Abstract
Background
Exposure to bisphenol A (BPA), a chemical widely used in consumer products, has been associated with in vitro Cyp19 gene expression.
Objective
To evaluate an in vivo human model of Cyp19 gene expression in granulosa cells.
Study Design
A subset of an ongoing prospective cohort study of women undergoing in vitro fertilization (IVF) at Massachusetts General Hospital.
Methods
Mixed effect models were used to evaluate the association of urinary BPA concentrations with granulosa cell Cyp19 mRNA expression.
Results
In 61 women undergoing 76 IVF cycles, adjusted changes in mean Cyp19 expression (β estimate (95% CI)) for quartiles 2,3 and 4 as compared to the lowest quartile were: −0.97 (− 2.22, 0.28); −0.97 (−2.18, 0.24) and −0.38 (−1.58, 0.82).
Conclusions
An in vivo model for evaluation of Cyp19 gene expression was developed for use in epidemiologic studies. In this pilot study, we found no statistically significant linear association between urinary BPA concentrations and Cyp19 expression.
Keywords: bisphenol A, follicular fluid, ovary, granulosa cells, Cyp19, P450 aromatase, gene expression, fertility, human
Introduction
Bisphenol A (BPA) is a ubiquitous chemical widely used in the manufacture of a variety of consumer products, including polycarbonate plastics [1,2], epoxy resins, the lacquer lining of food and beverage cans [3,4], some dental sealants and composites [5,6], and thermal receipt paper [7,8]. This has led to widespread and continuous exposure of BPA in the general population. BPA was detected in over 90% of spot urine samples collected from a representative sample of the general U.S. population in the 2003–2004 National Health and Nutrition Examination Survey (NHANES) [9]. Detectable concentrations of BPA have also been measured in human follicular fluid [10].
BPA has been shown to act as an endocrine disrupting chemical [11, 12]. While its estrogenic properties were discovered almost 80 years ago [13], specific mechanisms by which BPA causes endocrine disruption remain uncertain. One plausible explanation includes altered expression of hormone-responsive genes. However, there is a lack of in vivo evidence for changes in sex hormone-responsive gene expression associated with human exposure to BPA.
Previously, we found significant negative associations between urinary BPA concentrations and serum peak estradiol (E2) and oocyte yield on the day of egg retrieval [14] in the context of an in vitro fertilization (IVF) study. These findings were replicated in another IVF cohort at the University of San Francisco (UCSF) affiliated fertility center in which serum unconjugated BPA concentrations were used as a biomarker of exposure [15]. These findings motivated us to explore the underlying pathway by which BPA reduces serum E2 levels. While there is a paucity of human data, experimental animal and in vitro studies have consistently reported lower levels of Cyp19 messenger RNA (gene expression) with higher free BPA concentrations, ultimately leading to decreased P450 aromatase and ovarian E2 synthesis [16 - 19]. Based on these findings, we explored whether urinary BPA concentrations were associated with decreased Cyp19 gene expression in our IVF cohort.
We designed an in vivo human model to examine the association between urinary BPA concentrations and granulosa cell gene expression of cytochrome P450, family 19, subfamily A, polypeptide 1(Cyp19A1, Cyp19) in women undergoing an oocyte retrieval procedure prior to IVF. Cyp19 encodes aromatase, a cytochrome P450 superfamily enzyme, which converts androgens to estrogens and is highly expressed in granulosa cells. We hypothesized that the dose-dependent decreases in peak serum E2, previously observed in relation to urinary BPA concentrations in our larger prospective IVF cohort study cohort [14], are mediated by BPA-induced down-regulation of Cyp19 gene expression. A decrease in P450 aromatase synthesis would consequently result in decreased ovarian E2 synthesis by granulosa cells.
Methods
Study participants and data collection
The present analysis included women who had undergone IVF oocyte retrieval between October 2009 and June 2011 as part as part of their fertility treatment at the Massachusetts General Hospital (MGH) Fertility Center in Boston, MA USA. This sub-study was part of a larger prospective cohort study on the impact of environmental chemicals on fertility and pregnancy outcomes [20]. Follicular fluid samples were collected at the time of oocyte retrieval. Women between the ages of 18-45 years who used their own oocytes for IVF and who had BPA measurement available were eligible for the sub-study. Women were followed from study entry through each of their IVF cycles until either a live birth or the discontinuation of treatment at the MGH Fertility Center. The study was approved by the Institutional Review Boards of the MGH, Harvard School of Public Health (HSPH), and the Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA.
At recruitment, following informed consent, a nurse administered a brief questionnaire to collect data on demographics, medical history, and lifestyle. Clinical information was obtained from the electronic medical record and infertility diagnoses were assigned according to the Society for Assisted Reproductive Technology (SART) definitions.
