Background: Mammalian genomes encode two carotenoid oxygenases, but their contributions to vitamin A homeostasis remain undefined.
Results: Mammals employ symmetric and eccentric cleaving carotenoid oxygenases to convert different provitamin A carotenoids to vitamin A.
Conclusion: Both carotenoid oxygenases contribute to vitamin A production.
Significance: Carotenoids are the major source for vitamin A in the human diet.
Keywords: Carotenoid, Enzymes, Liver, Metabolism, Vitamin A
Abstract
Mammalian genomes encode two provitamin A-converting enzymes as follows: the β-carotene-15,15′-oxygenase (BCO1) and the β-carotene-9′,10′-oxygenase (BCO2). Symmetric cleavage by BCO1 yields retinoids (β-15′-apocarotenoids, C20), whereas eccentric cleavage by BCO2 produces long-chain (>C20) apocarotenoids. Here, we used genetic and biochemical approaches to clarify the contribution of these enzymes to provitamin A metabolism. We subjected wild type, Bco1−/−, Bco2−/−, and Bco1−/−Bco2−/− double knock-out mice to a controlled diet providing β-carotene as the sole source for apocarotenoid production. This study revealed that BCO1 is critical for retinoid homeostasis. Genetic disruption of BCO1 resulted in β-carotene accumulation and vitamin A deficiency accompanied by a BCO2-dependent production of minor amounts of β-apo-10′-carotenol (APO10ol). We found that APO10ol can be esterified and transported by the same proteins as vitamin A but with a lower affinity and slower reaction kinetics. In wild type mice, APO10ol was converted to retinoids by BCO1. We also show that a stepwise cleavage by BCO2 and BCO1 with APO10ol as an intermediate could provide a mechanism to tailor asymmetric carotenoids such as β-cryptoxanthin for vitamin A production. In conclusion, our study provides evidence that mammals employ both carotenoid oxygenases to synthesize retinoids from provitamin A carotenoids.
Introduction
Vitamin A (all-trans-retinol, ROL)4 is critical for vision, embryonic development, cellular homeostasis, and immunity (1–4). This isoprenoid lipid is metabolized into two biologically active derivatives as follows: all-trans-retinoic acid (RA) and 11-cis-retinal. RA is the ligand for retinoic acid receptors (RARs). These transcription factors form a heterodimeric complex with retinoid X receptors and regulate the expression of many genes throughout the mammalian life cycle (5). 11-cis-Retinal is the ligand for G protein-coupled receptors that mediate the phototransduction process by which light is converted into a neuronal signal in the retina (6).
Deficiency of vitamin A, especially prevalent in developing countries, leads to blindness in children as well as great increases in childhood morbidity (7). For most of the world's population, carotenoids with provitamin A activity are the main dietary source of vitamin A (8). The amount of vitamin A derived from carotenoids depends mainly on the following two factors: the bioavailability of the ingested carotenoid, and its metabolism by endogenous enzymes (9, 10). Consisting of compounds such as β,β-carotene (BC), α-carotene, and β-cryptoxanthin, these carotenoids must possess at least one nonsubstituted β-ionone ring. BC conversion to vitamin A was characterized in cell-free homogenates of the intestine (11, 12). From these analyses, a symmetric oxidative cleavage of BC at the 15,15′-double bond was proposed as the mode of vitamin A production. The gene encoding this β-carotene-15,15′-oxygenase (BCO1) was cloned from different species, including Drosophila, chicken, mouse, and humans (13–17). The primary BC cleavage product, retinaldehyde, can be converted to ROL, which is esterified by lecithin:retinol acyltransferase (LRAT) and/or acyl-CoA-dependent transferases (18). Then retinyl esters (REs) together with noncleaved BC are packaged into chylomicrons for secretion into lymph prior to body distribution and storage in stellate cells of the liver (19). Mobilization of vitamin A from hepatic stores depends on the 21-kDa retinol-binding protein (RBP) produced in hepatocytes and secreted into the circulation in an ROL-dependent manner (20). For uptake of vitamin A into peripheral tissues, an RBP receptor was identified to be encoded by the stimulated retinoic acid 6 gene (Stra6) (21).
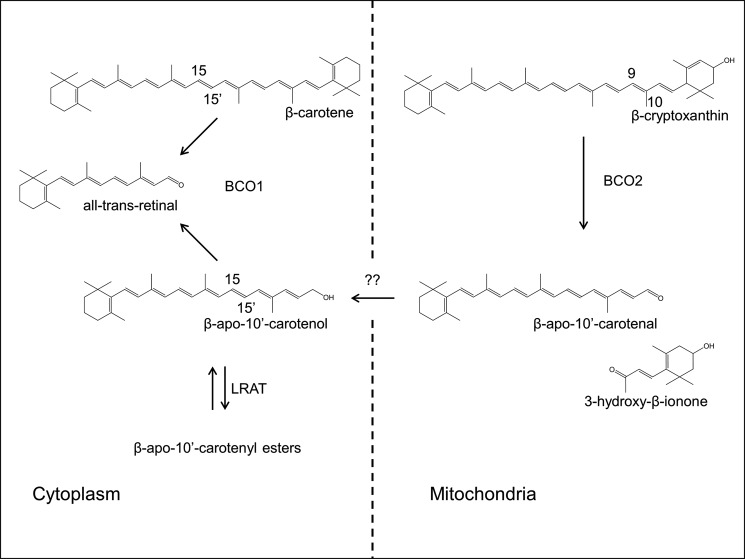
Biochemical studies indicate that mammals also can convert BC into β-apocarotenoids differently from retinoids (22–24). This eccentric cleavage of BC was proposed as an alternative method for producing vitamin A or its biologically active derivative, RA (25). Recently, β-apo-14-apocarotenal and β-apo-13-apocarotenone also have been implicated as selective antagonists of RARs (26, 27). Mammalian genomes encode a second putative BC-metabolizing enzyme (28). In contrast to BCO1, this β-carotene-9′,10′-oxygenase (BCO2) also can metabolize non-provitamin A compounds such as xanthophylls (29–31). BCO1 and BCO2 are localized in different intracellular compartments. BCO1 is a cytoplasmic protein, whereas BCO2 is found in mitochondria (17, 29). These differences in cellular localization may determine the substrate accessibility and reflect different physiological functions adopted by BCO1 and BCO2.
Whether BC can be metabolized by two different enzymes is of general interest for human health. Considering the vitamin A deficiency problem, it is clearly important to know what proportion of ingested BC can be converted to retinoids. Furthermore, the proposal that β-apocarotenoids function as naturally occurring retinoid antagonists has critical implications for the biological activities of BC as a provitamin (26). Thus, to study the role of the two carotenoid-cleaving enzymes in BC metabolism, we took advantage of previously generated knock-out mouse models for both enzymes (29, 32). Using single as well as compound mutants, we genetically dissected the function of these enzymes for provitamin A metabolism. Moreover, we analyzed the metabolic fate of apocarotenoids by performing both in vivo and in vitro experimental studies.
EXPERIMENTAL PROCEDURES
Animals, Husbandry, and Experimental Diets
Animal procedures and experiments were approved by the Case Western Reserve University Animal Care Committee and conformed to recommendations of both the American Veterinary Medical Association Panel on Euthanasia and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. In all experiments, mice were maintained at 24 °C in a 12:12-h light/dark cycle and had free access to food and water. The generation of Bco1−/−, Bco2−/−, and Lrat−/− mice has been previously described (29, 32, 33). Double knock-out Bco1−/−;Bco2−/− (ko/ko) mice were established by conventional cross-breeding. Female Bco1−/−, Bco2−/−, ko/ko, and wild type (WT) control mice with a C57/BL6;129Sv mixed genetic background were used for the described experiments. During breeding and weaning periods (up to 5 weeks of age), all mice were maintained on breeder chow containing ∼29,000 IU vitamin A/kg diet (Prolab RMH 3000). For BC intervention, mice then were fed a pelleted diet based on the AIN-93 formulation containing 50 mg of BC per kg dry pellet for 10 weeks as the sole source for vitamin A (data from this feeding study are presented in Figs. 1, 2, 5, and 6). As a control, female WT and Bco2−/− mice (n = 5) each were subjected to same diet for 10 weeks supplemented with 4000 IU of vitamin A instead of BC (experiment is presented in Fig. 7). The diet was prepared by Research Diets, Inc. (New Brunswick, NJ), by cold extrusion to protect BC from heat and incorporated a water-soluble formulation of beadlets (DSM Ltd., Sisseln, Switzerland). Because a previous study reported significant amounts of apocarotenoids in BC beadlets (34), we analyzed the diet for the presence of these compounds. However, β-apo-10′-carotenal content was below the detection limit (∼1 pmol) of our LC-MS system (data not shown). Weight gain and food intake of mice were measured as described previously (35). After 10 weeks of dietary intervention, mice were anesthetized by intraperitoneal injection of a mixture containing ketamine (80 mg/kg body weight) and xylazine (20 mg/kg body weight) in 10 mm sodium phosphate, pH 7.2, with 100 mm NaCl, and blood was drawn directly from the heart after snipping the right atrium. Then mice were perfused with 10 ml of PBS (137 mm NaCl, 2.7 mm KCl, 4.3 mm Na2HPO4, 1.4 mm KH2PO4, pH 7.3) and sacrificed by cervical dislocation before tissue collection.
