006; Haile and Kosten, 2009). Furthermore, polymorphisms in the DBH gene that confer low DBH activity in humans is associated with high levels of cocaine-induced paranoia (Cubells et al., 2000; Kalayasiri et al., 2007), and disulfiram is consistently reported to heighten aversive effects of cocaine (Hameedi et al., 1995; McCance-Katz et al., 1998a,b; Mutschler et al., 2009). We hypothesize that the increase in the discriminative stimulus effects of cocaine we observed in the present study is consistent with the reported enhancement of cocaine’s aversive effects in humans, and propose that DBH inhibition may produce an unexpected therapeutic benefit in cocaine abusers by potentiating the aversive effects of cocaine during a relapse episode. Thus, in addition to evidence that treatment with DBH inhibitors reduces craving for cocaine and promotes abstinence by interfering with stress, cue, and drug triggers to precipitate relapse, their use may also include the added benefit of producing an aversive reaction after cocaine use that will further deter future drug intake.
Given that nepicastat has recently entered phase II clinical trials for the treatment of cocaine dependence (ClinicalTrials.gov identifier NCT01704196), the present results provide a timely assessment of DBH inhibitor impact on the interoceptive stimulus effects of cocaine. The data indicate that DBH inhibitors should not mimic the subjective effects of cocaine and are therefore unlikely to promote craving or relapse in cocaine abusers, and have a possible added benefit to their therapeutic profile whereby the aversive effects of cocaine may be enhanced.
Acknowledgments
The authors thank Synosia Therapeutics for providing nepicastat, Pfizer Inc., for providing reboxetine, and the Emory University Division of Animal Care for their exceptional services.
Abbreviations
- DA
dopamine
- DAT
dopamine transporter
- DBH
dopamine β-hydroxylase
- FR
fixed ratio
- NAc
nucleus accumbens
- NE
norepinephrine
- NET
norepinephrine transporter
- PFC
prefrontal cortex
- TO
timeout
Authorship Contributions
Participated in research design: Manvich, Weinshenker.
Conducted experiments: Manvich, DePoy.
Performed data analysis: Manvich.
Wrote or contributed to the writing of the manuscript: Manvich, Weinshenker.
Footnotes
This research was funded by the National Institutes of Health National Institute on Drug Abuse [Grants DA015040 and DA027535].
This work was previously presented at the following workshop: Manvich DF, DeBrouse L, and Weinshenker D (2013) Modulation of the discriminative-stimulus effects of cocaine by dopamine beta-hydroxylase inhibitors in rats. American Society for Pharmacology and Experimental Therapeutics; 2013 Apr 20–24; Boston, MA.
References
- Baker JR, Jatlow P, McCance-Katz EF. (2007) Disulfiram effects on responses to intravenous cocaine administration. Drug Alcohol Depend 87:202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdélat-Parks BN, Anderson GM, Donaldson ZR, Weiss JM, Bonsall RW, Emery MS, Liles LC, Weinshenker D. (2005) Effects of dopamine beta-hydroxylase genotype and disulfiram inhibition on catecholamine homeostasis in mice. Psychopharmacology (Berl) 183:72–80 [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Bergman J. (2000) Effects of dopamine D1-like and D2-like agonists in rats trained to discriminate cocaine from saline: influence of experimental history. Exp Clin Psychopharmacol 8:404–414 [DOI] [PubMed] [Google Scholar]
- Callahan PM, De La Garza R, 2nd, Cunningham KA. (1997) Mediation of the discriminative stimulus properties of cocaine by mesocorticolimbic dopamine systems. Pharmacol Biochem Behav 57:601–607 [DOI] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, Di Chiara G. (1990) Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem 55:1067–1070 [DOI] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. (2004) Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry 61:264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Stratmann JA. (1996) Discriminative stimulus effects of cocaine in female versus male rats. Drug Alcohol Depend 42:27–37 [DOI] [PubMed] [Google Scholar]