Follicular fluid collection
On the day of oocyte retrieval, we collected aspirated follicular fluid from the consented study patient. Follicular fluid was aspirated at negative pressure (−130 mmHg) from the first 3 follicles. Three 1ml syringes were used to draw exactly 1ml of SAGE Quinn's Advantage Medium HEPES, which was added to each of the three collection tubes at the time of follicle aspiration. The embryologist then separated the oocytes from the follicular fluid aspirate and transferred the follicular fluid discard into three separate 15 ml BD polystyrene Falcon tubes, which we retrieved from the embryologist. The three Falcon tubes were kept on ice and then centrifuged at 1000 × g for 10 minutes until a granulosa cell pellet was formed. The supernatant was aliquoted into 2ml polypropylene cryovials and stored at −80°C, leaving 0.3 ml of supernatant in the Falcon tube, above the granulosa cell pellet. The Falcon tube was vortexed until the cells were in suspension. We prepared two cryostraws (IMV technologies unplugged mini straws) per aspirated follicle of 100 μL of granulosa cell suspension in follicular fluid. The straws were heat-sealed and stored in liquid nitrogen in plastic canes. Three sets of field blanks for the follicular fluid aspiration procedure all tested negative for BPA contamination at the CDC. The granulosa cell samples were shipped on dry ice over night to the Flaws laboratory at the University of Illinois-Urbana, Champaign in two separate batches; the first in April 2011, and second in January 2012.
Quantitative Polymerase Chain Reaction (qPCR) Analysis
Total RNA was extracted from follicles using the RNeasy Micro Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer's protocol. Reverse transcriptase generation of complementary DNA (cDNA) was performed with 0.40 μg of total RNA using an iScript RT kit (Bio-Rad Laboratories, Inc., Hercules, CA). Real-time PCR (qPCR) was conducted using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.) and accompanying software (CFX Manager Software) according to the manufacturer's instructions. The CFX96 quantifies the amount of PCR product generated by measuring a dye (SYBR Green) that fluoresces when bound to double-stranded DNA. A standard curve was generated from five serial dilutions of one of the samples, thus allowing analysis of the amount of cDNA in the exponential phase. Specific qPCR primer sequences were used as follows: human β-actin (forward) 5′-TCA TGA AGT GTG ACG TTG ACA TCC GT-3′, (reverse) 5′-CCT AGA AGC ATT TGC GGT GCA CGA TG-3′; human Cyp19(forward) 5′-TGT GGA CGT GTT GAC CCT TCT-3′, (reverse) 5′- ACC ACG ATA GAT AGC ACT TTC GTC C-3′. β-actin was used as an internal standard. qPCR analysis was performed using 2 μl cDNA, forward and reverse primers (5 pmol) for β-actin and Cyp19, in conjunction with a SsoFast EvaGreen Supermix qPCR Kit (Bio-Rad Laboratories). An initial incubation of 95°C for 10 min was followed by denaturing at 94°C for 10 s, annealing at 60°C for 10 s, and extension at 72°C for 10 s, for 40 cycles, followed by final extension at 72°C for 10 min. A melting curve was generated at 55–90°C to monitor the generation of a single product. The software also generated a standard curve. Final values of Cyp19 were calculated and expressed as the ratio normalized to β-actin, a housekeeping gene typically used for normalization of gene expression data in human studies [21, 22]. The assays were run in duplicate and the interassay variability for both β-actin and Cyp19 was low (0.18−0.33%).
Treatment Protocols and Clinical IVF Measures
Participants underwent one of three IVF treatment protocols, as described in detail previously [14]. Briefly, these included one of the following: 1) luteal phase gonadotropin releasing hormone (GnRH) agonist protocol using low, regular and high-dose leuprolide; 2) follicular phase GnRH-agonist/flare protocol; and 3) GnRH-antagonist protocol. Women who had responded poorly during past IVF cycles were the primary candidates for antagonist and flare protocols, with the flare protocol being indicated for women over age 40, whereas the antagonist protocol was assigned to women under age 40 with diminished ovarian reserve and poor ovarian response. Serum follicle stimulating hormone (FSH) was measured on cycle day 3, and measurements of serum E2 levels and details of oocyte retrieval have been previously described [14]. Peak serum E2 was measured from blood drawn approximately 36 hours prior to the patient's scheduled oocytes retrieval procedure.
Urine Sample Collection and Measurement of Urinary Bisphenol A Concentrations
Women provided up to two spot urine samples per IVF cycle, with the first one (not necessarily a fasting sample) collected between day 3 and day 9 of the gonadotropin phase, and the second, always a fasting sample, on the day of oocyte retrieval, typically prior to the procedure or administration of intravenous fluids. Urine was collected in a sterile clean polypropylene specimen cup. BPA concentrations were corrected for urine dilution by specific gravity (SG) using a conventional SG adjustment method [23]. SG was measured at room temperature using a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA), which was calibrated with deionized water before each measurement. The urine was divided into aliquots and frozen and stored at −80°C. Samples were shipped on dry ice overnight to the CDC where they were stored at or below −40°C until analysis for BPA concentrations.
