Abstract
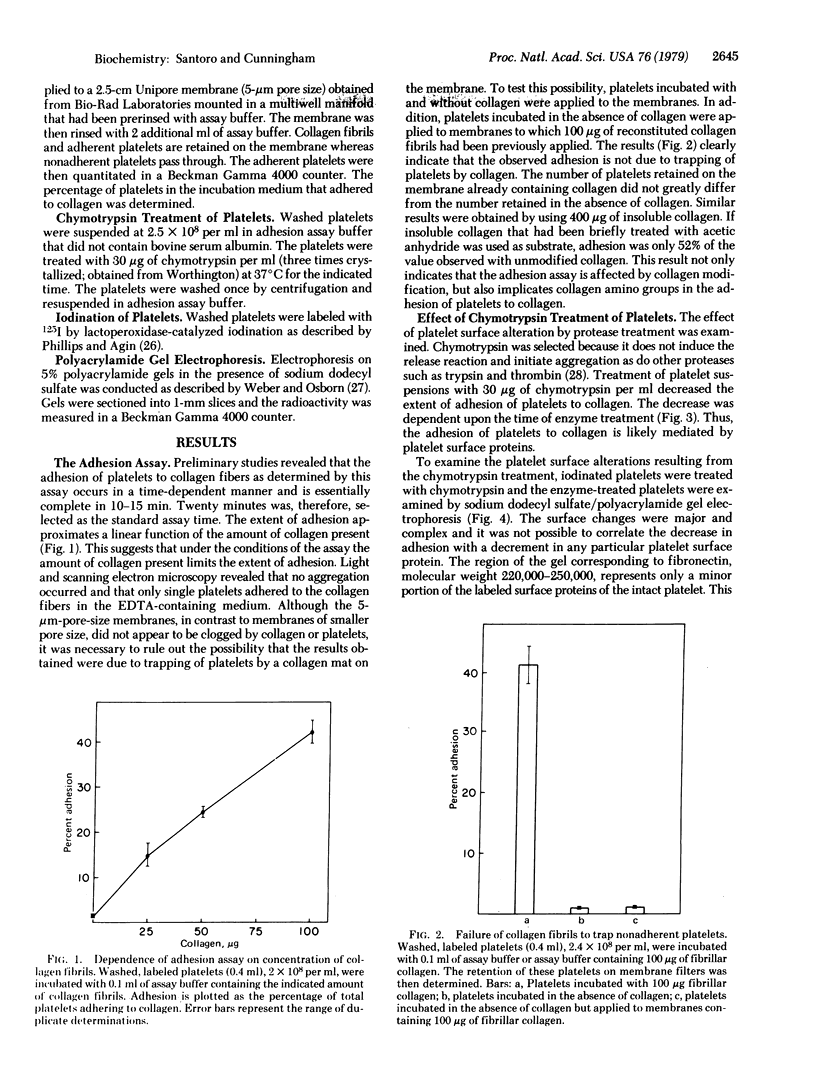
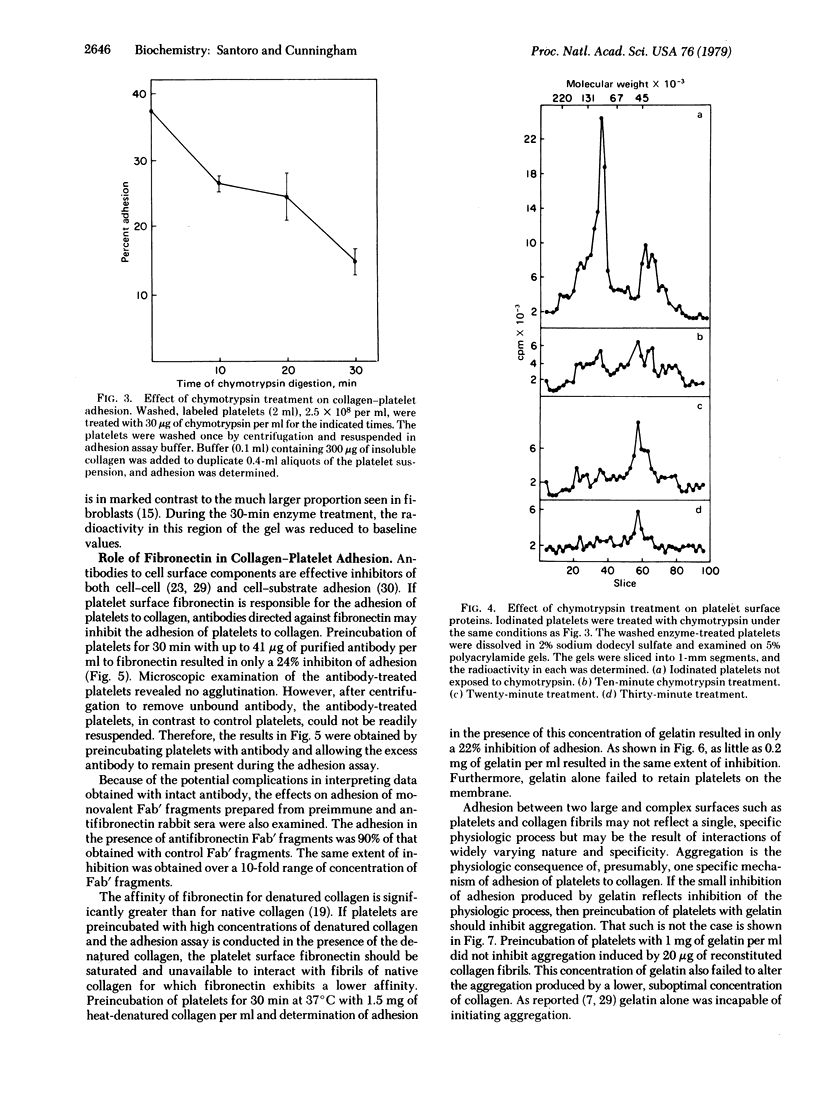
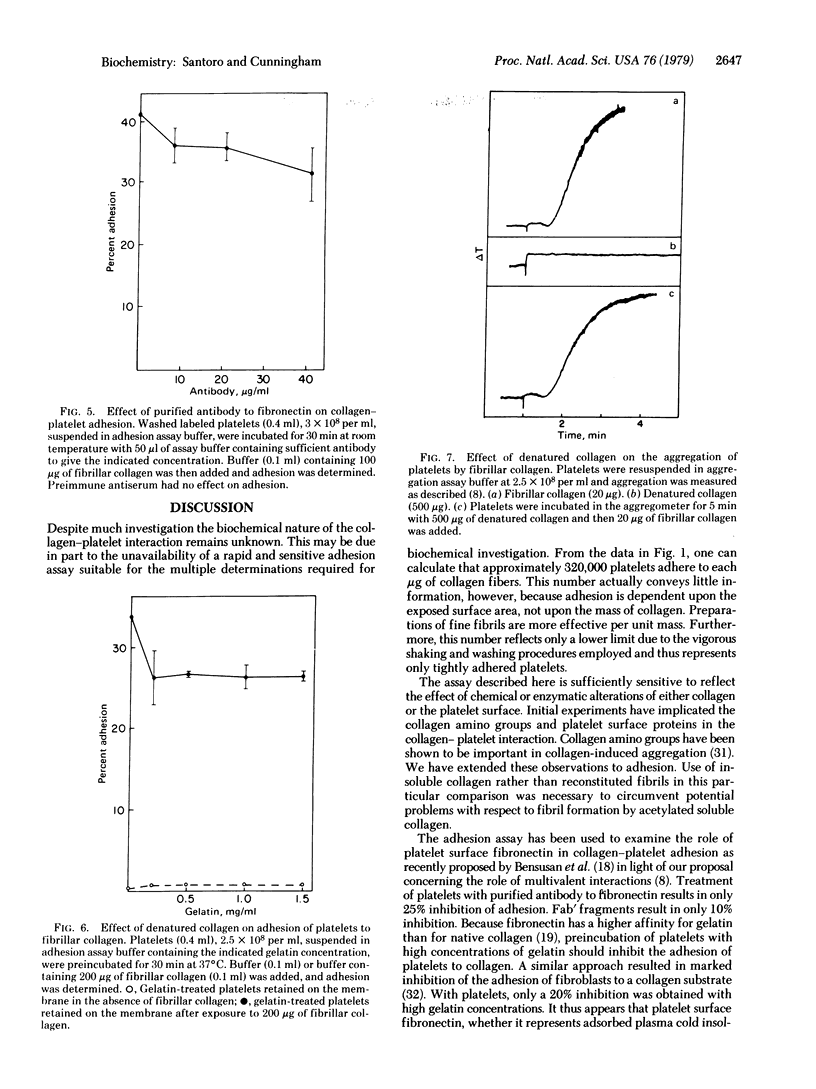
A rapid, sensitive, and reproducible assay to determine the adhesion of platelets to collagen has been developed. Collagen fibers and adherent platelets are retained on polycarbonate membrane filters. Chemical modification of collagen by acetylation and of platelets by treatment with chymotrypsin markedly reduces adhesion. The role of fibronectin in the collagen-platelet interaction has been examined. Treatment of platelets with purified antibody or Fab' fragments to fibronectin only slightly reduces adhesion. Preincubation of platelets with high concentrations of gelatin reduces adhesion by only 22% but fails to inhibit aggregation. Thus, fibronectin has only a limited role in the adhesion of platelets to collagen and is either not involved in the adhesion that leads to aggregation or is only one of several adhesion mechanisms, any of which alone can initiate aggregation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balleisen L., Gay S., Marx R., Kühn K. Comparative investigation on the influence of human and bovine collagen types I, II and III on the aggregation of human platelets. Klin Wochenschr. 1975 Oct 1;53(19):903–905. doi: 10.1007/BF01468982. [DOI] [PubMed] [Google Scholar]
- Balleisen L., Marx R., Kühn K. Platelet-collagen interaction. The influence of native and modified collagen (Type I) on the aggregation of human platelets. Haemostasis. 1976;5(3):155–164. doi: 10.1159/000214131. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Gordon J. L., MacIntyre D. E. Platelet-aggregating activity of type I and type III collagens from human aorta and chicken skin. Biochem J. 1976 Dec 15;160(3):647–651. doi: 10.1042/bj1600647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner H. R. Platelet interaction with collagen fibrils in flowing blood. I. Reaction of human platelets with alpha chymotrypsin-digested subendothelium. Thromb Haemost. 