- Cubells JF, Kranzler HR, McCance-Katz E, Anderson GM, Malison RT, Price LH, Gelernter J. (2000) A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Mol Psychiatry 5:56–63 [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Callahan PM. (1991) Monoamine reuptake inhibitors enhance the discriminative state induced by cocaine in the rat. Psychopharmacology (Berl) 104:177–180 [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Saba P, Cadeddu R, Gessa GL. (2012) Disulfiram stimulates dopamine release from noradrenergic terminals and potentiates cocaine-induced dopamine release in the prefrontal cortex. Psychopharmacology (Berl) 219:1153–1164 [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G, Saba P, Bini V, Gessa GL. (2013) The dopamine beta-hydroxylase inhibitor nepicastat increases dopamine release and potentiates psychostimulant-induced dopamine release in the prefrontal cortex. Addict Biol DOI:10.111/adb.12026 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Filip M, Papla I. (2001) Does combined treatment with novel antidepressants and a dopamine D3 receptor agonist reproduce cocaine discrimination in rats? Pol J Pharmacol 53:577–585 [PubMed] [Google Scholar]
- Fuller RK, Branchey L, Brightwell DR, Derman RM, Emrick CD, Iber FL, James KE, Lacoursiere RB, Lee KK, Lowenstam I, et al. (1986) Disulfiram treatment of alcoholism. A Veterans Administration cooperative study. JAMA 256:1449–1455 [PubMed] [Google Scholar]
- Gaval-Cruz M, Schroeder JP, Liles LC, Javors MA, Weinshenker D. (2008) Effects of disulfiram and dopamine beta-hydroxylase knockout on cocaine-induced seizures. Pharmacol Biochem Behav 89:556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaval-Cruz M, Weinshenker D. (2009) Mechanisms of disulfiram-induced cocaine abstinence: antabuse and cocaine relapse. Mol Interv 9:175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaval-Cruz M, Liles LC, Iuvone PM, Weinshenker D. (2012) Chronic inhibition of dopamine β-hydroxylase facilitates behavioral responses to cocaine in mice. PLoS ONE 7:e50583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Chawarski MC, Pakes J, Carroll KM, Kosten TR, Schottenfeld RS. (2000) Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: a preliminary trial. Biol Psychiatry 47:1080–1086 [DOI] [PubMed] [Google Scholar]
- Goldstein M. (1966) Inhibition of norepinephrine biosynthesis at the dopamine-beta-hydroxylation stage. Pharmacol Rev 18:77–82 [PubMed] [Google Scholar]
- Grassi MC, Cioce AM, Giudici FD, Antonilli L, Nencini P. (2007) Short-term efficacy of Disulfiram or Naltrexone in reducing positive urinalysis for both cocaine and cocaethylene in cocaine abusers: a pilot study. Pharmacol Res 55:117–121 [DOI] [PubMed] [Google Scholar]
- Haile CN and Kosten TA (2009) The dopamine beta-hydroxylase (DßH) inhibitor nepicastat blocks cocaine reward and is anxiolytic (Abstract 553.1/Z14), 2009 Neuroscience Meeting Planner Society for Neuroscience, Chicago, IL [Google Scholar]
- Haile CN, During MJ, Jatlow PI, Kosten TR, Kosten TA. (2003) Disulfiram facilitates the development and expression of locomotor sensitization to cocaine in rats. Biol Psychiatry 54:915–921 [DOI] [PubMed] [Google Scholar]
- Hald J, Jacobsen E. (1948) A drug sensitizing the organism to ethyl alcohol. Lancet 2:1001–1004 [DOI] [PubMed] [Google Scholar]
- Hameedi FA, Rosen MI, McCance-Katz EF, McMahon TJ, Price LH, Jatlow PI, Woods SW, Kosten TR. (1995) Behavioral, physiological, and pharmacological interaction of cocaine and disulfiram in humans. Biol Psychiatry 37:560–563 [DOI] [PubMed] [Google Scholar]
