Abstract
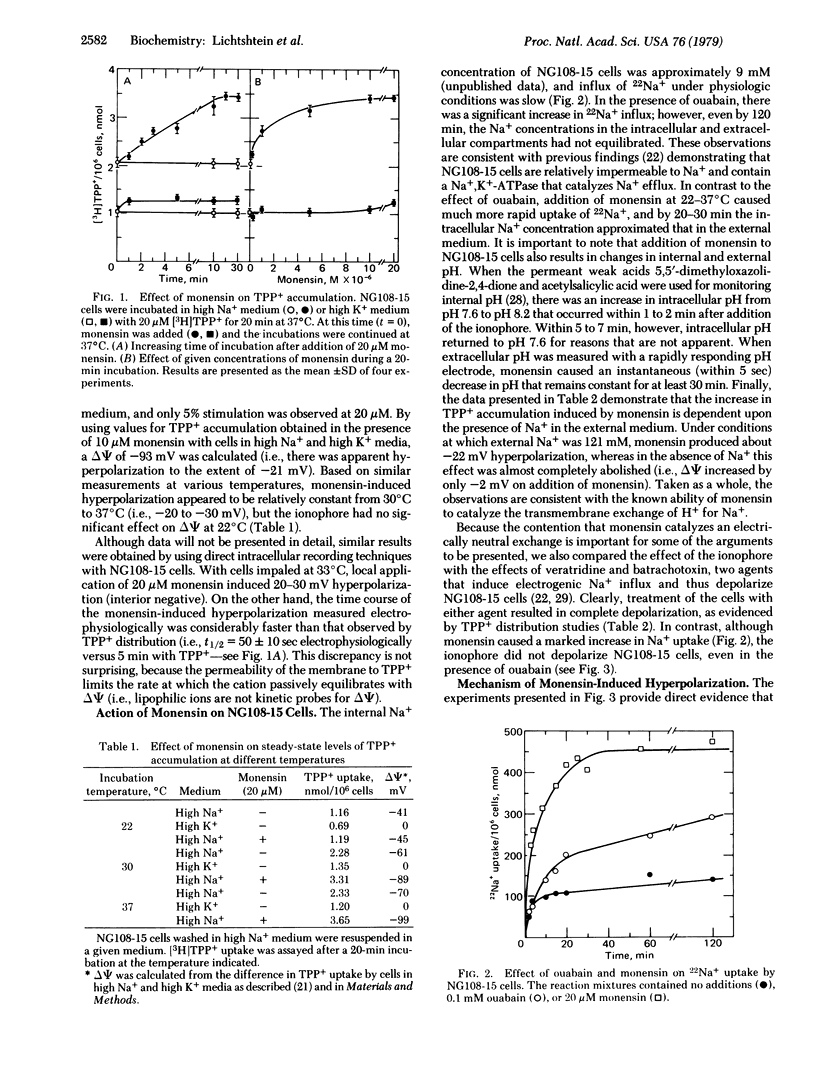
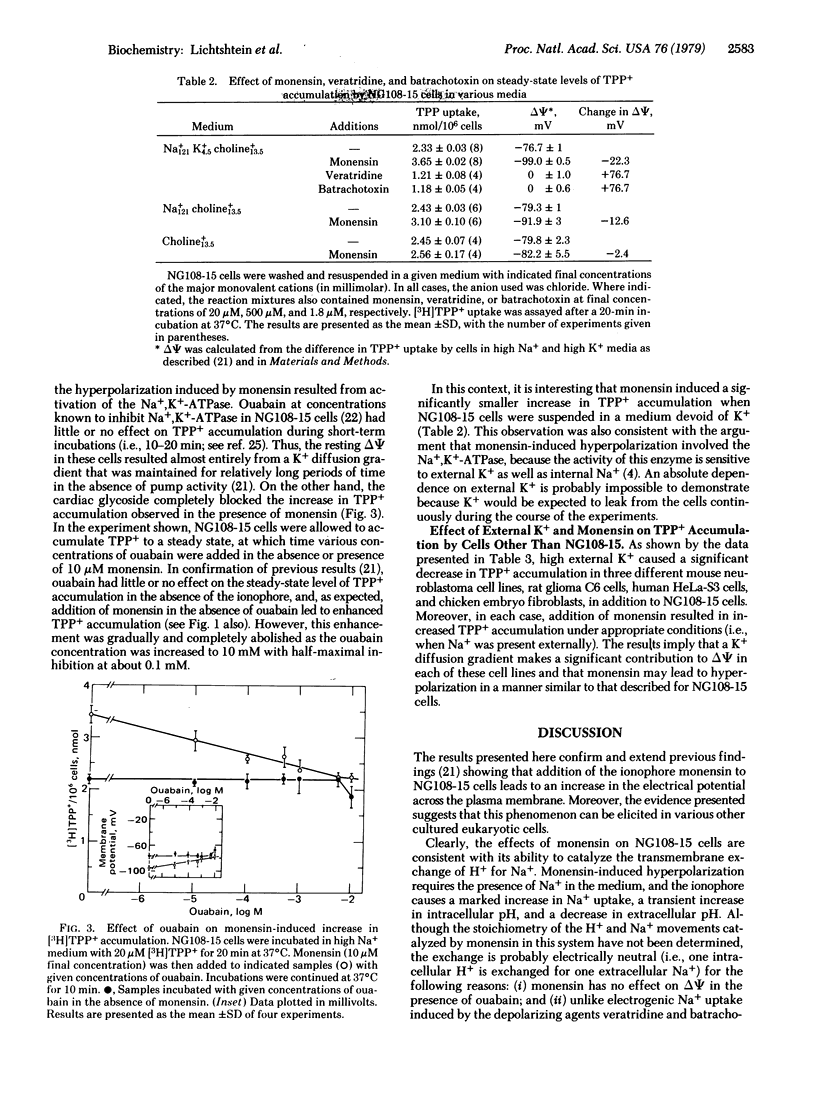
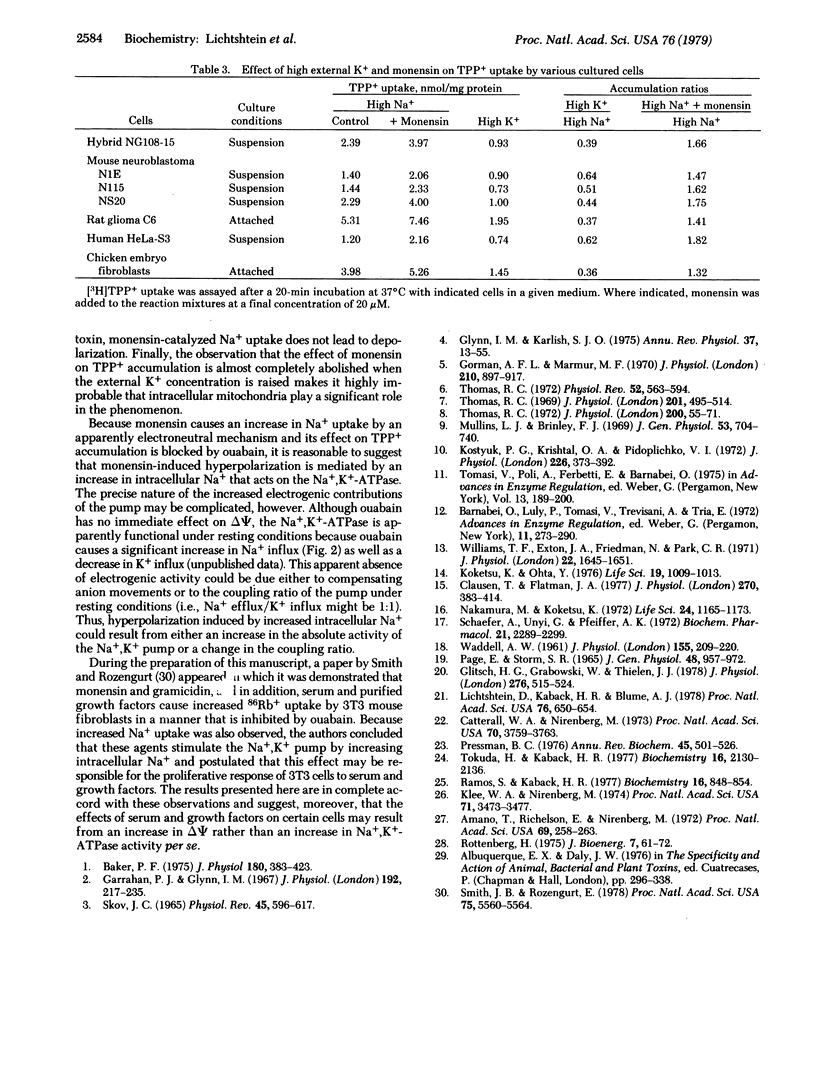
Addition of the ionophore monensin to mouse neuroblastoma-rat glioma hybrid NG108-15 cells leads to a 20 to 30-mV increase in the electrical potential across the plasma membrane as shown by direct intracellular recording techniques and by distribution studies with the lipophilic cation [3H]-tetraphenylphosphonium+ (TPP+) [Lichtshtein, D., Kaback, H.R. & Blume, A.J. (1979) Proc. Natl. Acad. Sci. USA 76, 650-654]. The effect is not observed with cells suspended in high K+ medium, is dependent upon the presence of Na+ externally, and the concentration of monensin that induces half-maximal stimulation of TPP+ accumulation is approximately 1 microM. The ionophore also causes rapid influx of Na+, a transient increase in intracellular pH, and a decrease in extracellular pH, all of which are consistent with the known ability of monensin to catalyze the transmembrane exchange of H+ for Na+. Although ouabain has no immediate effect on the membrane potential, the cardiac glycoside completely blocks the increase in TPP+ accumulation observed in the presence of monensin. Thus, the hyperpolarizing effect of monensin is mediated apparently by an increase in intracellular Na+ that acts to stimulate the electrogenic activity of the Na+,K+-ATPase. Because monensin stimulates TPP+ accumulation in a number of other cultured cell lines in addition to NG108-15, the techniques described may be of general use for studying the Na+,K+ pump and its regulation in situ.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Phosphorus metabolism of intact crab nerve and its relation to the active transport of ions. J Physiol. 1965 Sep;180(2):383–423. doi: 10.1113/jphysiol.1965.sp007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Nirenberg M. Sodium uptake associated with activation of action potential ionophores of cultured neuroblastoma and muscle cells. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3759–3763. doi: 10.1073/pnas.70.12.