1977 Feb 28;37(1):1–16. [PubMed] [Google Scholar]
- Bensusan H. B., Koh T. L., Henry K. G., Murray B. A., Culp L. A. Evidence that fibronectin is the collagen receptor on platelet membranes. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5864–5868. doi: 10.1073/pnas.75.12.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., Piez K. A. The nature of the intramolecular cross-links in collagen. The separation and characterization of peptides from the cross-link region of rat skin collagen. Biochemistry. 1966 Nov;5(11):3460–3473. doi: 10.1021/bi00875a012. [DOI] [PubMed] [Google Scholar]
- Brackenbury R., Thiery J. P., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. I. An immunological assay for molecules involved in cell-cell binding. J Biol Chem. 1977 Oct 10;252(19):6835–6840. [PubMed] [Google Scholar]
- Brass L. F., Bensusan H. B. On the role of the collagen carbohydrate residues in the platelet. Collagen interaction. Biochim Biophys Acta. 1976 Aug 24;444(1):43–52. doi: 10.1016/0304-4165(76)90222-1. [DOI] [PubMed] [Google Scholar]
- Brass L. F., Faile D., Bensusan H. B. Direct measurement of the platelet:collagen interaction by affinity chromatography on collagen/Sepharose. J Lab Clin Med. 1976 Mar;87(3):525–534. [PubMed] [Google Scholar]
- Chesney C. M., Harper E., Colman R. W. Critical role of the carbohydrate side chains of collagen in platelet aggregation. J Clin Invest. 