The urinary concentrations of free and conjugated BPA species (total BPA) were measured using online solid phase extraction coupled to isotope dilution-high-performance liquid chromatography)-tandem mass spectrometry as described before [24]. First, 100 μL of urine was treated with β-glucuronidase/sulfatase (Helix pomatia, H1; Sigma Chemical Co, St. Louis, MO) to hydrolyze the BPA-conjugated species. We added a solution of 13C4-4- methylumbelliferone, 4-methylumbelliferyl sulfate, and 4-methylumbelliferyl glucuronide to all samples, and used it as a deconjugation standard. The 4-methylumbelliferone/ 13C4-4- methylumbelliferone peak area ratio was monitored to check the extent of the deconjugation reaction [24]. After hydrolysis, BPA was retained and concentrated on a C18 reversed-phase size-exclusion solid phase extraction column (Merck KGaA, Germany), separated from other urine matrix components using a pair of monolithic HPLC columns (Merck KGaA), and detected by negative ion-atmospheric pressure chemical ionization-MS/MS. The limit of detection (LOD) for BPA was 0.4 μg/L. In addition to study samples, each analytical run included low-concentration and high-concentration quality control materials, prepared with pooled human urine spiked with BPA, and reagent blanks to assure the accuracy and reliability of the data [24]. BPA concentrations below the LOD were assigned a value equal to the LOD divided by the square root of 2 [25] prior to adjustment for urine dilution by SG as described previously.
Statistical Analysis
Characteristics of the women and IVF cycle characteristics were summarized using means, standard deviations and percentages, as appropriate. The percentile distributions of the first and second spot urine samples (both unadjusted and SG adjusted) were summarized, as was the distribution of Cyp19 gene expression.
Mixed effect models were applied to evaluate the association between SG-adjusted urine BPA concentration (on the day of oocyte retrieval) and Cyp19 gene expression. We used the urine BPA concentration from the morning on the day of oocyte retrieval due to its close proximity in time to the collection of the granulosa cells whose gene expression would be expected to change within hours if there was a response to BPA exposure. BPA concentrations were adjusted for SG and then modeled in 2 ways: 1) as a continuous variable (on the natural log scale) and 2) as a categorical variable (in quartiles). We also used a similar modeling strategy to confirm the association previously observed between SG-adjusted urine BPA concentration and peak serum E2 within this subset. Although our sample size was limited, we also investigated whether adjustment for Cyp19 gene expression in this latter model had an impact on the association between BPA and E2. We used a compound symmetry correlation structure to account for correlation between repeated IVF cycles in the same woman, and adjusted for selected covariates which could be confounders.
To account for the decreased statistical power in this pilot study, we considered covariates as potential confounders if associated with the outcome of interest with p-value <0.3 in univariate models. These covariates were chosen because of their clinical relevance to ovarian response and Cyp19 gene expression as evidenced in our earlier study [14]. Backward selection methods were used to identify variables for inclusion in the multivariate model. Covariates included IVF protocol type (flare/antagonist versus regular luteal phase protocol); day 3 serum FSH level (continuous measure); BMI (on a continuous scale); age and batch/shipment year (year 2012 versus year 2011). Characteristics represented by less than 5% of the cohort were not included as candidate predictors. Variables were retained in the final model if they had p-value ≤ 0.10. A test for trend was performed to determine if there was a linear dose-response relationship between quartiles of urinary BPA (coded ordinally as 1 to 4) and the log-transformed Cyp19 gene expression. All data analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
The 61 women included in our analyses were on average 35 years old at the time of recruitment to the study. These women underwent a total of 76 IVF cycles (48 women had a single IVF cycle, 11 women had two IVF cycles, and 2 women had 3 IVF cycles). Among these women, 88% were Caucasian and over 40% of the women were overweight or obese (BMI ≥ 25 kg/m2). Approximately one fifth had a primary SART diagnosis female factor, with 8% having a diagnosis of diminished ovarian reserve. Almost 40% of the 76 IVF cycles were assigned a low responder (flare or antagonist) protocol (Table I).
