Abstract
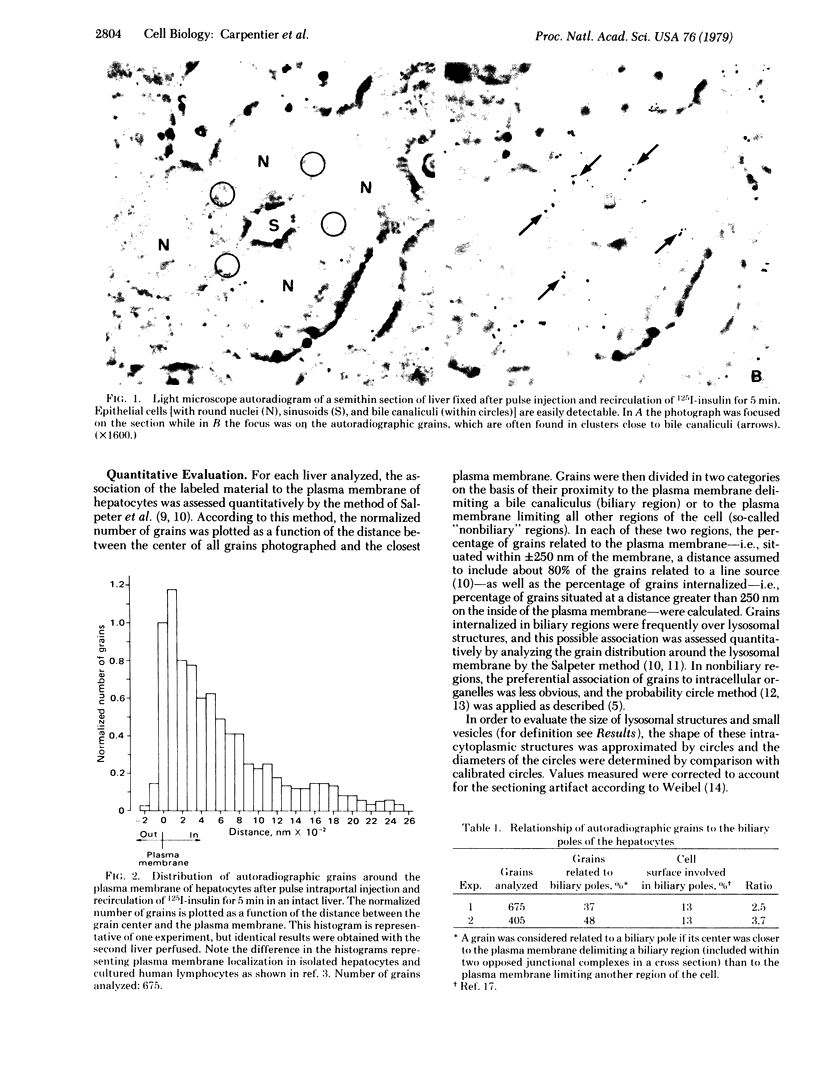
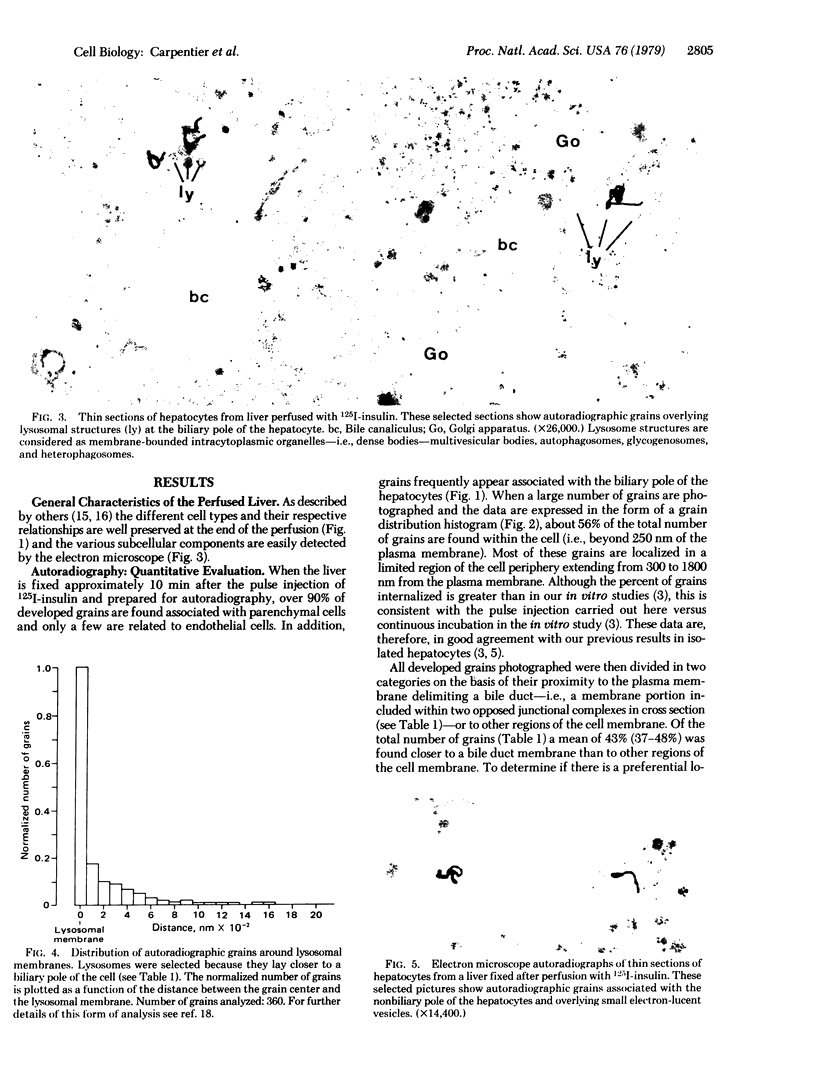
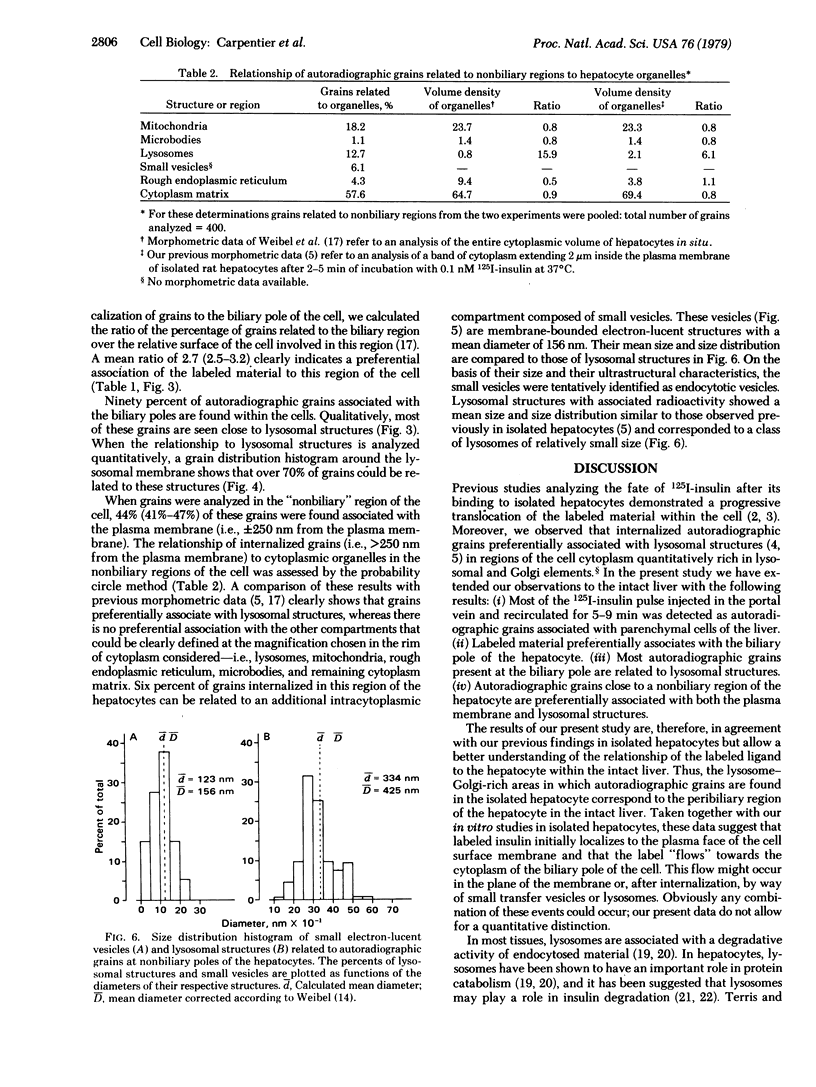
We have shown that 125I-labeled insulin initially localizes to the plasma membrane of isolated rat hepatocytes. The ligand is subsequently internalized and preferentially localizes to lysosomal structures. Further, we have observed that labeled insulin localizes to regions of the cell rich in lysosomal and Golgi elements. In the present study in intact rat liver we found that, approximately 10 min after a pulse injection of 125I-labeled insulin, 56% of the label was internalized by the cell. When all grains are considered there is a preferential localization of grains to the biliary pole of the cell and these grains are almost all internalized and preferentially associated with lysosomes. These data, therefore, demonstrate that the lysosome-Golgi-rich area of the isolated hepatocyte corresponds to the biliary pole of the cell and there is a movement of the labeled hormone from its initial binding site on the plasma face of the cell membrane toward the biliary pole of the cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansorge S., Bohley P., Kirschke H., Langner J., Hanson H. Metabolism of insulin and glucagon. Breakdown of radioiodinated insulin and glucagon in rat liver cell fractions. Eur J Biochem. 1971 Mar 11;19(2):283–288. doi: 10.1111/j.1432-1033.1971.tb01316.x. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Evans W. H., Geschwind I. I. Insulin binding to rat liver Golgi fractions. J Cell Biol. 1973 Dec;59(3):771–776. doi: 10.1083/jcb.59.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J., Levine G., Sikstrom R., O'Shaughnessy D., Kopriwa B., Nadler N. J., Posner B. I. Polypeptide hormone binding sites in vivo: initial localization of 125I-labeled insulin to hepatocyte plasmalemma as visualized by electron microscope radioautography. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5051–5055. doi: 10.1073/pnas.74.11.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J., Posner B. I., Josefsberg Z., Sikstrom R. Intracellular polypeptide hormone receptors. The demonstration of specific binding sites for insulin and human growth hormone in Golgi fractions isolated from the liver of female rats. J Biol Chem. 1978 Jun 10;253(11):4058–4066. [PubMed] [Google Scholar]
