Abstract
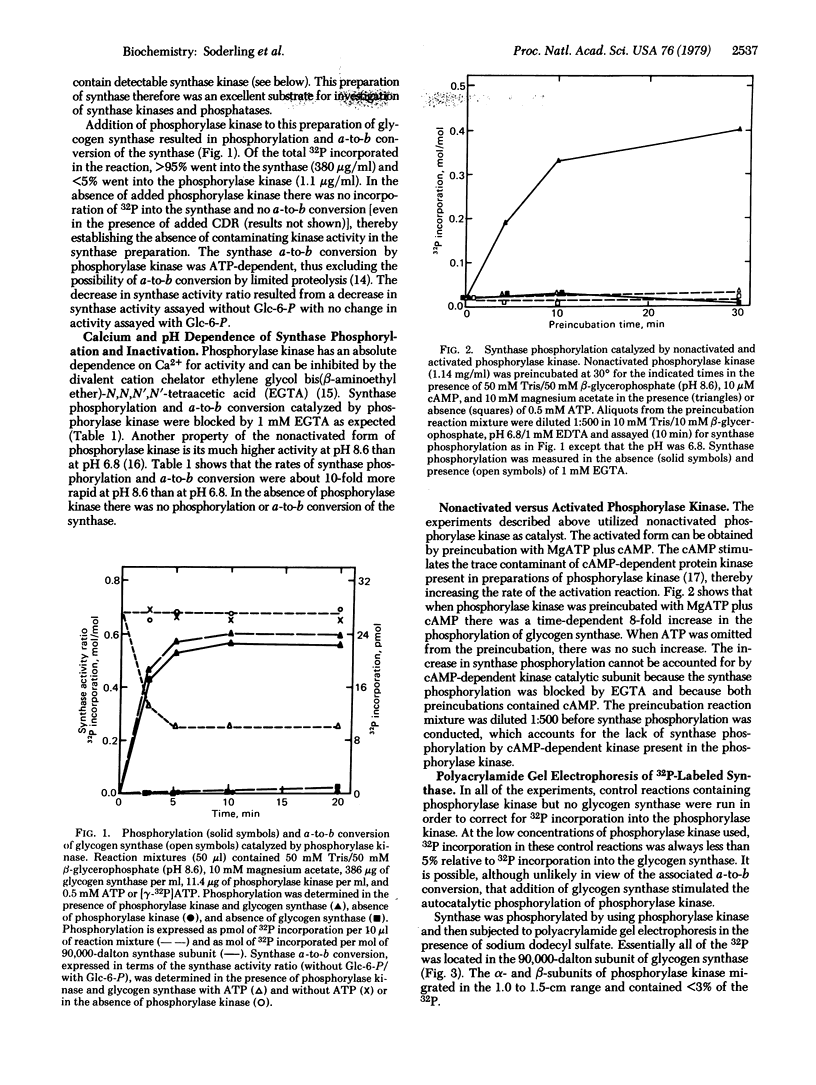
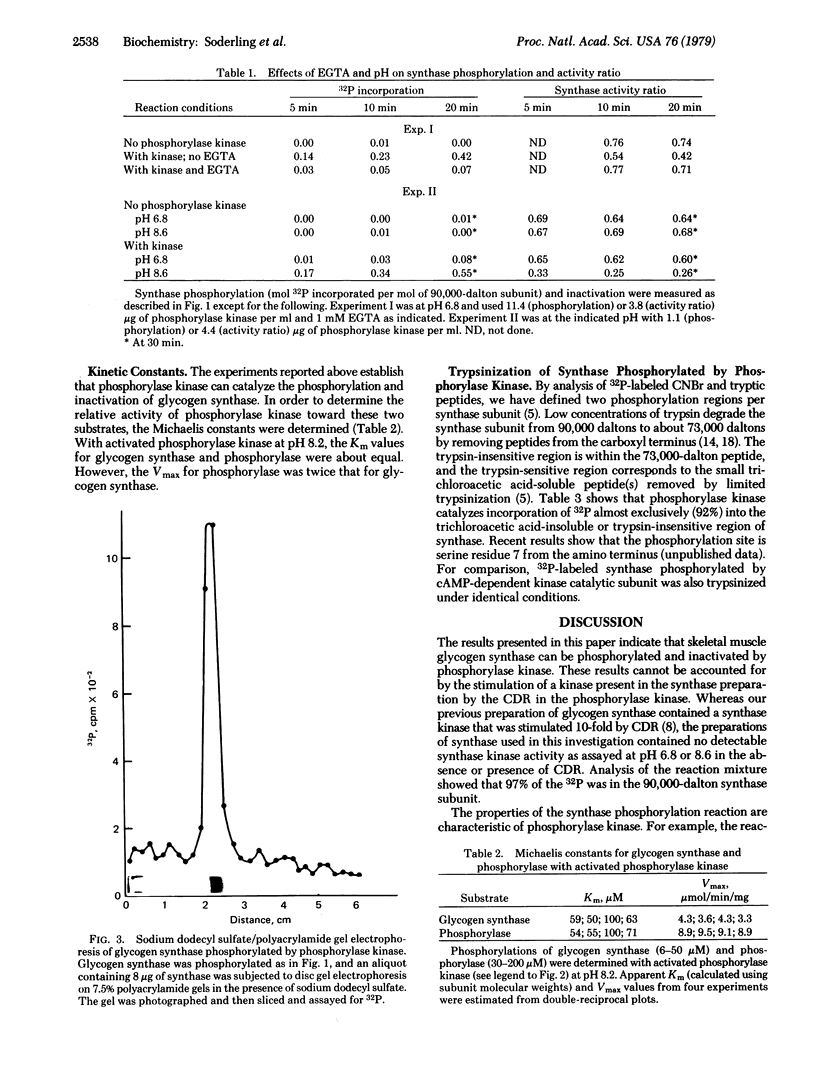
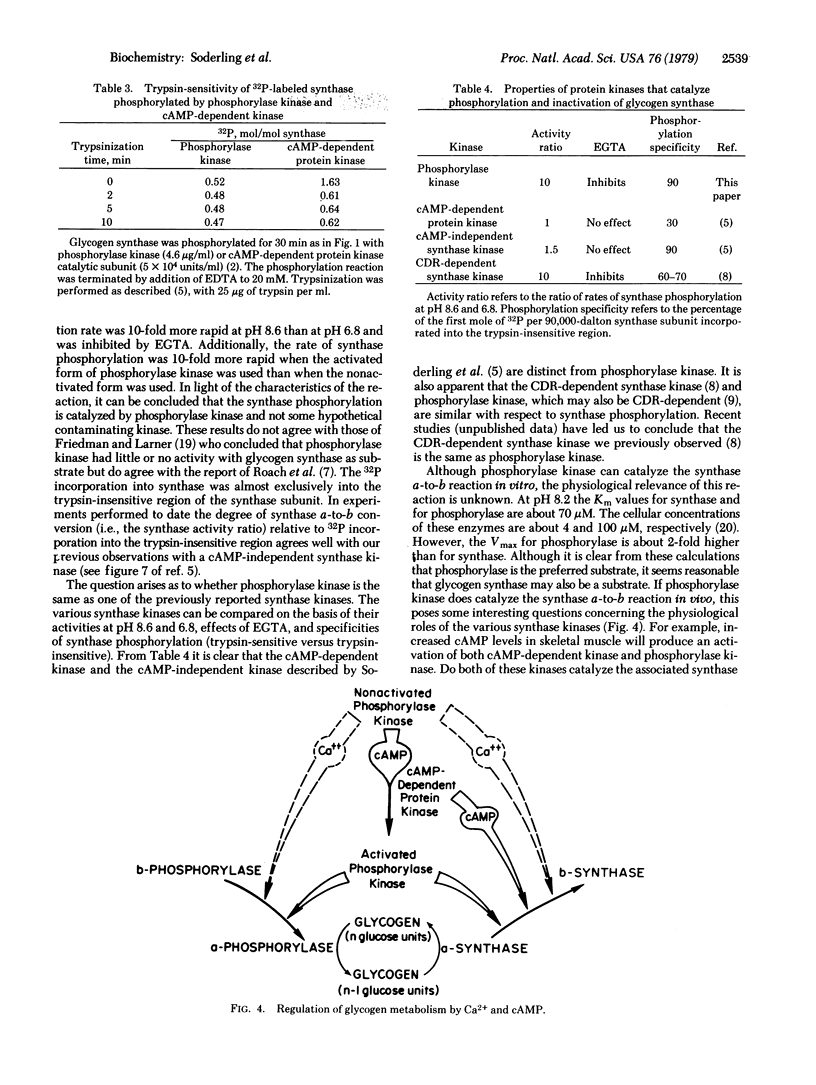
Skeletal muscle glycogen a4-synthase (EC 2.4.1.11) has been purified free of all synthase kinase and phosphatase activities by chromatography on a Glc-N-6-P-Sepharose affinity column and then on a phosphocellulose column. This preparation of glycogen synthase was tested as a substrate for purified skeletal muscle phosphorylase kinase (ATP:phosphorylase-b phosphotransferase, EC 2.7.1.38). Phosphorylase kinase (1-10 microgram/ml or 0.03-0.3 microM) catalyzes rapid phosphorylation of glycogen synthase (4.5 microM) associated with conversion of the active a form to the less active b form. In the reaction, greater than 95% of the 32P incorporation from [gamma-32P]ATP goes into the synthase subunit almost exclusively in the trypsin-insensitive region which is responsible for synthase a-to-b conversion. Synthase phosphorylation or inactivations catalyzed by phosphorylase kinase is blocked by ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid, is ATP dependent, is 10-fold more rapid at pH 8.6 than at pH 6.8, and is increased 10-fold by prior activation of the phosphorylase kinase with MgATP and cyclic AMP. With activated phosphorylase kinase at pH 8.2 the apparent Km and Vmax are approximately 70 microM and 4 mumol/min per mg with glycogen synthase and 70 microM and 9 mumol/min per mg with phosphorylase as substrate. It is concluded that glycogen synthase is a substrate in vitro for phosphorylase kinase, a Ca2+-dependent enzyme. The possible physiological significance of this reaction is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brostrom C. O., Hunkeler F. L., Krebs E. G. The regulation of skeletal muscle phosphorylase kinase by Ca2+. J Biol Chem. 1971 Apr 10;246(7):1961–1967. [PubMed] [Google Scholar]