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Flatman J. A. The effect of catecholamines on Na-K transport and membrane potential in rat soleus muscle. J Physiol. 1977 Sep;270(2):383–414. doi: 10.1113/jphysiol.1977.sp011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The stoicheiometry of the sodium pump. J Physiol. 1967 Sep;192(1):217–235. doi: 10.1113/jphysiol.1967.sp008297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Grabowski W., Thielen J. Activation of the electrogenic sodium pump in guinea-pig atria by external potassium ions. J Physiol. 1978 Mar;276:515–524. doi: 10.1113/jphysiol.1978.sp012250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. The sodium pump. Annu Rev Physiol. 1975;37:13–55. doi: 10.1146/annurev.ph.37.030175.000305. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., Marmor M. F. Contributions of the sodium pump and ionic gradients to the membrane potential of a molluscan neurone. J Physiol. 1970 Nov;210(4):897–917. doi: 10.1113/jphysiol.1970.sp009248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee W. A., Nirenberg M. A neuroblastoma times glioma hybrid cell line with morphine receptors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3474–3477. doi: 10.1073/pnas.71.9.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koketsu K., Ohta Y. Acceleration of the electrogenic Na+ pump by adrenaline in frog skeletal muscle fibres. Life Sci. 1976 Oct 1;19(7):1009–1013. doi: 10.1016/0024-3205(76)90292-7. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I. Potential-dependent membrane current during the active transport of ions in snail neurones. J Physiol. 1972 Oct;226(2):373–392. doi: 10.1113/jphysiol.1972.sp009989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtshtein D., Kaback H. R., Blume A. J. Use of a lipophilic cation for determination of membrane potential in neuroblastoma-glioma hybrid cell suspensions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):650–654. doi: 10.1073/pnas.76.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Potassium fluxes in dialyzed squid axons. J Gen Physiol. 1969 Jun;53(6):704–740. doi: 10.1085/jgp.53.6.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE E., STORN S. R. CAT HEART MUSCLE IN VITRO. 