Abstract
Rat submandibular gland cells have been obtained through enzymatic dispersion using chromatographically purified collagenase (EC 3.4.24.3) and hyaluronidase (EC 3.2.1.35) and gentle mechanical force. The recovery of viable cells after the isolation procedure was 59% on the basis of total glandular DNA content. Approximately 60% of the total cell population consisted of acinar cells; less than 8% were immature granular duct cells; and the remainder were intercalated duct, striated duct, and myoepithelial cells. Most of the acinar cells were in acinar-intercalated duct complexes. The integrity of the isolated cells was substantiated by their exclusion of trypan blue, intracellular electrolyte composition, incorporation of [14C]glucosamine into trichloroacetic acid + phosphotungstic acid precipitable material at a linear rate for 1.5 hr, secretory responses to parasympathomimetic and sympathomimetic stimulation, and morphologic integrity as determined by light and electron microscopy. The cholinergic receptors were characterized through investigation of the net transmembrane flux of K+ in response to carbamoylcholine. The alpha-adrenergic receptors were characterized by investigating the net transmembrane flux of K+ in response to norepinephrine stimulation and the beta-adrenergic receptors were characterized by determining the rate of secretion of 14C-labeled mucin after isoproterenol stimulation. A high degree of sensitivity to both cholinergic and adrenergic secretagogues was observed.
Full text
PDF

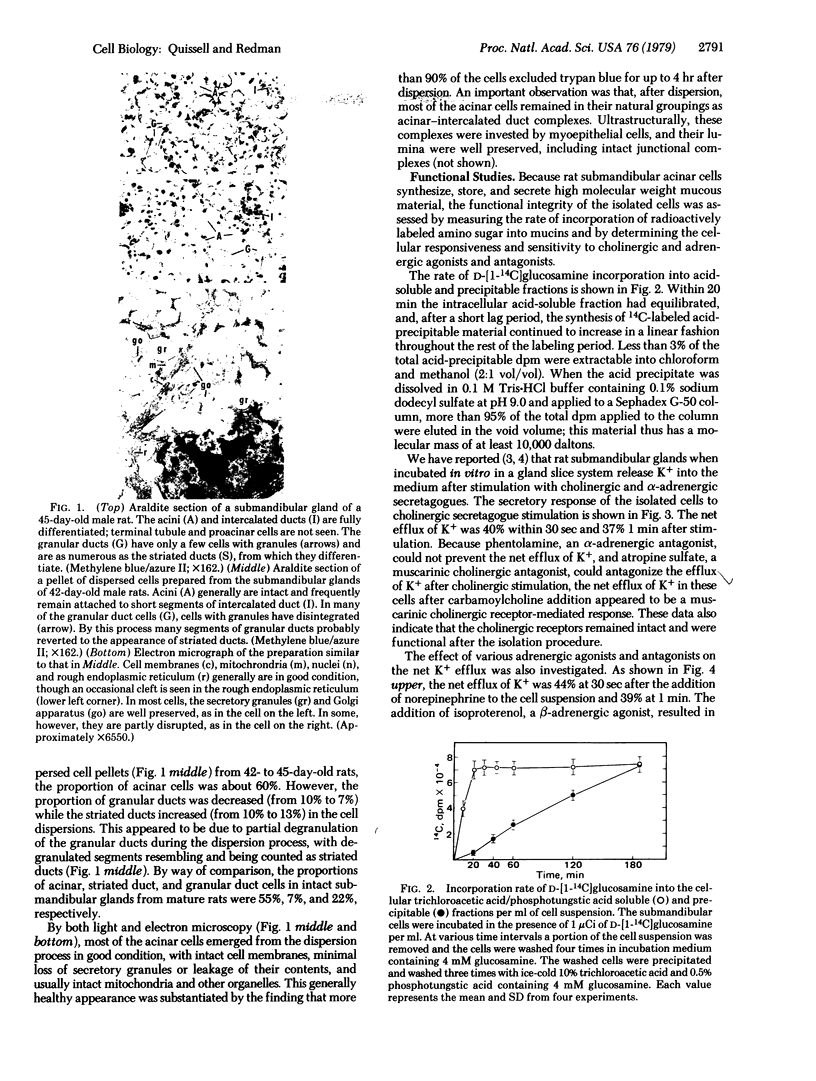