- Bergeron J. J., Sikstrom R., Hand A. R., Posner B. I. Binding and uptake of 125I-insulin into rat liver hepatocytes and endothelium. An in vivo radioautographic study. J Cell Biol. 1979 Feb;80(2):427–443. doi: 10.1083/jcb.80.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyns A. R., Pearce N., Mahler R. F. Insulin in bile. The effect of monosaccharides and hypoglycaemic agents. Diabetologia. 1969 Oct;5(5):304–308. doi: 10.1007/BF00452903. [DOI] [PubMed] [Google Scholar]
- CARO L. G., VAN TUBERGEN R. P., KOLB J. A. High-resolution autoradiography. I. Methods. J Cell Biol. 1962 Nov;15:173–188. doi: 10.1083/jcb.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Amherdt M., Van Obberghen E., Kahn C. R., Orci L. 125I-insulin binding to cultured human lymphocytes. Initial localization and fate of hormone determined by quantitative electron microscopic autoradiography. J Clin Invest. 1978 Apr;61(4):1057–1070. doi: 10.1172/JCI109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Jones A. L., Hradek G. T., Wong K. Y., Mooney J. S. Entry of insulin into human cultured lymphocytes: electron microscope autoradiographic analysis. Science. 1978 Nov 17;202(4369):760–763. doi: 10.1126/science.715440. [DOI] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Cohen S., Orci L. Epidermal growth factor: morphological demonstration of binding, internalization, and lysosomal association in human fibroblasts. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5025–5029. doi: 10.1073/pnas.75.10.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Freychet P., LeCam A., Orci L. Intracellular translocation of iodine-125-labeled insulin: direct demonstration in isolated hepatocytes. Science. 1978 May 19;200(4343):782–785. doi: 10.1126/science.644321. [DOI] [PubMed] [Google Scholar]