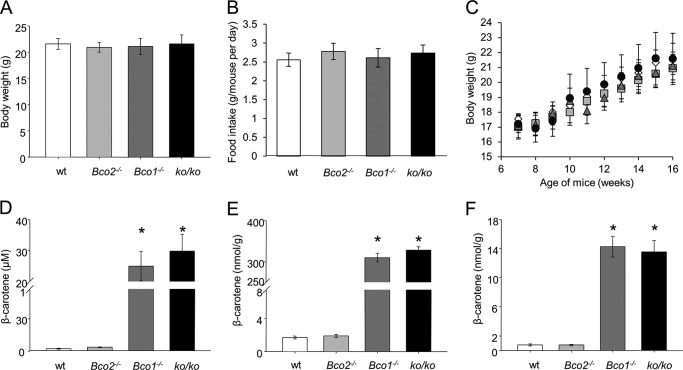
FIGURE 1.
BC selectively accumulates in mice lacking BCO1. Six-week-old wild type (wt), Bco2−/−, Bco1−/−, and ko/ko mice were provided a controlled diet for 10 weeks with BC (50 mg/kg) serving as the sole source for β-apocarotenoid production. A, body weights at sacrifice. B, food intakes during the feeding period. C, weight curves of WT (diamonds), Bco2−/− (squares), Bco1−/− (triangles), and ko/ko (circles) mice. D, BC serum levels. E, hepatic BC levels. F, pulmonary BC levels. Values indicate means ± S.E. of at least five female animals per tissue and genotype. Statistical significance was determined by the two-tailed Student's t test with results compared with the WT group. Threshold of significance was set at *, p < 0.05.
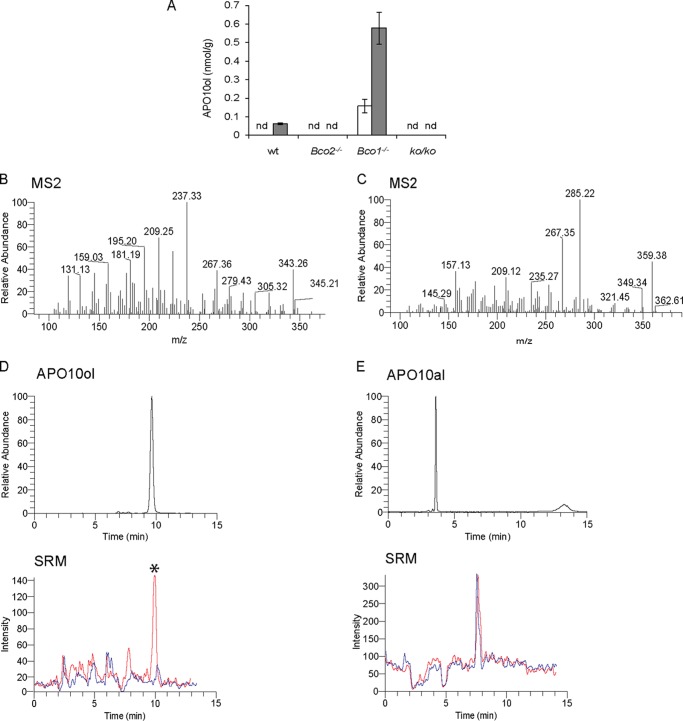
FIGURE 2.
APO10ol is the major long-chain β-apocarotenoid in mouse liver. Six-week-old wild type (wt), Bco2−/−, Bco1−/−, and ko/ko mice were provided a controlled diet for 10 weeks with BC (50 mg/kg) as the sole source for β-apocarotenoid production. A, hepatic levels of free APO10ol (white bars) and total APO10ol (free + esterified) (dark gray bars). nd, not detectable. Values indicate means ± S.E. for at least five female animals per tissue and genotype. Statistical significance was tested by the two-tailed Student's t test with results compared with the WT group. Threshold of significance was set at p < 0.05. B and C, MS/MS diffraction patterns of APO10ol (B) and β-apo-10′-carotenal (APO10al) (C). D and E, presence of APO10ol (D) and APO10al (E) extracted from livers of Bco1−/− (red trace) and ko/ko (blue trace) mice is indicated by an asterisk and identified by retention times and selected reaction monitoring (SRM) modes as compared with authentic standards (upper panel).
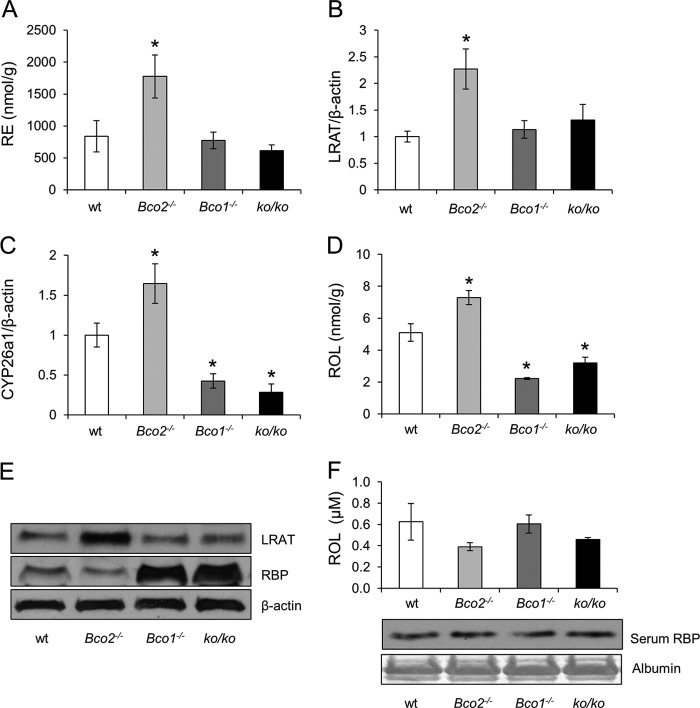
FIGURE 5.
Hepatic vitamin A status of WT, Bco2−/−, Bco1−/−, and ko/ko mice. Six-week-old WT, Bco2−/−, Bco1−/−, and ko/ko mice were provided a 10-week controlled diet with BC (50 mg/kg) as the sole source for β-apocarotenoid production. A, hepatic RE levels. B, qRT-PCR analysis of hepatic Lrat mRNA levels. C, qRT-PCR analysis of hepatic Cyp26a1 mRNA levels. D, hepatic ROL levels. E, immunoblots for hepatic LRAT and RBP protein levels. β-Actin was used as loading control. F, serum ROL levels (upper panel) and serum RBP protein levels (lower panel). Ponceau S staining for albumin was used as loading control. Values indicate mean ± S.E. from 5 to 6 animals per genotype. Statistical significance compared with the WT group was tested by the two-tailed Student's t test. Threshold of significance was set at *, p < 0.05.
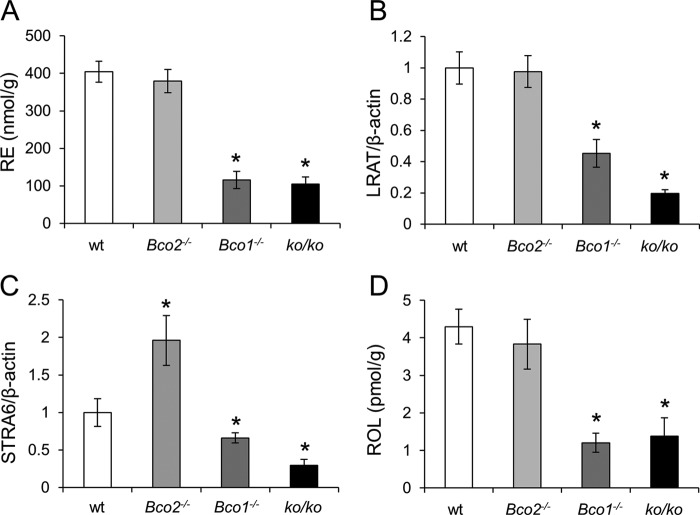
FIGURE 6.