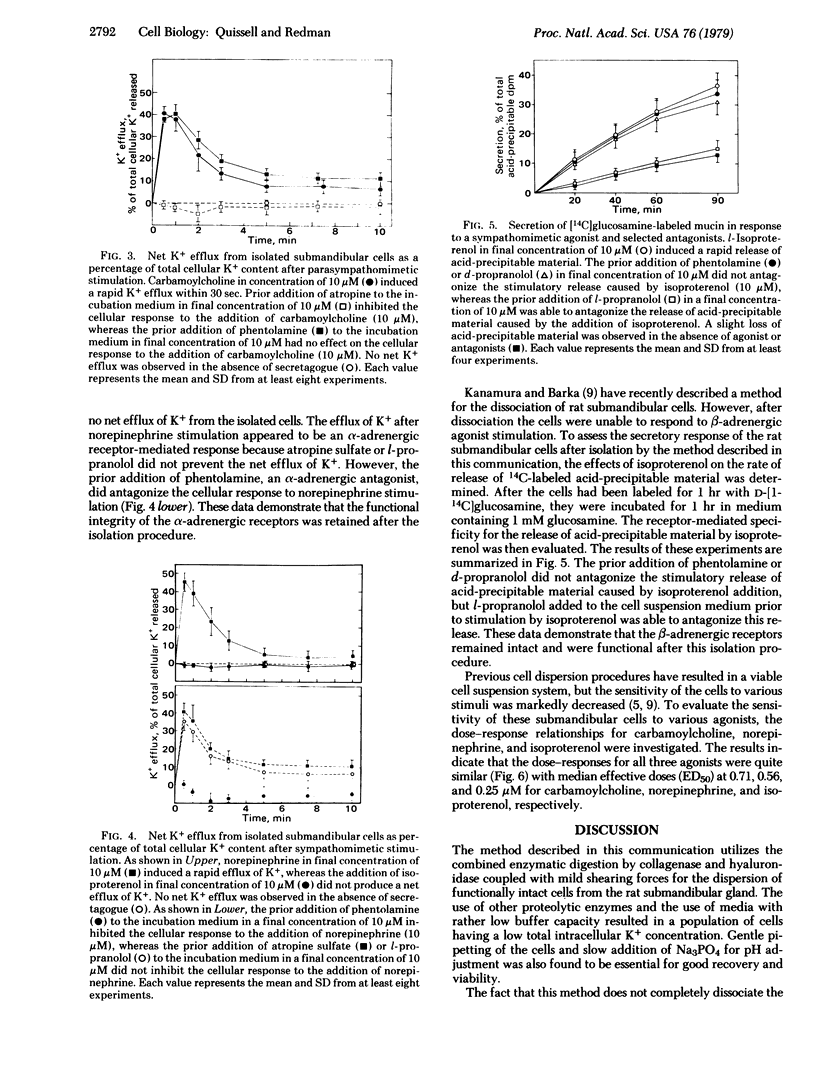
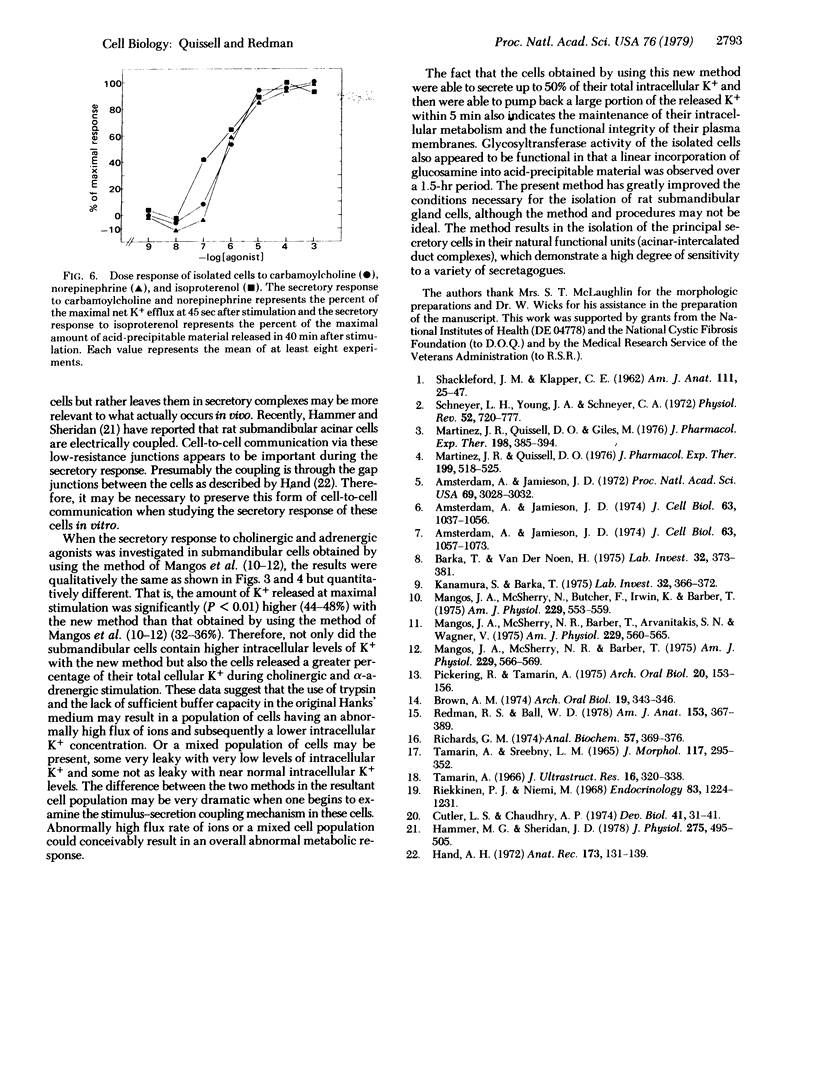
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam A., Jamieson J. D. Structural and functional characterization of isolated pancreatic exocrine cells. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3028–3032. doi: 10.1073/pnas.69.10.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A., Jamieson J. D. Studies on dispersed pancreatic exocrine cells. I. Dissociation technique and morphologic characteristics of separated cells. J Cell Biol. 1974 Dec;63(3):1037–1056. doi: 10.1083/jcb.63.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A., Jamieson J. D. Studies on dispersed pancreatic exocrine cells. II. Functional characteristics of separated cells. J Cell Biol. 1974 Dec;63(3):1057–1073. doi: 10.1083/jcb.63.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barka T., Van der Noen H. Dissociation of rat parotid gland. Lab Invest. 1975 Mar;32(3):373–380. [PubMed] [Google Scholar]
- Brown A. M. A method for the initiation and maintenance of permanent rat submandibular gland epithelial cell cultures. Arch Oral Biol. 1974 Apr;19(4):343–346. doi: 10.1016/0003-9969(74)90201-5. [DOI] [PubMed] [Google Scholar]
- Cutler L. S., Chaudhry A. P. Cytodifferentiation of the acinar cells of the rat submandibular gland. Dev Biol. 1974 Nov;41(1):31–41. doi: 10.1016/0012-1606(74)90280-2. [DOI] [PubMed] [Google Scholar]
