Abstract
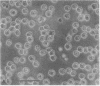
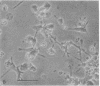
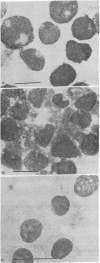
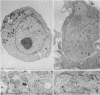
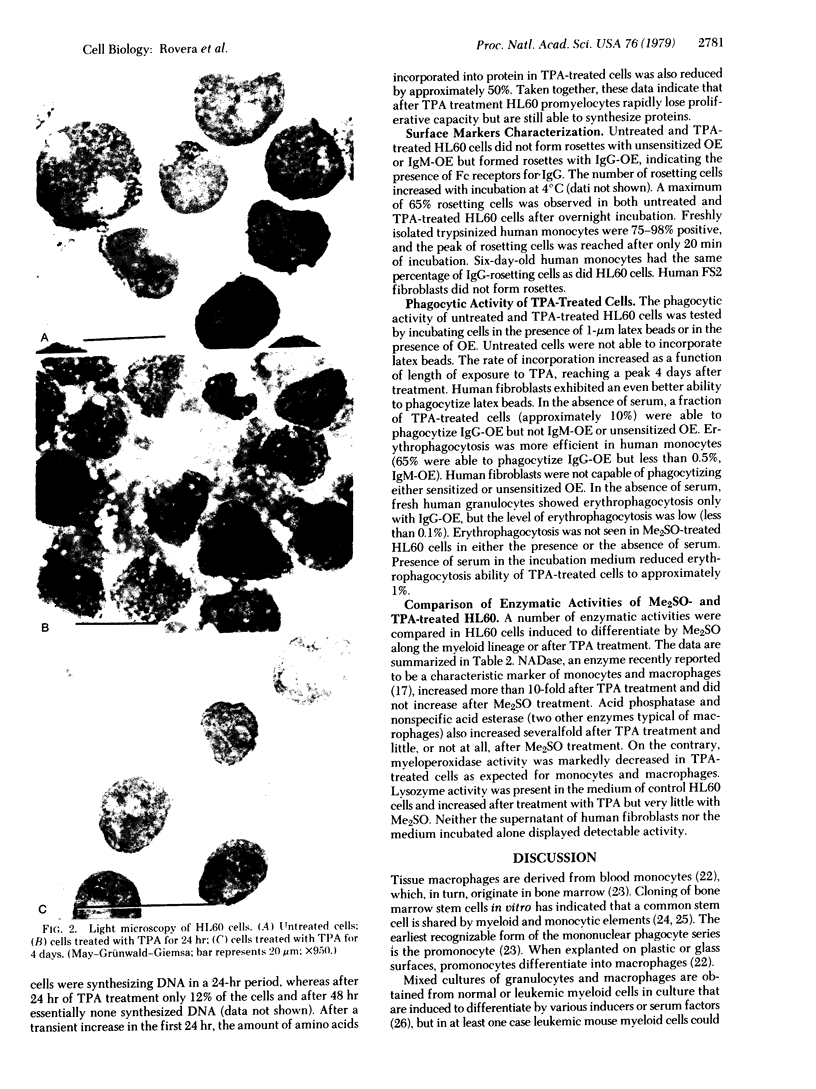
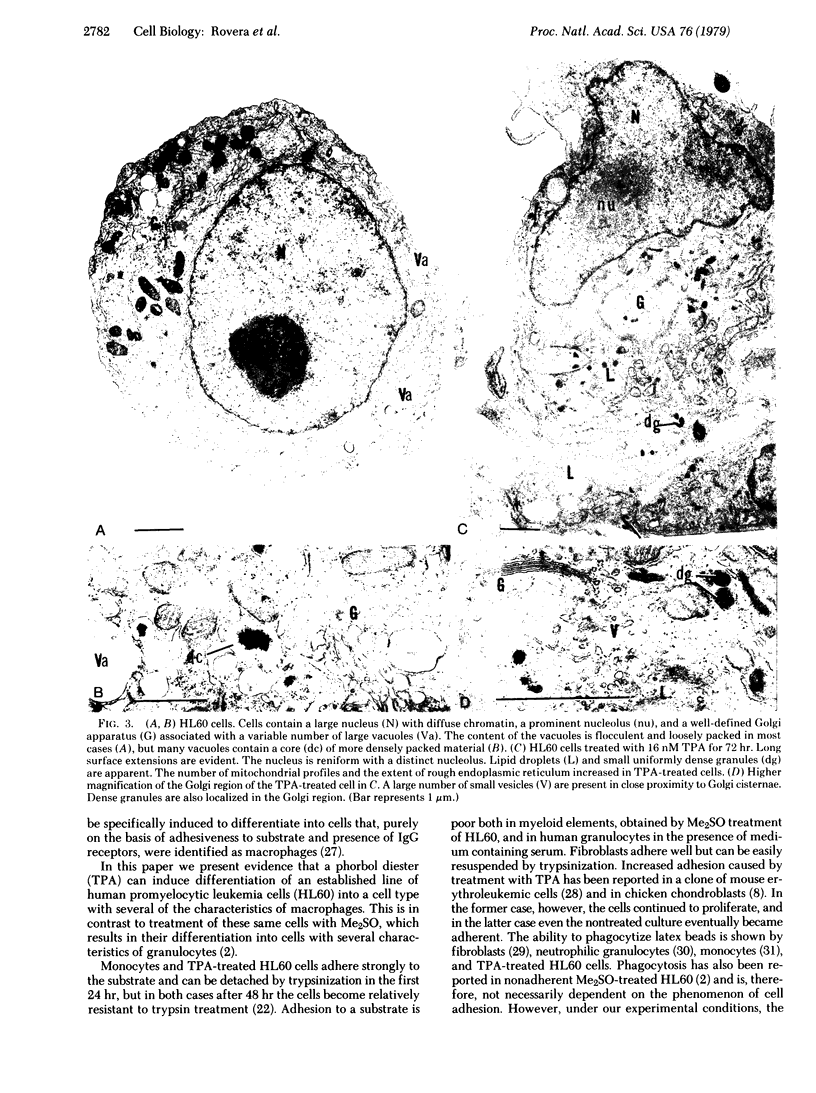
When suspension cultures of human promyelocytic leukemia cells (line HL60) were treated with 12-O-tetradecanoylphorbol 13-acetate (TPA; 1.6-160 nM), more than 80% of the cells adhered to the plastic substrate within 24 hr. Within the same time period the immature azurophilic granulations typical of HL60 promyelocytic cells disappeared and the nuclear chromatin became more condensed, but the nucleolus was retained. The attached cells stopped dividing and synthesizing DNA. The phenomenon was irreversible and independent of the continuous presence of TPA. Approximately 60% of the untreated cells and of TPA-treated cells bore surface Fc receptors for IgG. Under the experimental conditions used, about 10% of the TPA-treated cells were also able to phagocytize IgG-coated erythrocytes and more than 80% were able to phagocytize latex beads, but untreated controls were unable to do so. Cellular levels of NADase, acid phosphatase, and non-specific esterase were markedly increased after treatment with TPA, whereas little or no increase was seen after treatment with dimethyl sulfoxide (Me2SO), a drug that induces myeloid differentiation of HL60 cells. Peroxidase activity was lower in TPA-treated and Me2SO-treated cells than in HL60 cells. More lysozyme was found in the medium of TPA-treated cells than in the medium of untreated or Me2SO-treated cells. These data indicate that, after treatment with TPA, human promyelocytic leukemia cells can differentiate into cells that have several characteristics of macrophages.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artman M., Seeley R. J. Nicotinamide adenine dinucleotide splitting enzyme: a characteristic of the mouse macrophage. Science. 1978 Dec 22;202(4374):1293–1295. doi: 10.1126/science.214853. [DOI] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Differences in enzyme content of azurophil and specific granules of polymorphonuclear leukocytes. II. Cytochemistry and electron microscopy of bone marrow cells. J Cell Biol. 1968 Nov;39(2):299–317. doi: 10.1083/jcb.39.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D., Robinson W. Stimulation by leukaemic sera of colony formation in solid agar cultures by proliferation of mouse bone marrow cells. Nature. 1967 Mar 4;213(5079):926–927. doi: 10.1038/213926a0. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. Phagocytosis by human monocytes. Blood. 1968 Sep;32(3):423–435. [PubMed] [Google Scholar]