Pulmonary vitamin A status of WT, Bco2−/−, Bco1−/−, and ko/ko mice. Six-week-old WT, Bco2−/−, Bco1−/−, and ko/ko mice were provided a 10-week controlled diet containing BC (50 mg/kg) as the sole source for β-apocarotenoid production. A, pulmonary RE levels. B, qRT-PCR analysis of pulmonary Lrat mRNA levels. C, qRT-PCR analysis of pulmonary Stra6 mRNA levels. D, pulmonary ROL levels. Values indicate means ± S.E. of results from 5 to 6 animals per genotype. Statistical significance compared with the WT group was tested by the two-tailed Student's t test. Threshold of significance was set at *, p < 0.05.
FIGURE 7.
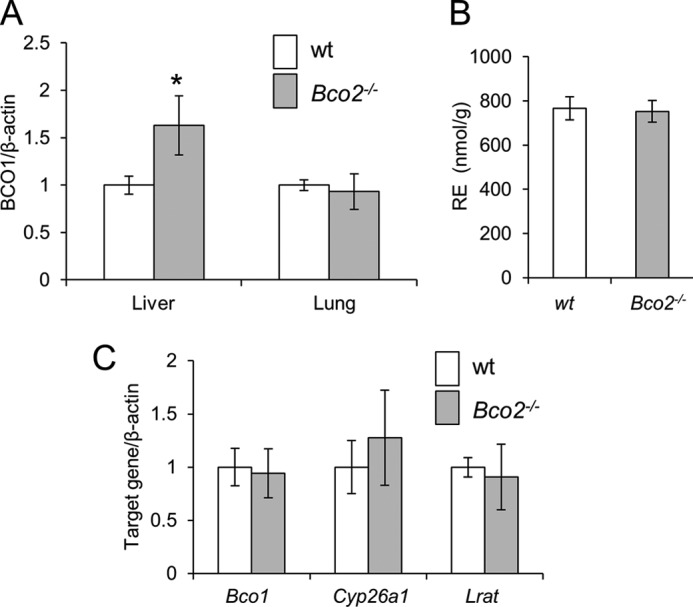
Bco1 expression and hepatic vitamin A status of Bco2−/− mice under different dietary conditions. A, hepatic Bco1 mRNA expression in female WT and Bco2−/− mice provided a 10-week controlled diet with BC (50 mg/kg) as the sole source for β-apocarotenoid production. B and C, female WT and Bco2−/− mice were provided a 10-week controlled diet with preformed vitamin A (4000 IU/kg). B, hepatic RE levels. C, qRT-PCR analyses of hepatic Bco1, Cyp26a1, and Lrat mRNA expression. Values indicate means ± S.E. from 5 to 6 animals per genotype. Statistical significance compared with the WT group was tested by the two-tailed Student's t test. Threshold of significance was set at *, p < 0.05.
For studying esterification, 12-week-old Lrat−/− and WT mice were gavaged with a pharmacological dose of β-10′-apocarotenal (20 mg/kg of body weight) dissolved in canola oil as a vehicle; prior to this, mice were raised on a standard chow diet containing ∼29,000 IU vitamin A/kg diet (Prolab RMH 3000) (the experiment is presented in Fig. 3). For assessing hepatic RBP release, 12- week- old female Bco1−/− mice were fed a diet based on the AIN-93 formulation without vitamin A (Research Diets). After 5 weeks, mice were treated either with pharmacological doses of ROL (Sigma) or β-apo-10′-carotenol (APO10ol) (each at 30 mg/kg of body weight) dissolved in canola oil. Four hours after treatment, mice were anesthetized and perfused with PBS, and tissues were harvested as described above. For studying β-cryptoxanthin metabolism, mice were raised on KLIBA 3430 chow (Provimi Kliba AG, Kaiseraugst, Switzerland) containing 14 IU/g vitamin A for 12 weeks and were injected with pharmacological doses of β-cryptoxanthin (20 mg/kg dissolved in DMSO) three times in 24-h intervals. Twenty four h after the last injection, mice were sacrificed, and livers were removed for further analysis (experiments are presented in Fig. 10).
FIGURE 3.
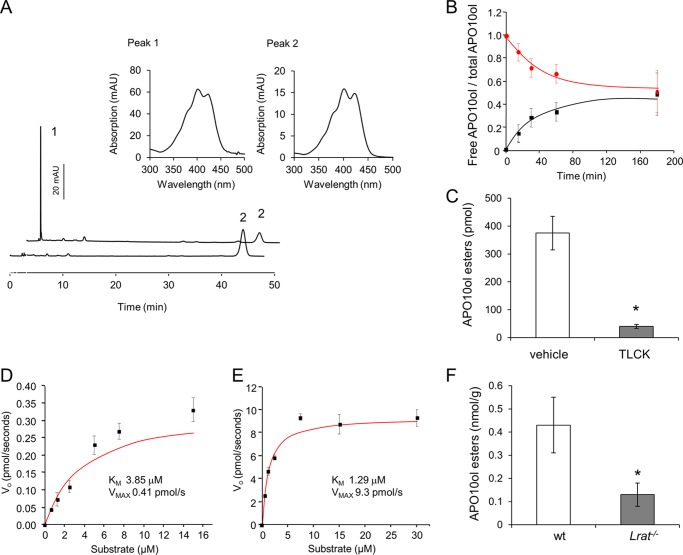
β-Carotene derivative APO10ol can be esterified by LRAT. A, HPLC traces of AP010ol before and after incubation with RPE microsomes (15 μg of protein per assay). Peak 1 represents APO10ol esters and peak 2 nonesterified APO10ol. The insets reveal the spectral characteristics of peaks 1 and 2. B, time course of APO10ol esterification by RPE microsomes. The red curve represents the decrease in free APO10ol, and the black curve represents the increase of APO10ol esters. Values are expressed as concentrations of APO10ol and/or APO10ol esters divided by the total APO10ol concentration. C, RPE microsomes (15 μg of protein each) were preincubated with TLCK (1 mm) and or vehicle for only 10 min. Then APO10ol was added to a final concentration of 15 μm. After 15 min, reactions were stopped, and APO10ol esterification was measured. D, RPE microsomes (15 μg of protein per assay) were incubated in the presence of increasing amounts of APO10ol for 10 min. APO10ol ester formation was measured by HPLC analysis, and initial reaction velocities were plotted against the substrate concentration. Vmax and Km values for APO10ol were calculated with Origin 9 software (OriginLab). E, RPE microsomes (15 μg of protein per assay) were incubated with increasing amounts of ROL for 45 s. RE formation was measured by HPLC analysis, and initial reaction velocities were plotted against the substrate concentration. Vmax and Km values for ROL were calculated with Origin 9 software. In all tests for enzymatic activity, values represent means ± S.E. of three independent assays. F, 12-week-old WT and Lrat−/− mice were gavaged with β-apo-10′-carotenal (20 mg/kg). Three hours later mice were sacrificed, and livers were isolated; lipids were extracted, and APO10ol esters were determined by HPLC analysis. Values present means ± S.E. of three animals per genotype. Statistical significance was tested by the two-tailed Student's t test with results compared with the WT group. Threshold of significance was set at p < 0.05.
FIGURE 10.

BCO2 converts β-cryptoxanthin to β-apo-10′-apocarotenal. A, protein extract containing recombinant murine BCO2 was incubated with increasing concentrations of β-cryptoxanthin. Lipophilic compounds were extracted and subjected to HPLC analysis. Amounts of products (β-apo-10′-carotenal (filled diamonds) and 3-OH-β-apo-10′-carotenal (open squares)) are plotted against the substrate concentration. B, β-cryptoxanthin levels in the liver of 12-week-old female WT, Bco2−/−, and Bco1−/− mice. Values indicate means ± S.E. from three animals per genotype. Statistical significance compared with the WT group was tested by the two-tailed Student's t test with p < 0.05 considered significant.