- Hammer M. G., Sheridan J. D. Electrical coupling and dye transfer between acinar cells in rat salivary glands. J Physiol. 1978 Feb;275:495–505. doi: 10.1113/jphysiol.1978.sp012203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand A. R. Adrenergic and cholinergic nerve terminals in the rat parotid gland. Electron microscopic observations on permanganate-fixed glands. Anat Rec. 1972 Jun;173(2):131–139. doi: 10.1002/ar.1091730202. [DOI] [PubMed] [Google Scholar]
- Kanamura S., Barka T. Short term culture of dissociated rat submandibular gland cells. Lab Invest. 1975 Mar;32(3):366–372. [PubMed] [Google Scholar]
- Mangos J. A., McSherry N. R., Barber T., Arvanitakis S. N., Wagner V. Dispersed rat parotid acinar cells. II. Characterization of adrenergic receptors. Am J Physiol. 1975 Sep;229(3):560–565. doi: 10.1152/ajplegacy.1975.229.3.560. [DOI] [PubMed] [Google Scholar]
- Mangos J. A., McSherry N. R., Barber T. Dispersed rat parotid acinar cells. III. Characterization of cholinergic receptors. Am J Physiol. 1975 Sep;229(3):566–569. doi: 10.1152/ajplegacy.1975.229.3.566. [DOI] [PubMed] [Google Scholar]
- Mangos J. A., McSherry N. R., Butcher F., Irwin K., Barber T. Dispersed rat parotid acinar cells. I. Morphological and functional characterization. Am J Physiol. 1975 Sep;229(3):553–559. doi: 10.1152/ajplegacy.1975.229.3.553. [DOI] [PubMed] [Google Scholar]
- Martinez J. R., Quissell D. O., Giles M. Potassium release from the rat submaxillary gland in vitro. I. Induction by catecholamines. J Pharmacol Exp Ther. 1976 Aug;198(2):385–394. [PubMed] [Google Scholar]
- Martinez J. R., Quissell D. O. Potassium release from the rat submaxillary gland in vitro. II. Induction by parasympathomimetic secretagogues. J Pharmacol Exp Ther. 1976 Dec;199(3):518–525. [PubMed] [Google Scholar]