- Cohen R., Pacifici M., Rubinstein N., Biehl J., Holtzer H. Effect of a tumour promoter on myogenesis. Nature. 1977 Apr 7;266(5602):538–540. doi: 10.1038/266538a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Rovera G. Inhibition of adipose conversion of 3T3 fibroblasts by tumour promoters. Nature. 1977 Sep 15;269(5625):247–249. doi: 10.1038/269247a0. [DOI] [PubMed] [Google Scholar]
- Ferrarini M., Moretta L., Abrile R., Durante M. L. Receptors for IgG molecules on human lymphocytes forming spontaneous rosettes with sheep red cells. Eur J Immunol. 1975 Jan;5(1):70–72. doi: 10.1002/eji.1830050115. [DOI] [PubMed] [Google Scholar]
- Gilfillan R. F., Robblee L. S., Bardawil W. A. Antigen as a function of RES blockade. J Reticuloendothel Soc. 1970 Oct;8(4):303–333. [PubMed] [Google Scholar]
- Gordon S., Cohn Z. A. The macrophage. Int Rev Cytol. 1973;36:171–214. doi: 10.1016/s0074-7696(08)60218-1. [DOI] [PubMed] [Google Scholar]
- Ishii D. N., Fibach E., Yamasaki H., Weinstein I. B. Tumor promoters inhibit morphological differentiation in cultured mouse neuroblastoma cells. Science. 1978 May 5;200(4341):556–559. doi: 10.1126/science.644318. [DOI] [PubMed] [Google Scholar]
- LITWACK G. Photometric determination of lysozyme activity. Proc Soc Exp Biol Med. 1955 Jul;89(3):401–403. doi: 10.3181/00379727-89-21824. [DOI] [PubMed] [Google Scholar]
- Levine M. R., Cox R. P. Use of latex particles for analysis of heterokaryon formation and cell fusion. Somatic Cell Genet. 1978 Jul;4(4):507–512. doi: 10.1007/BF01538870. [DOI] [PubMed] [Google Scholar]
- LoBuglio A. F., Cotran R. S., Jandl J. H. Red cells coated with immunoglobulin G: binding and sphering by mononuclear cells in man. Science. 1967 Dec 22;158(3808):1582–1585. doi: 10.1126/science.158.3808.1582. [DOI] [PubMed] [Google Scholar]
- Lowe M. E., Pacifici M., Holtzer H. Effects of phorbol-12-myristate-13-acetate on the phenotypic program of cultured chondroblasts and fibroblasts. Cancer Res. 1978 Aug;38(8):2350–2356. [PubMed] [Google Scholar]
- Maeda S., Sachs L. Control of normal differentiation of myeloid leukemic cells. XIII. Inducibility for some stages of differentiation by dimethylsulfoxide and its disassociation from inducibility by MGI. J Cell Physiol. 1978 Feb;94(2):181–185. doi: 10.1002/jcp.1040940207. [DOI] [PubMed] [Google Scholar]