Analysis of RBP Secretion by HepG2 Cells
Human HepG2 cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin sulfate and cultured at 37 °C in 5% CO2. When cells reached confluence, the media were removed, and cells were washed twice with PBS. To mimic either vitamin A deficiency (VAD) or vitamin A sufficiency (VAS), cells were incubated with DMEM containing 10 mg/ml of bovine serum albumin (BSA) (Sigma) either in the presence (VAS) or absence (VAD) of 0.02 μm ROL. The amount and chemical form of vitamin A added to the media were chosen based on retinoid analyses of three different batches of FBS routinely used in our laboratory. After 48 h of incubation, medium was replaced with medium containing either ROL or APO10ol at a final concentration of 4 μm for 2 h. Ethanol (up to 0.1% v/v) was used as the vehicle control. Cells were harvested and frozen at −80 °C until further analyses. For immunocytochemistry, HepG2 cells were seeded on coverslips after using the same treatment and media conditions described above. At collection time, the media were removed, and cells were immediately fixed with 4% paraformaldehyde in PBS for 1 h. Then cells were washed in PBS with 0.1% Triton X-100 (Roche Diagnostics) (PBS-T) and blocked for another hour with PBS-T supplemented with 10% BSA and 5% goat serum (Sigma). Afterward, cells were incubated overnight in blocking buffer containing a rabbit anti-human RBP serum (DakoCytomation, Glostrup, Denmark) diluted 1:200 at 4 °C. Then cells were washed and incubated for 1 h at room temperature with a goat anti-rabbit secondary antibody-conjugated Alexa 488 fluorophore (Invitrogen) diluted 1:400. DAPI was used to stain nuclei. Confocal images were acquired with a Zeiss LSM 510 META laser scanning confocal microscope (Carl Zeiss MicroImaging, Jena, Germany) by using a multiline argon laser (excitation 488 nm) or a 405 diode laser (excitation 405 nm) with a 63× C-Apochromat, NA 1.2-W objective.
mRNA Isolation and q-PCR Analysis
Total mRNA isolation was carried out with the TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA concentration and purity were measured with a Nano-drop spectrophotometer (ND-1000, Thermo Scientific, Marietta, OH). The retrotranscription kit (Applied BioSystems, Carlsbad, CA) was employed to reverse transcribe 0.5 μg of total RNA to cDNA. qRT-PCR was performed with TaqMan probes (Applied BioSystems) for Cyp26a1 (Mm00514486_m1), Stra6 (Mm00486457_m1), Lrat (Mm00469972_m1), Bco1 (Mm0151350_m1), and Bco2 (Mm00460051_m1). The β-actin (Mm01205647_g1) probe set was used as an endogenous control. All real time experiments were performed with an ABI Step-One Plus qRT-PCR instrument (Applied BioSystems).
Immunoblotting
Tissue samples were homogenized in M-PER mammalian protein extraction reagent (Thermo Scientific) following the manufacturer's instructions. For RBP and LRAT determinations in liver, 20 μg of total protein was used. To determine RBP serum levels, 2 μl of serum was diluted with 40 μl of PBS containing complete mini EDTA-free protease inhibitor (Roche Diagnostics), and 4 μl of this solution was used for immunoblot analysis. To quantify RBP levels in HepG2 cells, 10–20 μg of total protein cell lysate were subjected to immunoblot analyses. Protein samples were separated by SDS-PAGE and then electroblotted onto PVDF membranes (Bio-Rad). Membranes were blocked with fat-free milk powder (5% w/v) dissolved in Tris-buffered saline (15 mm NaCl and 10 mm Tris/HCl, pH 7.5) containing 0.01% Tween 100 (TBS-T), washed, and incubated overnight at 4 °C with the appropriate primary antibody. For RBP detection, a rabbit anti-human RBP serum (DakoCytomation) was used at a 1:1000 dilution. For LRAT detection, a noncommercial anti-LRAT monoclonal antibody was employed at a dilution of 1:2000 (33). β-Actin antiserum (Cell Signaling, Boston) at a dilution of 1:1000 served as a loading control. Secondary antibodies were either horseradish peroxidase-conjugated anti-rabbit IgG (Promega, Madison, WI) or anti-mouse IgG (Promega) used at a dilution of 1:5000. Immunoblots were developed with the ECL system (GE Healthcare).
HPLC Analyses of Apocarotenoids and Carotenoids
Nonpolar compounds were extracted from either 100 μl of serum or 30 mg of tissue under a dim red safety light as described previously (32). The extraction solution was composed of 200 μl of methanol, 400 μl of acetone, and 500 μl of hexane. The organic phase was removed, and the extraction was repeated with 500 μl of hexane. For saponification, selected tissues were incubated with 100 μl of 12% pyrogallol (Sigma) in ethanol, 200 μl of 30% KOH in water, and 1 ml of ethanol for 2 h at 37 °C. After saponification, 2 ml of ethanol and 2 ml of H2O were added, and samples were extracted twice with 3 ml of ether/hexane (2:1, stabilized with 1% ethanol). After centrifugation for 5 min at 800 × g, organic layers were collected, pooled, and dried in a SpeedVac (Eppendorf, Hamburg, Germany). The residue was dissolved in 200 μl of HPLC solvent (hexane/ethyl acetate 90:10, v/v). HPLC was performed with a normal phase Zobax Sil (5 μm, 4.6 × 150 mm) column (Agilent Technologies, Santa Clara, CA). For isocratic chromatographic separation of apocarotenal-oximes, 20% ethyl acetate was mixed with 80% hexane. For isocratic chromatographic separation of apocarotenals, 5% ethyl acetate was mixed with 95% hexane. For retinyl ester (RE) separation, a linear gradient of 1% ethyl acetate in hexane over 15 min followed by 10 min with 20% of ethyl acetate in hexane was used. In both cases, the flow rate was 1.4 ml/min. For molar quantification of retinoids, the HPLC was previously scaled with the pattern compounds ROL, RE (Sigma), BC (Calbiochem), and APO10ol.
Mass Spectroscopy Analyses
Polar and nonpolar apocarotenoids were separated and extracted from HepG2 and liver samples for mass spectrometry by previously described methodology (36). Briefly, either HepG2 cells grown on 6-well plates or 750 mg of liver was homogenized in 0.9% saline solution in a final volume of 0.75 ml. To separate nonpolar from polar apocarotenoids, the solution was alkalinized with 0.025 m KOH in 1 ml of ethanol. Then 1 ml of acetonitrile was added, followed by extraction of nonpolar apocarotenoids with 10 ml of hexane. After addition of 60 μl of 4 m HCl, polar retinoids were extracted with 10 ml of hexane. Lipid extracts were dried down in a SpeedVac, and residues were dissolved in hexane, ethyl acetate, and acetic acid (79.9:20:0.1 v/v) for further analysis.
Mass Spectrometry
MS-based detection of apocarotenoids was achieved with a LXQ linear ion trap mass spectrometer (Thermo Scientific, Waltham, MA) equipped with an atmospheric pressure chemical ionization interface coupled to an Agilent 1100 HPLC series and diode array detector (Agilent Technologies). Separation of compounds was achieved on a normal phase Zobax Sil (5 μm, 4.6 × 150 mm) column (Agilent Technologies) by isocratic flow of 20% ethyl acetate in hexane, 0.0175% acetic acid at rate of 1.4 ml/min. The HPLC effluent was directed into the MS via an atmospheric pressure chemical ionization probe operated in the positive ionization mode. Parameters of both ionization and detection were tuned with synthetic β-apocarotenoid standards to ensure the highest possible sensitivity. The detection limit (defined as a minimum of three times ion intensity over the background signal) was determined by titrations with known amounts of standards. The matrix had no gross influence on sensitivity, which was estimated to be ∼1 pmol for all of the tested apocarotenoids. RE, ROL, retinal, and RA were purchased from Sigma. β-Apo-10′-, β-apo-12′-, and β-apo-14′-apocarotenoic acid as well as β-apo-10′-carotenal were a gift from BASF (Ludwigshafen, Germany). APO10ol was prepared from β-apo-10′-carotenal by BH4 reduction. Retinoids and apocarotenoids were detected by a selected reaction monitoring mode using the following transitions: ROL and RE, m/z 269.2 → 213.2; retinal-oximes, m/z 300.3 → 208.2; RA, m/z 301.4 → 205.2; APO10ol and APO10ol esters, m/z 361.5 → 237.3; β-apo-10′-carotenal-oximes, m/z 392.4 → 285.2; β-apo-10′-carotenoic acid, m/z 393.4 → 347.3; β-apo-12′-carotenoic acid, m/z 367.4 → 349.3.0; and β-apo-14′-carotenoic acid, m/z 327.3 → 215.0.
ROL and APO10ol Uptake Assays
NIH3T3 cells expressing both LRAT and STRA6 were cultured in 12-well culture plates at a density of 1 × 106 cells per well as described previously (37). When cells reached ∼85% confluence, they were washed with PBS. Then serum-free medium containing human RBP (hRBP) with bound 2 μm ROL or APO10ol was added. As controls, separate sets of plates were treated with 2 μm of either free ROL or APO10ol. Cells were incubated at 37 °C. After collection, cells were washed twice with PBS, harvested in 250 μl of PBS, sonicated, and stored at −20 °C. The cell homogenate (200 μl) also was extracted with 200 μl of methanol, 400 μl of acetone, and 600 μl of hexane, and the entire organic phase was collected and dried down in a SpeedVac. Protein levels in the cell homogenate were measured by Bradford's method (Bio-Rad), and esters of ROL or APO10ol were analyzed by normal phase HPLC as described above. All experiments were performed at least in duplicate.