Table I. Subject demographics, infertility diagnoses and cycle characteristics among 61 women undergoing 76 IVF cycles.
| Characteristic | Among 61 women N (%) |
|---|---|
| Age (years) Mean ± SD (range) | 35.0 ± 4.1 (24 - 43) |
| Age ≥ 37 (years) | 22 (36%) |
| BMIa(Kg/m2) Mean ± SD (range) | 25.1 ± 4.6 (19 -37) |
| BMI ≥ 25 | 25 (42%) |
| Race | |
| Caucasian | 54 (88%) |
| other | 7 (11%) |
| SART diagnosisb | |
| Female factor | 13 (21%) |
| Diminshed ovarian reserve | 5 (8%) |
| Ovulation disorders | 6 (10%) |
| Endometriosis | 1 (2%) |
| Tubal factor | 1 (2%) |
| Male infertility | 25 (41%) |
| Unexplained | 21 (34%) |
|
| |
| Cycle-Specific Characteristics | Among 76 IVF Cycles, N (%) |
|
| |
| IVF Protocolc | |
| Luteal Phase (LDLL/RDLL) | 47 (63%) |
| Low responder (Flare/ Antagonist) | 28 (37%) |
| Mean ± SD (range) | |
|
|
|
| Serum Day 3 FSH (IU/L)* | 6.5 ± 2.2 (0.2-11.4) |
| Peak Serum Estradiol (pg/ml) | 1980 ± 878 (603-4665) |
| Number of Oocytes retrieved | 11 ± 6 (2-32) |
Totals may not sum to 100% due to rounding
Abbreviations: BMI, body mass index: IVF, in vitro fertilization, LDLL,
Low-dose leuprolide lupron; RDLL, regular-dose leuprolide lupron;
FSH, follicle stimulating hormone;
BMI missing for 1 subject;
Primary diagnosis of infertility (2 missing values);
IVF protocol missing for 1 cycles.
missing day 3 FSH on 17 women, therefore this covariate was excluded from multivariate analyses
The non SG-adjusted BPA concentrations were comparable to those of the general U.S. population, with a median of 2.59 μg/L for the early cycle (day 3-9) and 1.97 μg/L for the later cycle (retrieval day urine) spot urine samples, compared to 1.73 μg/L for females in NHANES 2009-2010 [26]. The early cycle urine sample was not necessarily collected following fasting, whereas the retrieval day urine sample was always a fasting sample. This could possibly explain the higher median urinary BPA concentration in the early cycle than at retrieval day. The distribution of BPA concentrations was also comparable to those with measured BPA urinary concentrations in the IVF study overall [20]. Detectable concentrations of BPA were measured in 91% of spot urine urines. Cyp19 gene expression ranged from 0.006 to 19.6, with a median (interquartile range) of 0.43 (0.15-2.40) for the 76 samples.
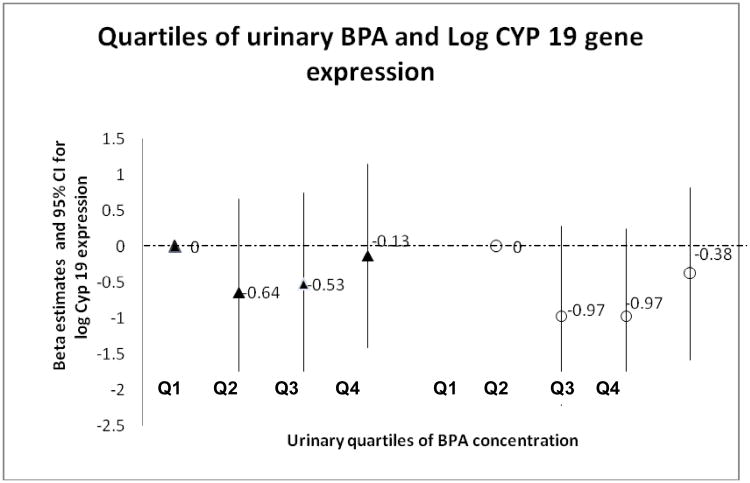
We observed no association between quartiles of urinary BPA concentration and log-transformed Cyp19 gene expression, either with or without adjustment for BMI and the year each batch of granulosa cell samples were shipped to the Flaws laboratory for analysis (Table II). Two shipments of granulosa cell samples were sent to the laboratory; samples from 55 cycles were shipped in 2011 and 21 cycles were sent in 2012. Initially, qPCR was performed upon receipt of each shipment, therefore analyzed in two batches. Cycle characteristics (median peak E2, urine BPA, age and BMI) did not differ significantly between batches, with the exception of median Cyp19 gene expression, which was significantly higher for samples sent in 2011 (Wilcoxon test p-value<0.001) compared to samples sent in 2012. qPCR was rerun in the same assay for all 76 samples to assess quality control and a similar difference between 2011 and 2012 shipment batches was observed (Wilcoxon test p-value =0.001) (Supplementary figure I). The Spearman correlation for Cyp19 gene expression between the first and repeat qPCR was high (r = 0.84, p-value<0.0001). Results from the qPCR rerun of Cyp19 gene expression were used for univariate and multivariate analyses.
Table II.