8. ACTIVE TRANSPORT OF SODIUM IN PAPILLARY MUSCLES. J Gen Physiol. 1965 May;48:957–972. doi: 10.1085/jgp.48.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The electrochemical proton gradient in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):848–854. doi: 10.1021/bi00624a006. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of transmembrane electrochemical proton gradients. J Bioenerg. 1975 May;7(2):61–74. doi: 10.1007/BF01558427. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Schaefer A., Unyi G., Pfeifer A. K. The effects of a soluble factor and of catecholamines on the activity of adenosine triphosphatase in subcellular fractions of rat brain. Biochem Pharmacol. 1972 Sep 1;21(17):2289–2294. doi: 10.1016/0006-2952(72)90379-6. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Rozengurt E. Serum stimulates the Na+,K+ pump in quiescent fibroblasts by increasing Na+ entry. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5560–5564. doi: 10.1073/pnas.75.11.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular sodium activity and the sodium pump in snail neurones. J Physiol. 1972 Jan;220(1):55–71. doi: 10.1113/jphysiol.1972.sp009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Membrane current and intracellular sodium changes in a snail neurone during extrusion of injected sodium. J Physiol. 1969 Apr;201(2):495–514. doi: 10.1113/jphysiol.1969.sp008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Kaback H. R. Sodium-dependent methyl 1-thio-beta-D-galactopyranoside transport in membrane vesicles isolated from Salmonella typhimurium. Biochemistry. 1977 May 17;16(10):2130–2136. doi: 10.1021/bi00629a013. [DOI] [PubMed] [Google Scholar]
- WADDELL A. W. Adrenaline, noradrenaline and potassium fluxes in rabbit auricles. J Physiol. 1961 Feb;155:209–220. doi: 10.1113/jphysiol.1961.sp006623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. F., Exton J. H., Friedmann N., Park C. R. Effects of insulin and adenosine 3',5'-monophosphate on K+ flux and glucose output in perfused rat liver. Am J Physiol. 1971 Dec;221(6):1645–1651. doi: 10.1152/ajplegacy.1971.221.6.1645. [DOI] [PubMed] [Google Scholar]