Expression, Purification, and Refolding of Human Serum RBP
RBP expression and purification from Escherichia coli was accomplished essentially as described previously (38). Briefly, hRBP cDNA was cloned into a pET3a expression vector and expressed in BL-21 DE3 cells according to a standard protocol. Bacterial cells were harvested and lysed by osmotic shock. Insoluble material was pelleted by centrifugation, washed, and solubilized in 7 m guanidine hydrochloride and 10 mm dithiothreitol (DTT). After overnight incubation, insoluble material was removed by ultracentrifugation, and the supernatant was used for the hRBP refolding procedure. hRBP was refolded by dropwise addition of solubilized material into a mixture containing 1 mm ROL (Sigma) or APO10ol. Refolded holo-hRBP was dialyzed against 10 mm Tris/HCl buffer, pH 8.0, and loaded onto a DE53 anion exchange chromatography column (Whatman). Holo-hRBP was eluted with linear gradient of NaCl (0–1 m) in 10 mm Tris/HCl buffer, pH 8.0. Collected fractions were examined by SDS-PAGE and UV-visible spectroscopy to determine the protein/apocarotenoid ratio. Fractions containing holo-hRBP were pooled together and concentrated in a Centricon centrifugal filter device (cutoff 10,000 Da) (Millipore, Billerica, MA).
LRAT Assays
Bovine RPE was used to measure LRAT enzymatic activity. Bovine RPE microsomes were obtained from fresh RPE isolated from bovine eye cups with 0.05 m MOPS, 0.25 m sucrose, 1 mm DTT, pH 7. The extract was homogenized in a glass homogenizer and then centrifuged (12,000 × g, 30 min, 4 °C). The resulting supernatant was collected for further ultracentrifugation at 4 °C for 60 min (100,000 × g). The RPE microsomal fraction was resuspended in 5 mm BisTris, 5 mm DTT, pH 7, and stored in small aliquots at −80 °C. To remove pre-existing RE and other retinoids that could interfere with the enzyme assay, 500-μl aliquots of the RPE microsomal fraction were irradiated in a quartz cuvette for 20 min on ice with a ChromatoUVE transilluminator (model TM-15 from UVP Inc.) at low intensity. Then 20 μl of RPE microsomal fractions (15 μg of protein) were added to a total volume of 200 μl of 10 mm Bistris propane buffer, 1 mm DTT, and 1% BSA, pH 7.5. The reaction was initiated by addition of variable amounts of ROL or APO10ol delivered in 1 μl of dimethylformamide. The reaction mixture was vigorously vortexed and incubated at 37 °C with 550 rpm rotation in a thermomixer (Eppendorf thermomixer compact; Eppendorf, Germany). After incubation, the reaction was stopped by addition of 0.3 ml of methanol and 0.3 ml of acetone followed by total retinoid extraction into 0.5 ml of hexane. The hexane extraction was repeated, and the combined organic phases were dried in a SpeedVac (Eppendorf). Dried residues were dissolved in solvent, and their β-apocarotenoid composition was analyzed by HPLC as described above.
Enzymatic Activity of Human BCO1
Twenty milliliters of baculovirus containing a human BCO1 plasmid (28) was added to 800 ml of Spodoptera frugiperda 9 cell culture in a 2-liter baffled flask. Cell pellets (20–30 g) were resuspended in 50 ml of sample buffer containing 20 mm Tricine, pH 7.5, 1 mm tris(2-carboxyethyl)phosphine hydrochloride (Hampton Research, Aliso Viejo, CA), and 1 tablet of Complete EDTA-free protease inhibitor mixture (Roche Diagnostics). Cells were lysed in a glass tissue grinder, and the lysate was centrifuged at 100,000 × g for 1 h at 4 °C (Beckman Coulter OptimaTM L-90K Ultracentrifuge). The supernatant was collected and used for assays of enzymatic activity as described previously (39). Briefly, APO10ol, β-8′-apocarotenal, and β-12′-apocarotenoic acid were mixed with 30 μl of 12% (w/v) n-octyl β-d-thioglucopyranoside micelles (3%), dissolved in ethanol, and dried in a SpeedVac. Then the enzyme solution (100 μl) or elution buffer only was added to the residues and vortexed for 20 s. Enzymatic assays were incubated at 28 °C with 300 rpm rotation in a thermomixer (Eppendorf) for 20 min under a dim red safety light. Reactions were stopped by the addition of 100 μl of NH2OH and 200 μl of methanol. Lipids then were extracted and analyzed by HPLC as described above. For enzyme tests with β-8′-apocarotenal, lipids were extracted without the addition of NH2OH and analyzed by HPLC.
Enzymatic Activity of Murine BCO2
For heterologous expression of murine BCO2, a plasmid containing the BCO2 full-length cDNA was transformed in the E. coli strain XL1-blue (Stratagene Inc., La Jolla, CA) as detailed previously (28). Enzymatic activity of crude extracts (100 μl of total protein) was determined in the presence of β-cryptoxanthin (CaroteNature, Lupsingen, Switzerland) in n-octyl β-d-thioglucopyranoside micelles (3%) at 28 °C. Reactions were stopped after 8 min by the addition of 300 μl of methanol, and lipophilic compounds were extracted and subjected to HPLC analysis as described above.
Statistical Analyses
Values are expressed as means ± S.E. Statistical significance of differences was derived from the two-tailed Student's t test with the threshold of significance set at p < 0.05. Vmax and Km values for APO10ol kinetics were calculated with software Origin 9 (OriginLab Corp., Northampton, MA).
RESULTS
BC Accumulation in Mice Depends on BCO1
We subjected 6-week-old and sex-matched WT, Bco1−/−, Bco2−/−, and ko/ko mice to dietary intervention with BC. During the 10-week feeding period, we found no differences in food intake and/or weight gain between genotypes (Fig. 1, A–C). Then mice were sacrificed, and blood and tissues were collected. HPLC analyses revealed that Bco1−/− and ko/ko mice had accumulated BC in the serum, liver, and lungs, but no significant differences were found in their total BC levels in serum and tissues. In contrast, WT and Bco2−/− mice showed about 100-fold lower levels of BC in tissues and serum when compared with Bco1−/− and ko/ko mice (Fig. 1, D–F). Again, no significant difference was observed in total BC levels between WT and Bco2−/− mice. Hence, we conclude that BCO2 did not affect bulk BC metabolism as indicated by comparable accumulation of BC in Bco1−/− and ko/ko mice.
BCO2 Catalyzes β-Apocarotenoid Production in Vivo
We next analyzed whether apocarotenoids other than retinoids were synthesized from BC by different mouse genotypes. After total hepatic lipid extraction and separation by HPLC, APO10ol was detected in Bco1−/− mice (Fig. 2A). APO10ol was identified by its retention time and mass as compared with an authentic standard (Fig. 2, B–D). The primary cleavage product β-apo-10′-carotenal was not detected by our LC-MS system (Fig. 2, E–G). Saponification of liver samples from Bco1−/− mice resulted in a 3-fold higher amount of APO10ol relative to nonsaponified liver samples, indicating that APO10ol exists largely in its esterified form (Fig. 2A). Also small amounts of APO10ol became detectable in saponified liver samples of WT mice. In contrast, APO10ol was absent in Bco2−/− and ko/ko mice, indicating that production of this compound depends on a functional Bco2 allele (Fig. 2A). Next, we investigated whether β-apo-carotenoic acids are formed from dietary BC in a BCO2-dependent manner. In Bco1−/− and ko/ko mice, apocarotenoids such as β-apo-12′- and β-apo-14′-carotenoic acid were not detected either in selected reaction monitoring or single ion monitoring modes (data not shown). However, trace amounts of β-10-apo-carotenoic acid became detectable in Bco1−/− mice (data not shown).
LRAT Can Esterify APO10ol
Our analyses revealed that APO10ol is the main eccentric cleavage product of BC in mice. In the liver of Bco1−/− and WT mice, this compound mainly existed in esterified form. The major hepatic enzyme for ROL esterification is LRAT (33). Because of the similar chemical structures of APO10ol and ROL, we tested whether APO10ol is a substrate for this enzyme by using bovine RPE microsomes as a source of LRAT. Upon incubation with APO10ol, we observed a time-dependent decrease of the substrate that corresponded to the formation of APO10ol esters (Fig. 3, A and B). To demonstrate that APO10ol esterification was indeed LRAT-dependent, we then performed assays in the presence of tosyl-lysine chloromethyl ketone (TLCK). TLCK is serine protease inhibitor that also inhibits enzymes such as LRAT with a thiol group in their active sites (40–42). After preincubation of microsomes with TLCK, esterification of APO10ol was greatly decreased (Fig. 3C). Next, we determined Km and Vmax values for APO10ol and ROL, respectively. LRAT displayed a 3-fold higher Kmvalue for APO10ol (3.85 μm) as compared with ROL (1.29 μm). But strikingly, the Vmax value was about 20-fold lower for APO10ol (0.41 pmol/s) relative to ROL (9.3 pmol/s) (Fig. 3, D and E). To determine whether APO10ol can be esterified by LRAT in vivo, we tested this hypothesis in Lrat knock-out mice. Lrat−/− and WT mice were gavaged with apo-10′-β-apocarotenal (20 mg/kg). After 3 h, they were sacrificed, and their livers were subjected to HPLC analysis. Livers of Lrat−/− mice showed significantly lower amounts of APO10ol esters as compared with WT controls. But small amounts of APO10ol esters were still formed in Lrat−/− mice, indicating that additional enzyme systems can esterify this compound (Fig. 3F).