Association between log transformed Cyp19 granulosa cell gene expression and urinary specific gravity adjusted BPA quartiles among 61 women (76 IVF cycles) based on mixed effects models, accounting for the correlation between outcomes.
| Covariates | Unadjusted | Adjusteda | Batch- adjustedb | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Parameter estimate | Parameter estimate | Parameter estimate | ||||
| (95% CI) | P-value | (95% CI) | P-value | (95% CI) | P-value | |
| Log SG-adjusted BPA | 0.12(−0.46, 0.69) | 0.67 | 0.06 (−0.53, 0.64) | 0.84 | 0.001 (−0.54, 0.54) | 1.00 |
|
| ||||||
| BPA quartiles (range ng/L) | ||||||
| Q1 (≤1.32) | ref | ref | ref | ref | ref | ref |
| Q2 (1.32-1.98) | −0.64(−1.94, 0.66) | 0.30 | −0.74(−2.09, 0.62) | 0.26 | −0.97 (-2.22, 0.28) | 0.11 |
| Q3 (1.98-3.27) | −0.53-1.81, 0.75) | 0.38 | −0.69 (−2.10, 0.62) | 0.27 | −0.97 (-2.18, 0.24) | 0.10 |
| Q4 (≥3.27) | −0.13(−1.41, 1.15) | 0.83 | −0.29 (−1.60, 1.03) | 0.64 | −0.38 (−1.58, 0.82) | 0.49 |
|
| ||||||
| Test for trend (p-value)c | 0.90 | 0.69 | 0.56 | |||
|
| ||||||
| BMI (continuous) | −0.06(−0.15, 0.04) | 0.21 | −0.07 (−0.17, 0.03) | 0.16 | −0.09 (−0.18, 0.01) | 0.06 |
| Day 3 FSH IU/L)* | 0.05(−0.20, 0.30) | 0.69 | --- | --- | --- | --- |
| IVF protocol (flare/ant. vs. luteal) | −0.04(−0.96, 0.88) | 0.93 | --- | --- | --- | --- |
| Age (years) | −0.05(−0.15, 0.05) | 0.35 | --- | --- | --- | --- |
| Batch (year 2012 vs year 2011) | −1.54(−2.47, -0.61) | 0.003 | --- | --- | −1.76 (−2.73, −0.80) | 0.002 |
Abbreviations: BPA, Bisphenol A; BMI, body mass index; Day 3 FSH, follicle stimulating hormone on 3rd day of menses; 95% CI=95% confidence intervals; batch = year of shipment
missing day 3 FSH on 17 women, therefore this covariate was excluded from multivariate analyses
adjusted for BMI;
adjusted for BMI and batch effect (year 2012 versus year 2011);
test for linear trend based on ordinal quartiles
To confirm prior observations among the larger IVF cohort, we used mixed effects models to examine the association between SG-adjusted BPA (log transformed) from spot urine samples collected at the start of the treatment cycle and peak serum E2 measurements. After adjusting for age (≥ 37 years vs. < 37 years) and BMI (≥ 25 kg/m2 vs. < 25 kg/m2), an increase in SG-adjusted BPA urinary concentration from 1.43 μg/L (25th percentile) to 3.90 μg/L (75th percentile) was associated with a decrease in peak serum E2 of 348 pg/ml (p-value= 0.03). The decrease in peak serum E2 did not substantially change (342 pg/ml, p-value=0.04) after adjusting for Cyp19 (natural log transformed). No significant associations were observed between Cyp19 and peak E2 (p-value=0.89).
Discussion
In this pilot study, we evaluated an in vivo human model designed to examine the association between exposure to BPA, estimated from urinary BPA concentrations, and ovarian granulosa cell Cyp19 gene expression. We made use of a larger ongoing prospective IVF cohort study for which granulosa cells had been extracted from follicular fluid aspirates (at oocyte retrieval) and appropriately stored in liquid nitrogen. Our study question was motivated by our previous study findings in the larger IVF cohort from which this sub-sample was drawn, in which we found a negative association between urinary BPA concentrations and peak serum E2 [14, 20]. We were able to confirm these findings in this subgroup of 61 women undergoing 76 IVF cycles. Similar findings have also been replicated in a separate IVF cohort study [15]. We hypothesized that BPA-associated decreases in peak serum E2 may be mediated through the Cyp19 pathway in the ovarian granulosa cell. However, contrary to evidence from animal and in vitro studies [16, 18, 28], we did not observe a negative linear association between urinary BPA concentrations and Cyp19 gene expression. While there was no significant association between quartiles of SG-adjusted urine BPA and Cyp19 expression, there was a suggestive nonmonotonic (U -shaped) trend in both the unadjusted and adjusted models (Figure I). Larger studies are needed to further examine dose-response patterns. It is possible that the dose response association in vivo would not be linear because of the potential action of negative feedback loops at given concentrations of BPA, similar to the well-established action of endogenous E2 on FSH during the menstrual cycle. In this example, during the follicular phase, higher serum E2 concentrations inhibit hypothalamic production of GnRH through negative feedback, resulting in a decrease in FSH and E2 production. Conversely, in the luteal phase, low serum E2 concentrations trigger an increase in FSH production via a positive feedback loop. Evidence of non-monotonic dose response associations at environmentally relevant doses of BPA support the possibility that BPA may have different (non-monotonic) actions at different doses. [29 - 32]. Because this was a pilot study, sample size was limited and consequently, we might have lacked statistical power to detect small, but possibly clinically relevant changes, in Cyp19 expression, although very limited data are available on what would be considered a clinically relevant change in Cyp 19 expression.