- Johansson B. (1992) A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr Scand Suppl 369:15–26 [DOI] [PubMed] [Google Scholar]
- Johnston CD. (1953) The in vitro reaction between tetraethylthiuram disulfide (antabuse) and glutathione. Arch Biochem Biophys 44:249–251 [DOI] [PubMed] [Google Scholar]
- Kalayasiri R, Sughondhabirom A, Gueorguieva R, Coric V, Lynch WJ, Lappalainen J, Gelernter J, Cubells JF, Malison RT. (2007) Dopamine beta-hydroxylase gene (DbetaH) -1021C—>T influences self-reported paranoia during cocaine self-administration. Biol Psychiatry 61:1310–1313 [DOI] [PubMed] [Google Scholar]
- Kapoor A, Shandilya M, Kundu S. (2011) Structural insight of dopamine β-hydroxylase, a drug target for complex traits, and functional significance of exonic single nucleotide polymorphisms. PLoS ONE 6:e26509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Anthony EW, Woolverton WL. (1990) Pharmacological characterization of the discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther 254:312–317 [PubMed] [Google Scholar]
- Kleven MS, Koek W. (1998) Discriminative stimulus properties of cocaine: enhancement by monoamine reuptake blockers. J Pharmacol Exp Ther 284:1015–1025 [PubMed] [Google Scholar]
- Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J, Nielsen DA. (2013) Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine β-hydroxylase. Biol Psychiatry 73:219–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas X, Negus SS, Gatch MB, Mello NK. (1998) Effects of heroin/cocaine combinations in rats trained to discriminate heroin or cocaine from saline. Pharmacol Biochem Behav 60:357–364 [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. (1998a) Chronic disulfiram treatment effects on intranasal cocaine administration: initial results. Biol Psychiatry 43:540–543 [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. (1998b) Disulfiram effects on acute cocaine administration. Drug Alcohol Depend 52:27–39 [DOI] [PubMed] [Google Scholar]
- Morón JA, Brockington A, Wise RA, Rocha BA, Hope BT. (2002) Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 22:389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio JM, Goldstein M, Anagnoste B, Poch G, Kopin IJ. (1966) Inhibition of dopamine-beta-hydroxylase by disulfiram in vivo. J Pharmacol Exp Ther 152:56–61 [PubMed] [Google Scholar]
- Mutschler J, Diehl A, Kiefer F. (2009) Pronounced paranoia as a result of cocaine-disulfiram interaction: case report and mode of action. J Clin Psychopharmacol 29:99–101 [DOI] [PubMed] [Google Scholar]
- Oliveto A, Poling J, Mancino MJ, Feldman Z, Cubells JF, Pruzinsky R, Gonsai K, Cargile C, Sofuoglu M, Chopra MP, et al. (2011) Randomized, double blind, placebo-controlled trial of disulfiram for the treatment of cocaine dependence in methadone-stabilized patients. Drug Alcohol Depend 113:184–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini CA, Beckstead MJ, Weinshenker D. (2007) Electrophysiological properties of catecholaminergic neurons in the norepinephrine-deficient mouse. Neuroscience 144:1067–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradisi R, Grossi G, Pintore A, Venturoli S, Porcu E, Capelli M, Flamigni C. (1991) Evidence for a pathological reduction in brain dopamine metabolism in idiopathic hyperprolactinemia. Acta Endocrinol (Copenh) 125:246–252 [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, Rounsaville BJ. (2000) Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction 95:219–228 [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237:1219–1223 [DOI] [PubMed] [Google Scholar]
- Ritz MC, Cone EJ, Kuhar MJ. (1990) Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci 46:635–645 [DOI] [PubMed] [Google Scholar]
- Rogers WK, Benowitz NL, Wilson KM, Abbott JA. (1979) Effect of disulfiram on adrenergic function. Clin Pharmacol Ther 25:469–477 [DOI] [PubMed] [Google Scholar]
