Abstract
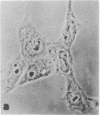
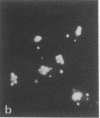
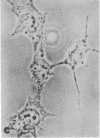
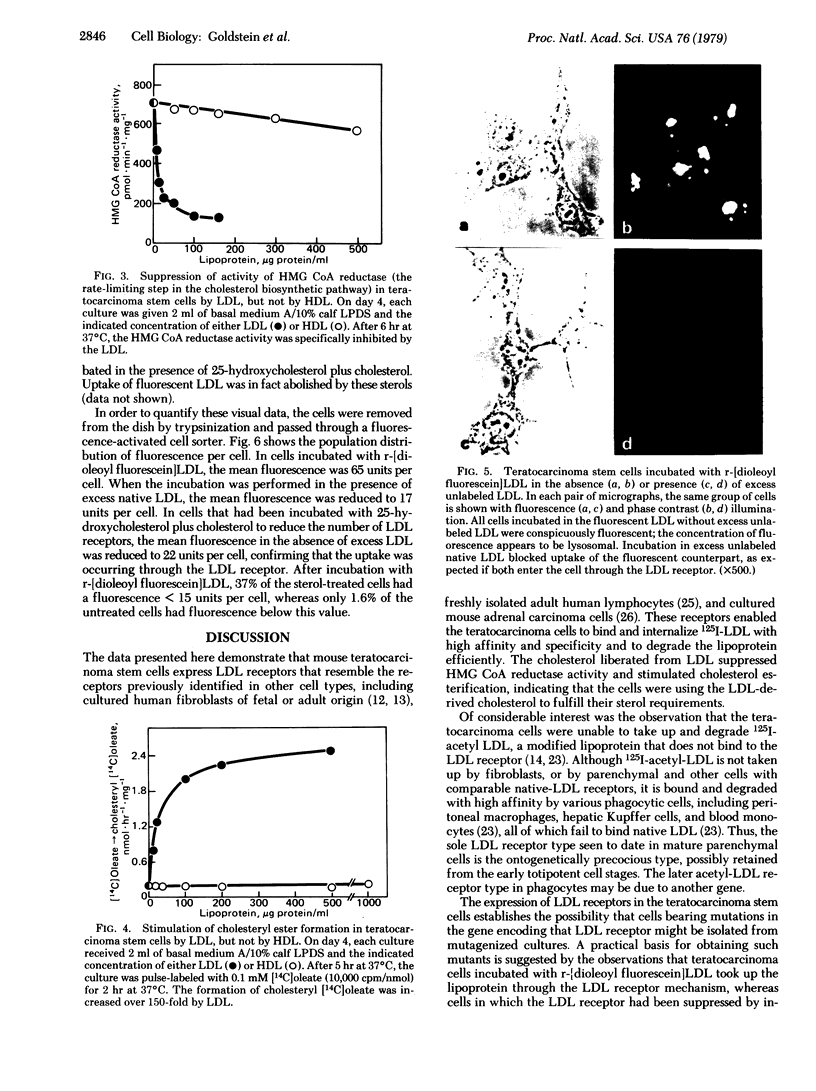
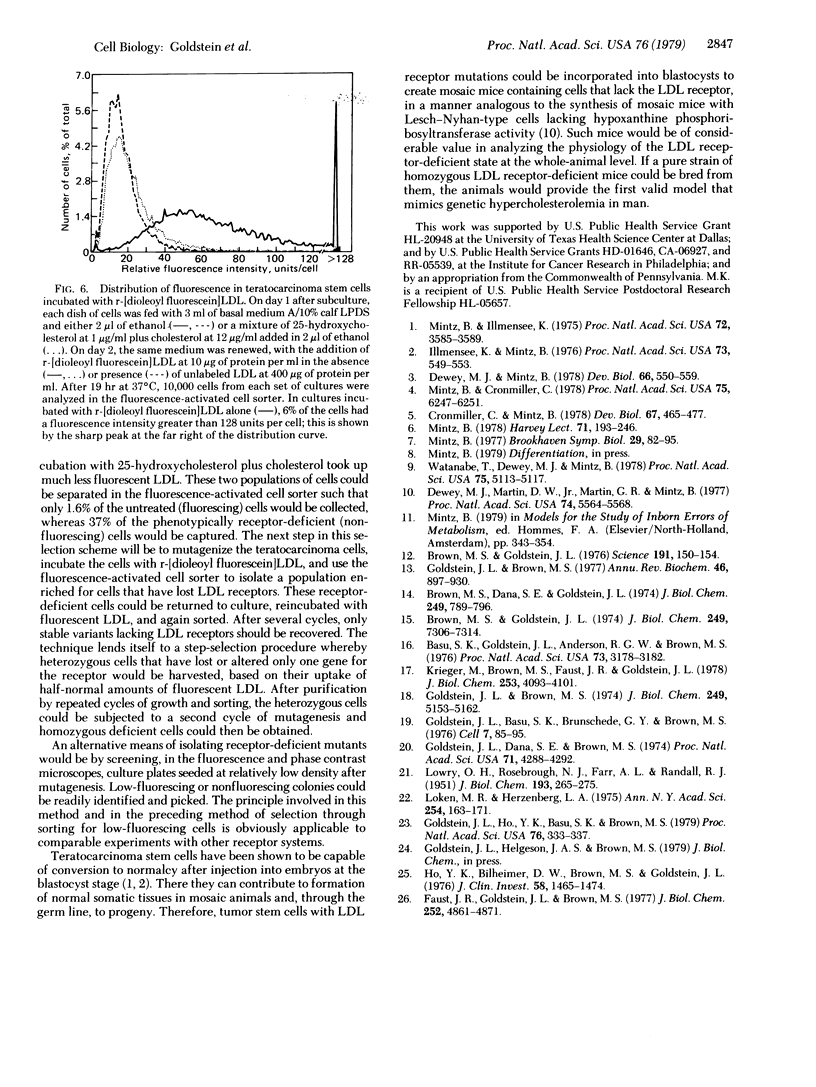
Familial hypercholesterolemia, a widespread human genetic disorder implicated in vascular and coronary disease, has had no laboratory animal counterpart that would enable the pathogenesis to be analyzed and drugs to be tested in vivo. The primary lesion in some patients is known to occur in the cells' initial handling of the major cholesterol-carrying lipoprotein of plasma. It entails a deficiency in the specific cell surface receptor that binds low density lipoprotein (LDL), with a consequent alteration in the control of cholesterol metabolism. The present study was undertaken to devise a practical scheme for producing, from developmentally versatile mouse teratocarcinoma stem cells, whole-animal models with a comparable genetic lesion. This requires first learning whether the tumor stem cells in culture express LDL receptors, and next establishing a selection or screening procedure to identify receptor-deficient mutants in mutagenized cell cultures. The results show that the teratocarcinoma cells do in fact have specific high-affinity LDL receptors which are similar to those reported for fibroblasts and the parenchymal cells of specialized tissues and different from those of phagocytic cells. Sterols suppressed the otherwise efficient binding, internalization, and degradation of LDL (125I-labeled) by the cells. Acetylation of LDL blocked the binding. Only LDL and not high density lipoprotein (HDL) was bound. After LDL uptake and degradation, the liberated cholesterol led, as expected, to increased cholesteryl ester formation; it also suppressed activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase [HMG CoA reductase; mevalonate: NADP+ oxidoreductase (CoA-acylating), EC 1.1.1.34], the rate-limiting step in cholesterol biosynthesis. Cells with LDL receptors were readily visualized by administering a fluorescent derivative of LDL; in the fluorescence microscope, labeling was seen in all cells. Cells with experimentally depressed receptors, yielding little fluorescence, were separable from those with normal fluorescence in the fluorescence-activated cell sorter. Thus, two methods for isolating receptor-deficient cells from mutagenized cultures are now available, either by visual recognition of low-fluorescing or nonfluorescing colonies in culture plates or by electronic cell sorting. Such mutants in an appropriate line of teratocarcinoma cells can then be passaged into blastocysts for full somatic tissue differentiation and germ-line development into mice.