- Miao R. M., Filedsteel A. H., Fodge D. W. Opposing effects of tumor promoters on erythroid differentiation. Nature. 1978 Jul 20;274(5668):271–272. doi: 10.1038/274271a0. [DOI] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Durante M. L., Mingari M. C. Expression of a receptor for IgM by human T cells in vitro. Eur J Immunol. 1975 Aug;5(8):565–569. doi: 10.1002/eji.1830050812. [DOI] [PubMed] [Google Scholar]
- Mueller J., Brun del Re G., Buerki H., Keller H. U., Hess M. W., Cottier H. Nonspecific acid esterase activity: a criterion for differentiation of T and B lymphocytes in mouse lymph nodes. Eur J Immunol. 1975 Apr;5(4):270–274. doi: 10.1002/eji.1830050411. [DOI] [PubMed] [Google Scholar]
- Nichols B. A., Bainton D. F., Farquhar M. G. Differentiation of monocytes. Origin, nature, and fate of their azurophil granules. J Cell Biol. 1971 Aug;50(2):498–515. doi: 10.1083/jcb.50.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici M., Holtzer H. Effects of a tumor-promoting agent on chondrogenesis. Am J Anat. 1977 Sep;150(1):207–212. doi: 10.1002/aja.1001500116. [DOI] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The cloning of normal "mast" cells in tissue culture. J Cell Physiol. 1965 Dec;66(3):319–324. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- Rabellino E. M., Ross G. D., Trang H. T., Williams N., Metcalf D. Membrane receptors of mouse leukocytes. II. Sequential expression of membrane receptors and phagocytic capacity during leukocyte differentiation. J Exp Med. 1978 Feb 1;147(2):434–445. doi: 10.1084/jem.147.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Tumor promoters inhibit spontaneous differentiation of Friend erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2894–2898. doi: 10.1073/pnas.74.7.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukaemia. Nature. 1978 Aug 10;274(5671):535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- Santoli D., Trinchieri G., Lief F. S. Cell-mediated cytotoxicity against virus-infected target cells in humans. I. Characterization of the effector lymphocyte. J Immunol. 1978 Aug;121(2):526–531. [PubMed] [Google Scholar]
- Schnyder J., Baggiolini M. Secretion of lysosomal hydrolases by stimulated and nonstimulated macrophages. J Exp Med. 1978 Aug 1;148(2):435–450. doi: 10.1084/jem.148.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H., Fibach E., Nudel U., Weinstein I. B., Rifkind R. A., Marks P. A. Tumor promoters inhibit spontaneous and induced differentiation of murine erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3451–3455. doi: 10.1073/pnas.74.8.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]