- Rosen GF, Lobo RA. (1987) Further evidence against dopamine deficiency as the cause of inappropriate gonadotropin secretion in patients with polycystic ovary syndrome. J Clin Endocrinol Metab 65:891–895 [DOI] [PubMed] [Google Scholar]
- Sanders JD, Szot P, Weinshenker D, Happe HK, Bylund DB, Murrin LC. (2006) Analysis of brain adrenergic receptors in dopamine-beta-hydroxylase knockout mice. Brain Res 1109:45–53 [DOI] [PubMed] [Google Scholar]
- Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, Seeman P, Weinshenker D. (2006) Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology 31:2221–2230 [DOI] [PubMed] [Google Scholar]
- Schank JR, Liles LC, Weinshenker D. (2008) Norepinephrine signaling through beta-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biol Psychiatry 63:1007–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter MD. (1997) Discrete versus cumulative dosing in dose-response discrimination studies. Eur J Pharmacol 326:113–118 [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Cooper DA, Schank JR, Lyle MA, Gaval-Cruz M, Ogbonmwan YE, Pozdeyev N, Freeman KG, Iuvone PM, Edwards GL, et al. (2010) Disulfiram attenuates drug-primed reinstatement of cocaine seeking via inhibition of dopamine β-hydroxylase. Neuropsychopharmacology 35:2440–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Alisha Epps S, Grice TW, Weinshenker D. (2013) The selective dopamine β-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology 38:1032–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD. (2000) Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. J Comp Neurol 420:211–232 [PubMed] [Google Scholar]
- Schuster CR, Johanson CE. (1988) Relationship between the discriminative stimulus properties and subjective effects of drugs. Psychopharmacol Ser 4:161–175 [DOI] [PubMed] [Google Scholar]
- Spealman RD. (1995) Noradrenergic involvement in the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 275:53–62 [PubMed] [Google Scholar]
- Stanley WC, Li B, Bonhaus DW, Johnson LG, Lee K, Porter S, Walker K, Martinez G, Eglen RM, Whiting RL, et al. (1997) Catecholamine modulatory effects of nepicastat (RS-25560-197), a novel, potent and selective inhibitor of dopamine-beta-hydroxylase. Br J Pharmacol 121:1803–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Frau R, Di Chiara G. (1997) Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur J Neurosci 9:2077–2085 [DOI] [PubMed] [Google Scholar]
- Tella SR, Goldberg SR. (2001) Subtle differences in the discriminative stimulus effects of cocaine and GBR-12909. Prog Neuropsychopharmacol Biol Psychiatry 25:639–656 [DOI] [PubMed] [Google Scholar]
- Terry P, Witkin JM, Katz JL. (1994) Pharmacological characterization of the novel discriminative stimulus effects of a low dose of cocaine. J Pharmacol Exp Ther 270:1041–1048 [PubMed] [Google Scholar]
- Weinshenker D, White SS, Javors MA, Palmiter RD, Szot P. (2002) Regulation of norepinephrine transporter abundance by catecholamines and desipramine in vivo. Brain Res 946:239–246 [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. (2007) There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology 32:1433–1451 [DOI] [PubMed] [Google Scholar]
- Wenger GR. (1980) Cumulative dose-response curves in behavioral pharmacology. Pharmacol Biochem Behav 13:647–651 [DOI] [PubMed] [Google Scholar]
- Wise RA. (2009) Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. Trends Neurosci 32:517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DM, Emmett-Oglesby MW. (1989) Mediation in the nucleus accumbens of the discriminative stimulus produced by cocaine. Pharmacol Biochem Behav 33:453–457 [DOI] [PubMed] [Google Scholar]
- Woolverton WL. (1991) Discriminative stimulus effects of cocaine. NIDA Res Monogr 116:61–74 [PubMed] [Google Scholar]
- Yamamoto BK, Novotney S. (1998) Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem 71:274–280 [DOI] [PubMed] [Google Scholar]