- Brown J. H., Thompson B., Mayer S. E. Conversion of skeletal muscle glycogen synthase to multiple glucose 6-phosphate dependent forms by cyclic adenosine monophosphate dependent and independent protein kinases. Biochemistry. 1977 Dec 13;16(25):5501–5508. doi: 10.1021/bi00644a017. [DOI] [PubMed] [Google Scholar]
- Cohen P., Burchell A., Foulkes J. G., Cohen P. T., Vanaman T. C., Nairn C. Identification of the Ca2+-dependent modulator protein as the fourth subunit of rabbit skeletal muscle phosphorylase kinase. FEBS Lett. 1978 Aug 15;92(2):287–293. doi: 10.1016/0014-5793(78)80772-8. [DOI] [PubMed] [Google Scholar]
- FISCHER E. H., KREBS E. G. The isolation and crystallization of rabbit skeletal muscle phosphorylase b. J Biol Chem. 1958 Mar;231(1):65–71. [PubMed] [Google Scholar]
- Hayakawa T., Perkins J. P., Walsh D. A., Krebs E. G. Physiochemical properties of rabbit skeletal muscle phosphorylase kinase. Biochemistry. 1973 Feb;12(4):567–573. doi: 10.1021/bi00728a001. [DOI] [PubMed] [Google Scholar]
- Khatra B. S., Soderling T. R. Reversible inhibition of skeletal muscle phosphoprotein phosphatase by ATP, phosphate and fluoride. Biochem Biophys Res Commun. 1978 Nov 29;85(2):647–654. doi: 10.1016/0006-291x(78)91211-1. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., DeLange R. J., Kemp R. G., Riley W. D. Activation of skeletal muscle phosphorylase. Pharmacol Rev. 1966 Mar;18(1):163–171. [PubMed] [Google Scholar]
- Nimmo H. G., Proud C. G., Cohen P. The phosphorylation of rabbit skeletal muscle glycogen synthase by glycogen synthase kinase-2 and adenosine-3':5'-monophosphate-dependent protein kinase. Eur J Biochem. 1976 Sep;68(1):31–44. doi: 10.1111/j.1432-1033.1976.tb10762.x. [DOI] [PubMed] [Google Scholar]
- Piras R., Staneloni R. In vivo regulation of rat muscle glycogen synthetase activity. Biochemistry. 1969 May;8(5):2153–2160. doi: 10.1021/bi00833a056. [DOI] [PubMed] [Google Scholar]