In our multivariable model (Table II), we adjusted for BMI and batch year. BMI is an important covariate because in premenopausal women, in addition to adipose tissue being a known precursor of E2 [33], it has also been associated with ovulatory infertility [34] and decreased sex hormone binding globulin (SHBG), which in turn results in decreased total serum E2 in both the follicular and luteal phase [35]. In our final batch-adjusted multivariate model (Table II), the association between BMI and Cyp19 gene expression (log transformed) was statistically significant (p-value=0.06) at the 0.10 significance level that we a priori established for this pilot study. Treatment protocol was not associated with Cyp19 gene expression and was therefore not included in our final multivariate model.
We found a batch year effect and noted a significant difference in Cyp19 gene expression depending on when the batch of samples was shipped to the laboratory (Supp figure I). This difference could potentially have been due to conditions during shipment (more RNA loss in the second shipment), although identical processing, storage and shipment protocols were followed [36]. Ideally, future studies should consider shipment of replicate samples in different batches for quality control. However, due to feasibility reasons, we were unable to conduct quality control experiments across shipments of samples. The RNA expression experiments were conducted twice. First, independently, i.e. as soon as each batch was received at the Flaws laboratory. Then, at a later date, the experiments were repeated on both batches in the same experiment. The correlation between the repeated measures was high (Spearman correlation coefficient = 0.84).
Our study was limited in sample size given that it was a pilot study. We had 80% power to detect differences in mean log transformed Cyp19 gene expression of 1.41 for women with high (above 1.97 μg/L) versus low (<1.97 μg/L) urine BPA concentrations at a 5% significance level. A post hoc power calculation (statistical program nQUery Advisor 7.0) was performed and in order to detect a difference in means, characterized by a variance of means of Cyp19 gene expression by urine BPA quartile. A sample size of 260 would be required at the 0.05 significance level, assuming the association found in this pilot study is a reflection of the true pattern of association. It is therefore difficult to draw definitive conclusions regarding the lack of association we observed between urine BPA concentration and Cyp19 expression. We cannot however dismiss the possibility that Cyp19 may not be on the causal pathway in humans. A recent animal study used an in vitro follicle culture system, administering BPA concentrations of 44 μM and 440 μM and found decreased E2 production in mouse ovarian antral follicles, with corresponding decreased gene expression of steroid acute regulatory protein (Star) and cytochrome P450 side chain cleavage [37]. It is possible that this follicle culture system rather than a granulosa cell culture system better translates to our human in vivo model. In our follicular fluid aspirates, we were not able to examine Star gene expression because of the absence of theca cells in the aspirates. However, it is important to note that in humans BPA may affect different enzymes in the ovarian two-cell system (theca/granulosa cell) than those previously demonstrated in rats and mice.
Another consideration is that we do not know how well the BPA concentration in the urine reflects the BPA concentration at the relevant target, which is the follicle. Future experiments could involve measuring BPA concentrations in the follicular fluid [10, 37] and explore associations with gene expression. It is possible that free BPA concentration at the target tissue is a more accurate measure of exposure than urine conjugated BPA concentration. In addition, since gene expression occurs within hours, it is possible that timing of our urine sample is not representative of the relevant biological window of exposure. We suggest replicating our Cyp19 analyses in a larger sample size in the future and also examining upstream genes in the granulosa cells (e.g., FSH receptors (FSHR), estrogen receptor beta (ESRβ), progesterone receptor (PGR) and others). This would provide important insights as to how BPA may potentially cause impaired ovarian steroidogenesis inwomen undergoing fertility treatment.
Conclusions
We evaluated an in vivo human model to study the underlying mechanism by which BPA decreases serum peak E2 in an IVF cohort. Although we observed no clear dose-response association between urinary SG-adjusted BPA and Cyp19 granulosa gene expression, our study does not rule out the possibility of a non-monotonic dose response association between quartiles of urinary BPA concentration and Cyp19 gene expression. Based on these preliminary data, a linear dose-response association was unlikely, but further studies are warranted to explore the relationships of Cyp19 gene expression with BPA exposure.