APO10ol Can Trigger RBP Release
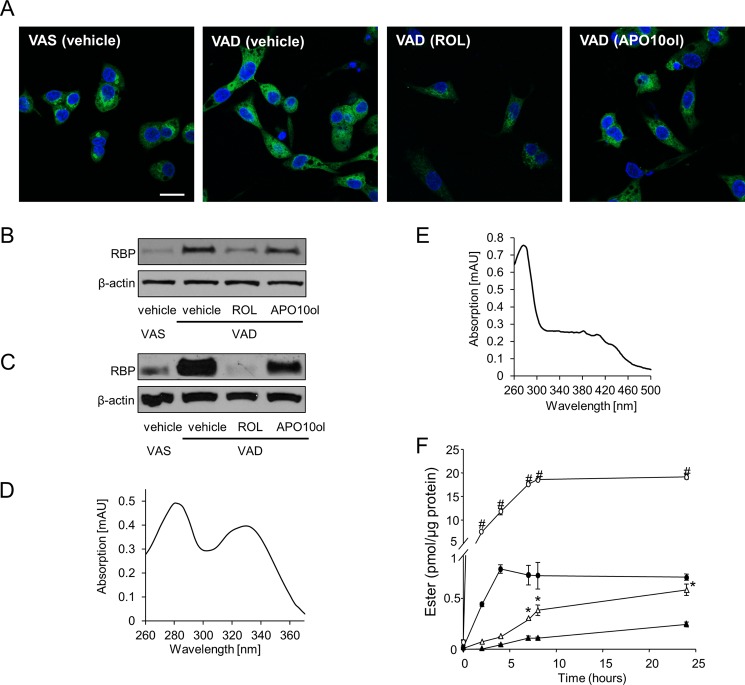
We showed that APO10ol, like ROL, existed in esterified form in mouse liver. Therefore, we next wondered whether APO10ol also can be released from hepatocytes like vitamin A. To experimentally address this question, we employed human hepatoma HepG2 cells used as a reliable model to study RBP-mediated vitamin A release (43) and cultured these cells in serum-free medium supplemented with and without vitamin A. Cells then were treated with either ROL or APO10ol. By immunocytochemistry and confocal imaging we visualized cellular RBP levels. As expected, cells cultured in vitamin A-free medium accumulated the vitamin A carrier RBP (Fig. 4, A and B). ROL treatment of cells decreased intracellular RBP levels, indicative of its release (Fig. 4, A and B). Similarly, APO10ol treatment also decreased cellular RBP levels although this decrease was less pronounced.
FIGURE 4.
APO10ol transport and uptake. HepG2 cells were maintained at 37 °C in the presence (VAS) or absence (VAD) of vitamin A in the cell culture medium for 48 h. Cells then were incubated in 4 μm ROL or APO10ol with ethanol used as a vehicle control for 2 h. A, immunostaining for RBP (green). Nuclei were stained with DAPI (blue). Scale bar, 20 μm. B, immunoblot analysis for RBP in protein extracts of HepG2 cells, using β-actin as the loading control. Ten μg of total protein were loaded per lane. C, 12-week-old Bco1−/− mice were maintained on a VAD or VAS diet for 5 weeks. Then vitamin A-deprived mice were each gavaged with 30 mg/kg APO10ol or ROL dissolved in canola oil as a vehicle. Four hours later, mice were sacrificed; their livers were removed, and RBP levels were determined by immunoblot analysis. Ten μg of total protein were loaded per lane with β-actin used as a loading control. At least three mice were used per experimental group. D and E, spectral characteristics of purified ROL-hRBP (D) and APO10ol-hRBP (E). F, NIH3T3 cells expressing human LRAT and STRA6 were incubated in either 2 μm of ROL-hRBP (open circles), APO10ol-hRBP (open triangles), free ROL (filled circles), or free APO10ol (filled triangles). Uptake efficiency was measured as RE and APO10ol ester formation per μg of protein at different time points. Values shown for cell culture experiments indicate means ± S.E. from three independent experiments. Statistical significance was tested by the two-tailed Student's t test with results compared with free ROL (#) and APO10ol (*), respectively. Threshold of significance was set at p < 0.05.
We next subjected 12-week-old Bco1−/− mice to dietary vitamin A restriction for 5 weeks. We then gavaged these mice with a pharmacological dose (30 mg/kg) of either ROL or APO10ol. Mice were sacrificed 4 h later, and RBP protein levels were measured in their livers. Siblings maintained on a vitamin A-sufficient diet were used as controls. In vitamin A-deprived mice treated with vehicle only, RBP levels were significantly higher than in vitamin A-sufficient mice. Moreover, ROL treatment of vitamin A-deprived Bco1−/− mice decreased hepatic RBP levels (Fig. 4C). Gavage with APO10ol also resulted in a reduction of RBP levels, indicating that APO10ol can trigger the release of RBP from the liver of these animals (Fig. 4C).
Cellular Uptake of APO10ol
We next tested whether APO10ol bound to RBP can be taken up by target cells. Accordingly, recombinant human RBP was produced in E. coli and refolded in the presence of ROL or APO10ol. Refolded RBP and free apocarotenoid fractions were purified by size exclusion chromatography to ensure the absence of free ROL or APO10ol in both protein preparations (Fig. 4, D and E) (38). We used NIH3T3 cells expressing STRA6 and LRAT previously established to measure ROL and APO10ol uptake (37, 38). These cells were incubated with 2 μm free and RBP-bound APO10ol as well as with 2 μm free and RBP-bound ROL. Uptake efficiency was measured as the amount of esterified AP010 or ROL at different time points (Fig. 4F). Both RBP-bound ROL and APO10ol were taken up more rapidly than their unbound forms. However, RBP-bound ROL was taken up far more rapidly than its APO10ol counterpart. This difference might be explained in part by the enzymatic activity of LRAT that can catalyze ROL esterification more rapidly than APO10ol esterification (Fig. 3). Additionally, a lower affinity of RBP-bound APO10ol to STRA6 may slow down its transfer to LRAT and its accumulation as ester.
BCO1, but Not BCO2, Is Critical for Vitamin A Homeostasis
We showed that APO10ol is BCO2-dependently produced from BC and can be esterified and transported by similar mechanisms as ROL. Thus, to learn whether dietary APO10ol affects vitamin A homeostasis, we determined retinoid levels in the liver and lungs of different mouse mutants supplemented with BC and compared them with those of WT mice. Additionally, we measured dietary BC effects on the expression levels of key proteins for vitamin A homeostasis such as RBP, STRA6, LRAT, and CYP26a1. CYP26a1 is an RA-catabolizing enzyme widely used as a surrogate marker for RA status in tissues (44). Bco2−/− mice displayed increased hepatic RE stores as compared with the other genotypes (Fig. 5A). LRAT and CYP26a1 expression also were significantly elevated as compared with WT animals (Fig. 5, B, C and E). Furthermore, hepatic free ROL levels also were increased (Fig. 5D). In lungs of Bco2−/− mice, no differences in RE levels were detectable when compared with WT mice (Fig. 6A). Additionally, LRAT mRNA expression levels and free ROL levels were not significantly altered as compared with WT mice (Fig. 6, B and D). Only a slight but significant increase of Stra6 mRNA level was observed (Fig. 6C). Thus, genetic disruption of BCO2 increased vitamin A status of the liver but not the lungs on the BC diet.
In Bco1−/− mice, no significant differences were observed for hepatic RE stores as compared with WT mice (Fig. 5A). Similarly, LRAT expression was not altered in the liver of this mutant (Fig. 5, B and E). However, the expression level of Cyp26a1 was significantly decreased in Bco1−/− as compared with WT animals (Fig. 5C). Additionally, these mice showed increased hepatic RBP levels (Fig. 5E) and a substantial reduction of free hepatic ROL levels (Fig. 5D). Serum vitamin A and RBP levels were comparable between different genotypes (Fig. 5F). In the lungs of Bco1−/− mice, RE stores were significantly reduced as compared with WT mice (Fig. 6A). The decreased vitamin A status of this organ also was reinforced by the reduced Lrat and Stra6 mRNA levels (Fig. 6, B and C) and reduced free ROL levels in this organ (Fig. 6D). Thus, these analyses indicated that genetic disruption of BCO1 impaired vitamin A homeostasis, whereas genetic disruption of BCO2 resulted in increased hepatic but not pulmonary vitamin A status when BC was provided as the sole dietary source of this vitamin. To determine whether BCO2 impacted vitamin A status in BCO1 deficiency, we analyzed ko/ko mice. In livers of these mice, Cyp26a1 mRNA expression was reduced to a similar extent as in Bco1−/− mice (Fig. 5C). Additionally, hepatic free ROL and RBP protein levels were also comparable between the double and single BCO1 mutant (Fig. 5, D and E). Finally, pulmonary RE and free ROL levels (Fig. 6, A and D) as well as Lrat and Stra6 mRNA expression levels (Fig. 6, B and C) were all reduced to a similar extent as in Bco1−/− mice. Thus, we excluded that BCO2 alone can improve vitamin A status on BC diet.