1972 Oct;51(10):2693–2701. doi: 10.1172/JCI107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham L. W., Ford J. D. A comparison of glycopeptides derived from soluble and insoluble collagens. J Biol Chem. 1968 May 10;243(9):2390–2398. [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M. G., Lüscher E. F. Actions of thrombin and other coagulant and proteolytic enzymes on blood platelets. Nature. 1967 Dec 2;216(5118):857–858. doi: 10.1038/216857a0. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E., Miller E. J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978 Jun 1;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper E., Simons E. R., Chesney C. M., Colman R. W. The effect of chemical or enzymatic modifications upon the ability of collagen to form multimers and to initiate platelet aggregation. Thromb Res. 1975 Jul;7(1):113–122. doi: 10.1016/0049-3848(75)90129-2. [DOI] [PubMed] [Google Scholar]
- Jaffe R., Deykin D. Evidence for a structural requirement for the aggregation of platelets by collagen. J Clin Invest. 1974 Mar;53(3):875–883. doi: 10.1172/JCI107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson G. A., Urban C. L., Barber A. J. Enzymatic basis for platelet: collagen adhesion as the primary step in haemostasis. Nat New Biol. 1971 Nov 3;234(44):5–7. doi: 10.1038/newbio234005a0. [DOI] [PubMed] [Google Scholar]
- Klebe R. J. Isolation of a collagen-dependent cell attachment factor. Nature. 1974 Jul 19;250(463):248–251. doi: 10.1038/250248a0. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGoodwin E. B. Localization of the cell attachment region in types I and II collagens. Biochem Biophys Res Commun. 1976 Sep 20;72(2):426–432. doi: 10.1016/s0006-291x(76)80060-5. [DOI] [PubMed] [Google Scholar]
- Menashi S., Harwood R., Grant M. E. Native collagen is not a substrate for the collagen glucosyltransferase of platelets. Nature. 1976 Dec 16;264(5587):670–672. doi: 10.1038/264670a0. [DOI] [PubMed] [Google Scholar]