- Pickering R., Tamarin A. Morphologic and biochemical study of dissociated rat parotid gland acini stimulated in vitro. Arch Oral Biol. 1975 Mar;20(3):153–156. doi: 10.1016/0003-9969(75)90001-1. [DOI] [PubMed] [Google Scholar]
- Redman R. S., Ball W. D. Cytodifferentiation of secretory cells in the sublingual gland of the prenatal rat: a histological, histochemical and ultrastructural study. Am J Anat. 1978 Nov;153(3):367–389. doi: 10.1002/aja.1001530304. [DOI] [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Riekkinen P. J., Niemi M. Androgen-dependent salivary gland protease in the rat. Endocrinology. 1968 Dec;83(6):1224–1231. doi: 10.1210/endo-83-6-1224. [DOI] [PubMed] [Google Scholar]
- SHACKLEFORD J. M., KLAPPER E. C. Structure and carbohydrate histochemistry of mammalian salivary glands. Am J Anat. 1962 Jul;111:25–47. doi: 10.1002/aja.1001110104. [DOI] [PubMed] [Google Scholar]
- Schneyer L. H., Young J. A., Schneyer C. A. Salivary secretion of electrolytes. Physiol Rev. 1972 Jul;52(3):720–777. doi: 10.1152/physrev.1972.52.3.720. [DOI] [PubMed] [Google Scholar]
- Tamarin A. Myoepithelium of the rat submaxillary gland. J Ultrastruct Res. 1966 Oct;16(3):320–338. doi: 10.1016/s0022-5320(66)80066-7. [DOI] [PubMed] [Google Scholar]
- Tamarin A., Sreebny L. M. The rat submaxillary salivary gland. A correlative study by light and electron microscopy. J Morphol. 1965 Nov;117(3):295–352. doi: 10.1002/jmor.1051170303. [DOI] [PubMed] [Google Scholar]