Keywords: cholesterol biosynthesis, dioleoyl fluorescein labeling, fluorescence-activated cell sorting, differentiation, hypercholesterolemia disease model
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated control of cholesterol metabolism. Science. 1976 Jan 16;191(4223):150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and inhibition of growth of human fibroblasts by 7-ketocholesterol. J Biol Chem. 1974 Nov 25;249(22):7306–7314. [PubMed] [Google Scholar]
- Cronmiller C., Mintz B. Karyotypic normalcy and quasi-normalcy of developmentally totipotent mouse teratocarcinoma cells. Dev Biol. 1978 Dec;67(2):465–477. doi: 10.1016/0012-1606(78)90212-9. [DOI] [PubMed] [Google Scholar]
- Dewey M. J., Martin D. W., Jr, Martin G. R., Mintz B. Mosaic mice with teratocarcinoma-derived mutant cells deficient in hypoxanthine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5564–5568. doi: 10.1073/pnas.74.12.5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey M. J., Mintz B. Direct visualization, by beta-galactosidase histochemistry, of differentiated normal cells derived from malignant teratocarcinoma in allophenic mice. Dev Biol. 1978 Oct;66(2):550–559. doi: 10.1016/0012-1606(78)90259-2. [DOI] [PubMed] [Google Scholar]
- Faust J. R., Goldstein J. L., Brown M. S. Receptor-mediated uptake of low density lipoprotein and utilization of its cholesterol for steroid synthesis in cultured mouse adrenal cells. J Biol Chem. 1977 Jul 25;252(14):4861–4871. [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brunschede G. Y., Brown M. S. Release of low density lipoprotein from its cell surface receptor by sulfated glycosaminoglycans. Cell. 1976 Jan;7(1):85–95. doi: 10.1016/0092-8674(76)90258-0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Brown M. S. Esterification of low density lipoprotein cholesterol in human fibroblasts and its absence in homozygous familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4288–4292. doi: 10.1073/pnas.71.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. K., Brown S., Bilheimer D. W., Goldstein J. L. Regulation of low density lipoprotein receptor activity in freshly isolated human lymphocytes. J Clin Invest. 1976 Dec;58(6):1465–1474. doi: 10.1172/JCI108603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illmensee K., Mintz B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc Natl Acad Sci U S A. 1976 Feb;73(2):549–553. doi: 10.1073/pnas.73.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M., Brown M. S., Faust J. R., Goldstein J. L. Replacement of endogenous cholesteryl esters of low density lipoprotein with exogenous cholesteryl linoleate. Reconstitution of a biologically active lipoprotein particle. J Biol Chem. 1978 Jun 25;253(12):4093–4101. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loken M. R., Herzenber L. A. Analysis of cell populations with a fluorescence-activated cell sorter. Ann N Y Acad Sci. 1975 Jun 30;254:163–171. doi: 10.1111/j.1749-6632.1975.tb29166.x. [DOI] [PubMed] [Google Scholar]
- Mintz B., Cronmiller C. Normal blood cells of anemic genotype in teratocarcinoma-derived mosaic mice. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6247–6251. doi: 10.1073/pnas.75.12.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B. Gene expression in neoplasia and differentiation. Harvey Lect. 1978;71:193–246. [PubMed] [Google Scholar]
- Mintz B., Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B. Teratocarcinoma cells as vehicles for mutant and foreign genes. Brookhaven Symp Biol. 1977 May 12;(29):82–95. [PubMed] [Google Scholar]
- Watanabe T., Dewey M. J., Mintz B. Teratocarcinoma cells as vehicles for introducing specific mutant mitochondrial genes into mice. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5113–5117. doi: 10.1073/pnas.75.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]