- Roach P. J., DePaoli-Roach A. A., Larner J. Ca2+-stimulated phosphorylation of muscle glycogen synthase by phosphorylase b kinase. J Cyclic Nucleotide Res. 1978 Aug;4(4):245–257. [PubMed] [Google Scholar]
- Roach P. J., Takeda Y., Larner J. Rabbit skeletal muscle glycogen synthase. I. Relationship between phosphorylation state and kinetic properties. J Biol Chem. 1976 Apr 10;251(7):1913–1919. [PubMed] [Google Scholar]
- Schlender K. K., Reimann E. M. Isolation of a glycogen synthase I kinase that is independent of adenosine 3':5'-monophosphate. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2197–2201. doi: 10.1073/pnas.72.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling T. R., Jett M. F., Hutson N. J., Khatra B. S. Regulation of glycogen synthase. Phosphorylation specificities of cAMP-dependent and cAMP-independent kinases for skeletal muscle synthase. J Biol Chem. 1977 Nov 10;252(21):7517–7524. [PubMed] [Google Scholar]
- Soderling T. R. Regulation of glycogen synthetase. Effects of trypsin on the structure, activity, and phosphorylation of the skeletal muscle enzyme. J Biol Chem. 1976 Jul 25;251(14):4359–4364. [PubMed] [Google Scholar]
- Soderling T. R. Regulation of glycogen synthetase. Specificity and stoichiometry of phosphorylation of the skeletal muscle enzyme by cyclic 3':5'-AMP-dependent protein kinase. J Biol Chem. 1975 Jul 25;250(14):5407–5412. [PubMed] [Google Scholar]
- Srivastava A. K., Waisman D. M., Brostrom C. O., Soderling T. R. Stimulation of glycogen synthase phosphorylation by calcium-dependent regulator protein. J Biol Chem. 1979 Feb 10;254(3):583–586. [PubMed] [Google Scholar]
- Takeda Y., Larner J. Structural studies on rabbit muscle glycogen synthase. II. Limited proteolysis. J Biol Chem. 1975 Dec 10;250(23):8951–8956. [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Brosom C. O., Ho E. S., Kreb E. G. Catlysis of the phosphrylaseinase actition reaction. J Biol Chem. 1971 Apr 10;246(7):1968–1976. [PubMed] [Google Scholar]