Increased Hepatic RA Status Is Associated with BCO1 Expression
Our analyses revealed that hepatic RE stores were significantly elevated in Bco2−/− as compared with WT mice. Additionally, Cyp26a1 mRNA levels were significantly elevated, indicative of increased hepatic RA levels in this mouse mutant. A putative explanation for these findings is the abolition of BCO2-dependent production of apocarotenoids that antagonize vitamin A action. However, our biochemical analyses appeared to exclude that such compounds were produced in mice (see above). Thus, we speculated that improved vitamin A status could be related to BCO1 and retinaldehyde production, since retinaldehyde is the direct precursor of RA that regulates Lrat and Cyp26a1 expression. qRT-PCR analysis revealed that Bco1 mRNA levels were significantly elevated in the livers of Bco2−/− as compared with WT mice (Fig. 7A). This difference was not observed in the lungs of these mice wherein vitamin A homeostasis was not altered relative to WT mice (Fig. 7A). To demonstrate more directly that the altered hepatic vitamin A status of Bco2−/− mice is causatively related to BCO1, we raised mice in a similar fashion as described above but on diet containing preformed vitamin A (4000 IU per kg) instead of BC. No differences should become apparent between Bco1−/− and WT mice if BC conversion caused this phenotype. In fact, our HPLC analysis revealed comparable levels of RE in the livers of Bco2−/− and WT mice (Fig. 7B). Additionally, expression of Bco1, Cyp26a1, and Lrat was comparable between Bco2−/− and WT mice under this dietary regimen (Fig. 7C).
APO10ol Can Be Metabolized to Vitamin A by BCO1
Bco1−/− mice displayed higher levels of APO10ol than WT mice. Thus, we tested whether this difference might be related to further metabolic conversion of APO10ol by assaying the effect of purified recombinant human BCO1 on APO10ol. This enzymatic analysis revealed that BCO1 converted APO10ol to retinaldehyde (Fig. 8A). We also performed BCO1 enzymatic assays with other β-apocarotenoids. These analyses revealed that β-8′-apocarotenal as well as β-12′-apocarotenoic acid were converted to retinal (Fig. 8, B and C), indicating that BCO1 can catalyze the conversion of β-apocarotenoids with different chain lengths and oxidation states. We next determined the specific activity of the protein crude extract for different apocarotenoid substrates and compared it with the established substrate BC. This analysis revealed that apocarotenoids were converted to retinal with comparable efficiency as BC (Fig. 8). The specific activity was determined to be 183.5, 79.7, 27.6, and 80.4 pmol·min−1·mg−1, respectively, for APO10ol, β-8′-apocarotenal, β-12′-apocarotenoic acid, and BC. To confirm this finding in a cellular environment, we added APO10ol to a culture medium of human hepatoma HepG2 cells. After incubation, cells were harvested, and lipophilic compounds were extracted and analyzed, which revealed that various retinoids were produced from APO10ol, including ROL, RE, and retinaldehyde (Fig. 9A). We also tested by LC-MS analyses whether β-apo-12′- and β-apo-14′-apocarotenoic acids were synthesized from APO10ol (Fig. 9B). Except for trace amounts of β-apo-12′-apocarotenoic acid, none of these compounds were detectable indicating that conversion to retinoids is the main metabolic fate of APO10ol in these cells.
FIGURE 8.
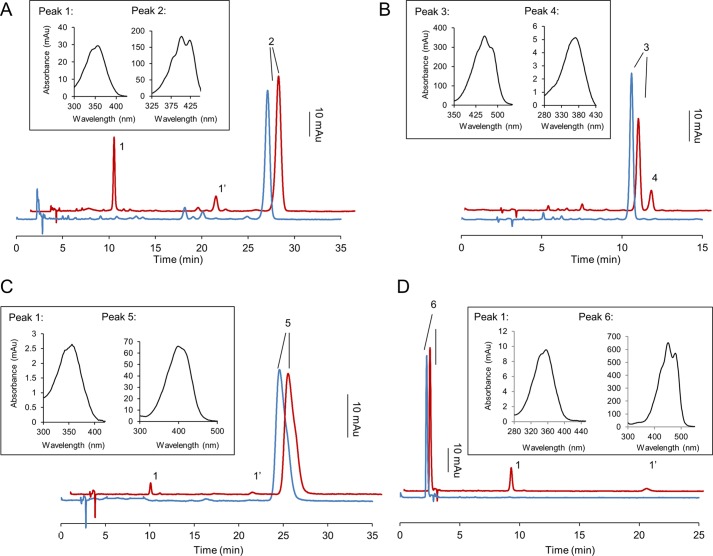
BCO1 converts long-chain β-apocarotenoids into retinoids. Recombinant human BCO1 was incubated with different β-apocarotenoids and BC (20 μm each) for 15 min. Lipophilic compounds were extracted and subjected to HPLC analyses. The panels show HPLC traces of substrates (blue traces) as compared with substrates incubated with recombinant human BCO1 (red traces). The insets give the spectral characteristics of different peaks. A, APO10ol; peak 1, all-trans-retinal-oxime (syn); peak 1′, all-trans-retinal-oxime (anti); peak 2, APO10ol. B, β-Apo-8′-carotenal; peak 3, β-apo-8′-carotenal; peak 4, all-trans-retinal. Note that apocarotenoids were not converted to the corresponding oxime because apo-8′-carotenal-oxime and all-trans-retinal-oxime (syn) co-eluted on the HPLC system. C, β-apo-12′-carotenoic acid; peak 1, all-trans-retinal-oxime peak (syn); peak 1′, all-trans-retinal-oxime (anti); peak 5, β-apo-12′-carotenoic acid. D, BC, peak 1, all-trans-retinal-oxime peak (syn); peak 1′, all-trans-retinal-oxime (anti); peak 6, BC.
FIGURE 9.
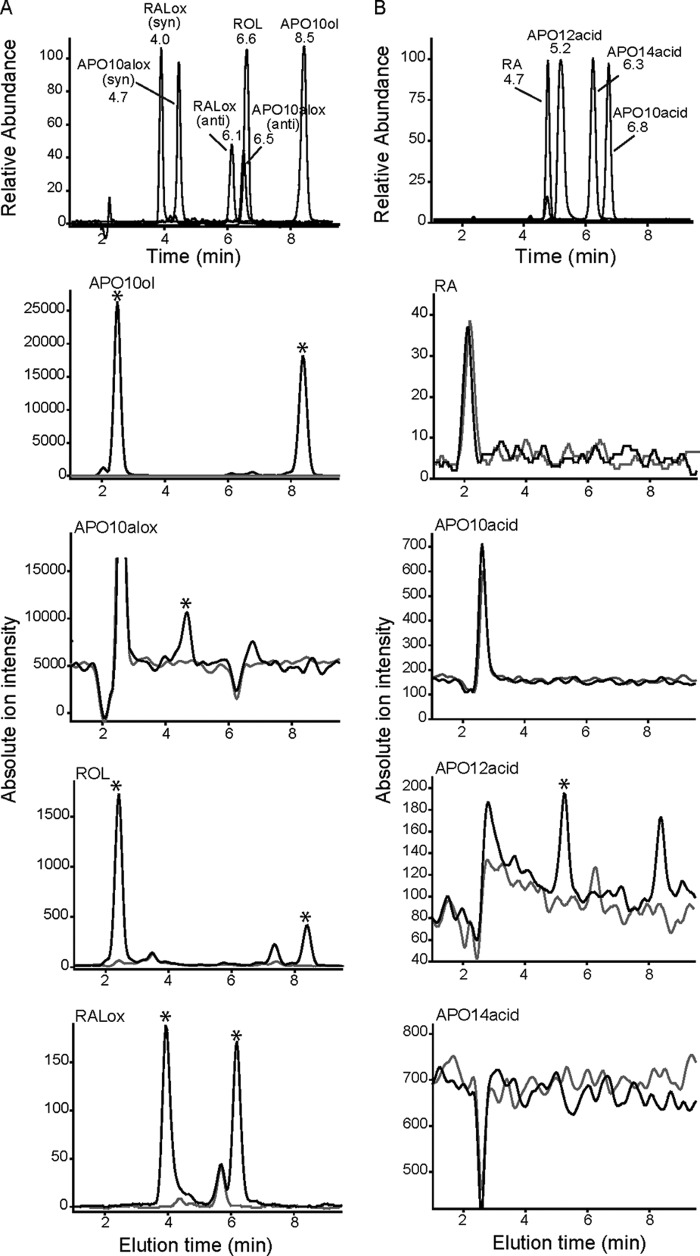
APO10ol is metabolized into retinoids by HepG2 cells. HepG2 cells were incubated with APO10ol (2 μm) (black line) or vehicle (ethanol) (gray line) for 12 h at 37 °C. Then cells were harvested, and lipophilic compounds were extracted and subjected to LC-MS analysis. A, analysis of nonpolar apocarotenoids. The presence of APO10ol, β-apo-10′-carotenal-oximes (APO10alox), ROL, and all-trans-retinal-oximes (RALox) extracted from HepG2 cells treated with APO10ol are indicated by asterisks and were identified by retention times and selected reaction monitoring modes as compared with authentic standards (upper panel). Esters of APO10ol and ROL are evident in examined samples by the presence of distinct peaks at 2.2 min of elution. B, analysis of acidic apocarotenoids. The presence of RA, β-apo-12′-carotenoic acid (APO12 acid), APO14 acid, β-apo-14′-carotenoic acid; APO10 acid, β-apo-10′-carotenoic acid extracted from HepG2 cells treated with APO10ol or vehicle are indicated by asterisks and were identified by retention times and selected reaction monitoring modes as compared with authentic standards (upper panel).