Supplementary Material
Figure I. Quartiles of urinary BPA and Log CYP 19 gene expression.
▲ Unadjusted
○ Adjusted for BMI and Batch/shipment (year 2012 versus year 2011)
Q1, lowest quartile (≤1.32 μg/L);
Q2, 2nd quartile (1.33-1.97 μg/L);
Q3, 3rd quartile Q3 (1.98-3.26 μg/L);
Q4, 4th quartile Q4 (≥3.27 μg/L)
An in vivo human model looking at the underlying mechanism for BPA's action at the target tissue
Translational pilot study on a subsample of a larger IVF cohort
Possible non-monotonic dose-response association between BPA and Cyp 19 gene expression in human ovarian granulosa cells
Acknowledgments
This work was supported by grant ES009718, ES000002, ES019178 from the National Institute of Environmental Health Sciences and grant OH008578 from the National Institute for Occupational Safety and Health.
We thank the research team at the Hauser lab, Flaws lab and also Xiaoyun Ye, Xiaoliu Zhou, Ryan Hennings and Lily Jia for technical assistance in measuring urinary BPA. Finally, special thanks to the patients who participated in the study while undergoing their IVF treatment cycles.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Competing Financial Interests: None of the authors has any actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brede C, Fjeldal P, Skjevrak I, Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food additives and contaminants. 2003;20(7):684–689. doi: 10.1080/0265203031000119061. [DOI] [PubMed] [Google Scholar]
- 2.Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, Chang JY, Ye X, Calafat AM, Michels KB. Polycarbonate bottle use and urinary bisphenol A concentrations. Environmental Health Perspectives. 2009;117(9):1368–72. doi: 10.1289/ehp.0900604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae B, Jeong JH, Lee SJ. The quantification and characterization of endocrine disruptor bisphenol-A leaching from epoxy resin. Water science and technology : a journal of the International Association on Water Pollution Research. 2002;46(11-12):381–387. [PubMed] [Google Scholar]
- 4.Carwile JL, Ye X, Zhou X, Calafat AM, Michels KB. Canned soup consumption and urinary bisphenol A: a randomized crossover trial. JAMA. 2011 Nov 23;306(20):2218–20. doi: 10.1001/jama.2011.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joskow R, Barr DB, Barr JR, Calafat AM, Needham LL, Rubin C. Exposure to bisphenol A from bis-glycidyl dimethacrylate-based dental sealants. J Am Dent Assoc. 2006;137(3):353–362. doi: 10.14219/jada.archive.2006.0185. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki N, Okuda K, Kato T, Kakishima H, Okuma H, Abe K, et al. Salivary bisphenol-A levels detected by ELISA after restoration with composite resin. Journal of materials science Materials in medicine. 2005;16(4):297–300. doi: 10.1007/s10856-005-0627-8. [DOI] [PubMed] [Google Scholar]
- 7.Vom Saal FS, Myers JP. Bisphenol A and risk of metabolic disorders. JAMA : the journal of the American Medical Association. 2008;300(11):1353–1355. doi: 10.1001/jama.300.11.1353. [DOI] [PubMed] [Google Scholar]
- 8.Biedermann S, Tschudin P, Grob K. Transfer of bisphenol A from thermal printer paper to the skin. Analytical and bioanalytical chemistry. 2010;398(1):571–576. doi: 10.1007/s00216-010-3936-9. [DOI] [PubMed] [Google Scholar]
- 9.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environmental health perspectives. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17(11):2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 11.Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S, Kimura M, Shimohigashi Y. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma. J Biochem. 2007 Oct;142(4):517–24. doi: 10.1093/jb/mvm158. http://www.ncbi.nlm.nih.gov.ezp-prod1.hul.harvard.edu/pubmed/17761695. [DOI] [PubMed] [Google Scholar]
- 12.Quesada I, Fuentes E, Viso-León MC, Soria B, Ripoll C, Nadal A. FASEB J. 2002 Oct;16(12):1671–3. doi: 10.1096/fj.02-0313fje. [DOI] [PubMed] [Google Scholar]
- 13.Dodds EC, L W. Synthetic estrogenic agents without the phenanthrene nucleus. Nature. 1936;137:996. [Google Scholar]
- 14.Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, et al. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. International journal of andrology. 2010;33(2):385–393. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloom MS, Kim D, vom Saal FS, Taylor JA, Cheng G, Lamb JD, Fujimoto VY. Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertil Steril. 2011;96(3):672–677. doi: 10.1016/j.fertnstert.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mlynarcikova A, Kolena J, Fickova M, Scsukova S. Alterations in steroid hormone production by porcine ovarian granulosa cells caused by bisphenol A and bisphenol A dimethacrylate. Molecular and Cellular Endocrinology. 2005;244:57–62. doi: 10.1016/j.mce.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Osuga Y, Yano T, Morita Y, Tang X, Fujiwara T, Takai Y, Matsumi H, Koga K, Taketani Y, Tsutsumi O. Bisphenol A induces apoptosis and G2-to-M arrest of ovarian granulosa cells. Biochemical and Biophysical Research Communications. 2002;29:456–462. doi: 10.1006/bbrc.2002.6644. [DOI] [PubMed] [Google Scholar]