- Müller K., Gerisch G. A specific glycoprotein as the target site of adhesion blocking Fab in aggregating Dictyostelium cells. Nature. 1978 Aug 3;274(5670):445–449. doi: 10.1038/274445a0. [DOI] [PubMed] [Google Scholar]
- Pearlstein E. Plasma membrane glycoprotein which mediates adhesion of fibroblasts to collagen. Nature. 1976 Aug 5;262(5568):497–500. doi: 10.1038/262497a0. [DOI] [PubMed] [Google Scholar]
- Puett D., Wasserman B. K., Ford J. D., Cunningham L. W. Collagen-mediated platelet aggregation. Effects of collagen modification involving the protein and carbohydrate moieties. J Clin Invest. 1973 Oct;52(10):2495–2506. doi: 10.1172/JCI107440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro S. A., Cunningham L. W. Collagen-mediated platelet aggregation. Evidence for multivalent interactions of intermediate specificity between collagen and platelets. J Clin Invest. 1977 Nov;60(5):1054–1060. doi: 10.1172/JCI108856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Mosher D. F. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiss H. J. Platelet physiology and abnormalities of platelet function (first of two parts). N Engl J Med. 1975 Sep 11;293(11):531–541. doi: 10.1056/NEJM197509112931105. [DOI] [PubMed] [Google Scholar]
- Wilner G. D., Nossel H. L., LeRoy E. C. Aggregation of platelets by collagen. J Clin Invest. 1968 Dec;47(12):2616–2621. doi: 10.1172/JCI105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie D. E., Damsky C. H., Buck C. A. Studies on the function of cell surface glycoproteins. I. Use of antisera to surface membranes in the identification of membrane components relevant to cell-substrate adhesion. J Cell Biol. 1979 Feb;80(2):385–402. doi: 10.1083/jcb.80.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]