- Grisolia S., Wallace R. Insulin degradation by lysosomal extracts from rat liver; model for a role of lysosomes in hormon degradation. Biochem Biophys Res Commun. 1976 May 3;70(1):22–27. doi: 10.1016/0006-291x(76)91103-7. [DOI] [PubMed] [Google Scholar]
- Nadler N. J. The interpretation of grain counts in electron microscope radioautography. J Cell Biol. 1971 Jun;49(3):877–882. [PubMed] [Google Scholar]
- Posner B. I., Josefsberg Z., Bergeron J. J. Intracellular polypeptide hormone receptors. Characterization of insulin binding sites in Golgi fractions from the liver of female rats. J Biol Chem. 1978 Jun 10;253(11):4067–4073. [PubMed] [Google Scholar]
- Roth J. Peptide hormone binding to receptors: a review of direct studies in vitro. Metabolism. 1973 Aug;22(8):1059–1073. doi: 10.1016/0026-0495(73)90225-4. [DOI] [PubMed] [Google Scholar]
- Salpeter M. M., Bachmann L., Salpeter E. E. Resolution in electron microscope radioautography. J Cell Biol. 1969 Apr;41(1):1–32. doi: 10.1083/jcb.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M., Fertuck H. C., Salpeter E. E. Resolution in electron microscope autoradiography. III. Iodine-125, the effect of heavy metal staining, and a reassessment of critical parameters. J Cell Biol. 1977 Jan;72(1):161–173. doi: 10.1083/jcb.72.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stäubli W., Schweizer W., Suter J., Weibel E. R. The proliferative response of hepatic peroxidomes of neonatal rats to treatment with SU-13 437 (nafenopin). J Cell Biol. 1977 Sep;74(3):665–689. doi: 10.1083/jcb.74.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Retention and degradation of 125I-insulin by perfused livers from diabetic rats. J Clin Invest. 1976 Apr;57(4):885–896. doi: 10.1172/JCI108365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward W. F., Mortimore G. E. Compartmentation of intracellular amino acids in rat liver. Evidence for an intralysosomal pool derived from protein degradation. J Biol Chem. 1978 May 25;253(10):3581–3587. [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]