- 18.Zhou W, Liu J, Liao L, Han S, Liu J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Molecular and Cellular Endocrinology. 2008;283:12–18. doi: 10.1016/j.mce.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Peretz J, Craig ZR, Flaws JA. Bisphenol a inhibits follicle growth and induces atresia in cultured mouse antral follicles independently of the genomic estrogenic pathway. Biol Reprod. 2012 Sep 21;87(3):63. doi: 10.1095/biolreprod.112.101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, Petrozza JC, Wright D, Hauser R. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod. 2012 Dec;27(12):3583–92. doi: 10.1093/humrep/des328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadek KH, Cagampang FR, Bruce KD, Shreeve N, Macklon N, Cheong Y. Variation in stability of housekeeping genes in endometrium of healthy and polycystic ovarian syndrome women. Hum Reprod. 2012;27(1):251–256. doi: 10.1093/humrep/der363. [DOI] [PubMed] [Google Scholar]
- 22.van Duursen MB, Nijmeijer SM, de Morree ES, de Jong PC, van den Berg M. Genistein induces breast cancer-associated aromatase and stimulates estrogen-dependent tumor cell growth in in vitro breast cancer model. Toxicology. 2011;289(2-3):67–73. doi: 10.1016/j.tox.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Meeker JD, Ehrlich S, Toth TL, Wright DL, Calafat AM, Trisini AT, et al. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod Toxicol. 2010;30(4):532–539. doi: 10.1016/j.reprotox.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Analytical chemistry. 2005;77(16):5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- 25.Hornung RW, Reed L. Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene. 1990;5(1):46–51. [Google Scholar]
- 26.CDC. [accessed 1 April, 2013];Fourth National Report on Human Exposure to Environmental Chemicals - Updated tables. 2013 Mar; http://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Mar2013.pdf.
- 27.Watanabe M, Ohno S, Nakajin S. Effects of bisphenol A on the expression of cytochrome P450 aromatase (CYP19) in human fetal osteoblastic and granulosa cell-like cell lines. Toxicol Lett. 2012 Feb 4;210(1):95–99. doi: 10.1016/j.toxlet.2012.01.020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, Dhar MD, Ganjam VK, Parmigiani S, Welshons WV. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):2056–61. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weltje L, vom Saal FS, Oehlmann J. Reproductive stimulation by low doses of xenoestrogens contrasts with the view of hormesis as an adaptive response. Hum Exp Toxicol. 2005 Sep;24(9):431–7. doi: 10.1191/0960327105ht551oa. [DOI] [PubMed] [Google Scholar]
- 30.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, Vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr Rev. 2012 Mar 14; doi: 10.1210/er.2011-1050. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birnbaum LS. Environmental Chemicals: Evaluating Low-Dose Effects. Environ Health Perspect. 2012 Mar 14;120:a143–a144. doi: 10.1289/ehp.1205179. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fritz MA, S L. Clinical Gynecologic Endocrinology and Infertility. Lippincott Wilkins and Williams 2010 [Google Scholar]
- 33.Rich-Edwards JW, Spiegelman D, Garland M, Hertzmark E, Hunter DJ, Colditz GA, et al. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology. 2002 Mar;13(2):184–190. doi: 10.1097/00001648-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Tworoger SS, Eliassen AH, Missmer SA, Baer H, Rich-Edwards J, Michels KB, et al. Birthweight and body size throughout life in relation to sex hormones and prolactin concentrations in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2006 Dec;15(12):2494–2501. doi: 10.1158/1055-9965.EPI-06-0671. [DOI] [PubMed] [Google Scholar]
- 35.Tworoger SS, Hankinson SE. Use of biomarkers in epidemiologic studies: minimizing the influence of measurement error in the study design and analysis. Cancer Causes Control. 2006 Sep;17(7):889–99. doi: 10.1007/s10552-006-0035-5. [DOI] [PubMed] [Google Scholar]
- 36.Peretz J, Gupta RK, Singh J, Hernández-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci. 2011 Jan;119(1):209–17. doi: 10.1093/toxsci/kfq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenie S, Cortvrindt R, Eichenlaub-Ritter U, Smitz J. Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Mutat Res. 2008 Mar 12;651(1-2):71–81. doi: 10.1016/j.mrgentox.2007.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.