Eccentric Cleavage by BCO2 Converts β-Cryptoxanthin to Vitamin A
We showed that APO10ol produced by BCO2 can be further converted to vitamin A via a second cleavage reaction carried out by BCO1. This stepwise cleavage pathway plays a minor role in BC metabolism. However, it could prove critical for the metabolism of asymmetric carotenoids such as β-cryptoxanthin. This carotenoid possesses a nonsubstituted β-ionone, necessary for vitamin A formation, and a 3-OH-β-ionone ring. Stepwise cleavage of β-cryptoxanthin by BCO2 and BCO1 would first remove the 3-OH-ring site and yield APO10ol. The latter compound would then be converted to vitamin A by BCO1. Thus, we incubated recombinant murine BCO2 with β-cryptoxanthin and extracted lipophilic compounds for HPLC analysis. In fact, β-apo-10′-carotenal was the main cleavage product of this carotenoid, and only small amounts of 3-OH-β-apocarotenal were formed (Fig. 10A). To analyze the metabolic fate of β-cryptoxanthin in an animal model, we injected 12-week-old female WT, Bco2−/−, and Bco1−/− mice (n = 3 each) with pharmacological doses of β-cryptoxanthin. After three consecutive daily injections, we analyzed β-cryptoxanthin levels in the livers of these mice. Bco2−/− mice showed a significant hepatic accumulation of β-cryptoxanthin as compared with Bco1−/− and WT mice (Fig. 10B). This accumulation suggested that BCO2 is critically required for the metabolism of β-cryptoxanthin in mouse liver.
DISCUSSION
There has been a long lasting controversy about symmetric versus eccentric cleavage of BC in mammalian vitamin A biology (24, 45). Ganguly and co-workers (46, 47) first demonstrated that β-apocarotenoids other than retinoids can impact vitamin A-dependent processes in experimental animal models (25). These observations were later confirmed by other groups, and, upon the discovery of RARs, such compounds were implicated as agonists of these transcription factors (25). More recently, β-apocarotenoids also have been implicated as specific antagonists of these nuclear receptors (26, 27). β-Apocarotenoids exist in the diet as plant metabolites and/or oxidative breakdown products of carotenoids in food (48, 49). Additionally, eccentric oxidative enzymatic cleavage of BC has been proposed as the method to produce these compounds (23). A decade ago BCO2, an eccentric cleaving enzyme, was molecularly identified (28). However, whether this enzyme is part of a pathway for the production of β-apocarotenoids remains to be defined.
By a comprehensive study in single and double mutants for BCO1 and BCO2, we documented here the contributions of these enzymes to BC metabolism. This analysis revealed that BCO1 is the major BC-metabolizing enzyme in mice. Furthermore, it showed that BCO2 does not influence vitamin A-dependent processes. These conclusions are based on the following observations. Bco1−/− and ko/ko mice accumulated large amounts of hepatic BC, although no such accumulation took place in Bco2−/− and WT mice. Because no differences were observable between Bco1−/− and ko/ko animals, we conclude that BCO2 alone does not significantly contribute to BC homeostasis in mice. Nevertheless, small amounts of APO10ol were produced in Bco1−/− mice. Upon saponification, hepatic APO10ol content increased in Bco1−/− mice, and trace amounts of APO10ol also became detectable in WT mice. The lack of APO10ol in Bco2−/− and ko/ko mice clearly demonstrated that this production was BCO2-dependent. We did not detect other apocarotenoids in the liver of these mice, suggesting that BCO2 displays high regional selectivity for the C9,C10 double bond of BC. Although minor amounts of APO10ol were produced, our analysis failed to provide evidence that this production impacted vitamin A homeostasis and retinoid signaling. Bco1−/− and ko/ko mice showed similar reductions of retinoids and expression levels of key components of vitamin A metabolism, although the single knock-out mice expressed BCO2 and produced APO10ol. This finding also agrees with the results of previous studies in Bco1−/− mice. BC supplementation did not influence global transcriptional activity of white adipose tissue in Bco1−/− mice as compared with siblings maintained on the same diet without BC (35). When compared with nonsupplemented Bco1−/− mouse siblings, BC supplementation also had no gross effect on global gene expression profiles in other tissues, including the liver and the lungs (50). Together, these findings make it improbable that BCO2 is a component of a pathway for the production of β-apocarotenoid signaling molecules that agonize and/or antagonize the activity of nuclear receptors such as RARs.
Several studies showed that β-apocarotenoids can interact with proteins that bind vitamin A derivatives, including nuclear receptors (24). This finding is not surprising when it is considered that these compounds are chemically and structurally related to vitamin A. In fact, we also observed that APO10ol can interact with molecular components of vitamin A metabolism. APO10ol was esterified by LRAT, bound by the vitamin A carrier RBP, and taken up by target cells from its RBP-bound form by STRA6. Therefore, high concentrations of APO10ol presumably should compete with vitamin A for the same metabolic pathways. However, our study indicated instead that such ambiguity and competition between BC metabolites is limited in mammals. One possible explanation involves the substrate specificity of BCO2. In Bco1−/− mice, BC existed in 1000-fold excess over APO10ol, indicating that BC is a poor substrate for BCO2 in vivo. This conclusion is further corroborated by enzymatic assays with the asymmetric carotenoid, β-cryptoxanthin, that contains a 3-OH and a nonhydroxylated β-ionone ring site. Our results showed that BCO2 preferentially removed the 3-OH-β-ionone ring site from this carotenoid. This preference for hydroxylated ring sites of carotenoids also has been reported for BCO2 from ferrets (30). Moreover, structural analyses and enzymatic properties of carotenoid oxygenases indicate that these enzymes specifically interact with one ionone ring side of their carotenoid substrates (51–53). This specificity of BCO2 for carotenoids with 3-OH-β-ionone ring sites is also indicated by β-cryptoxanthin accumulation in BCO2-deficient mice as we found here and by accumulation of zeaxanthin and lutein as we demonstrated previously in this mouse mutant (29). The preference of BCO2 for 3-hydroxyionone rings also explains why no hydroxylated apocarotenoids accumulate in mice. These compounds are further converted by BCO2 (29, 30). In contrast, the accumulation of nonhydroxylated apocarotenoids such as APO10ol and probably also other long chain β-apocarotenoids in mice is prevented by another mechanism. As we demonstrated here, not only APO10ol but also β-8′- and β-12′-apocarotenoids were readily converted by recombinant human BCO1 into retinoids. This conversion was also the major metabolic fate of APO10ol in human HepG2 cells. Such BCO1 action prevents the accumulation of long chain β-apocarotenoids in blood and tissues that may interact with components of vitamin A metabolic pathways. Simultaneously, this BCO1 activity ensures that even long chain β-apocarotenoids can be utilized for vitamin A production, thereby providing an explanation for the observed vitamin A activity of these compounds (24). Physiologically, this pathway may play a role after consumption of plants rich in β-apocarotenoids such as melons (48). Additionally, it also might be important for the tailoring of asymmetric carotenoids such as β-cryptoxanthin for vitamin A production. In this last pathway, the 3-OH-β-ionone ring site is removed by BCO2, and the resulting APO10ol is then further metabolized by BCO1. We propose that this stepwise cleavage also is the method to convert other asymmetric carotenoids with provitamin A activity into retinoids (Fig. 11).
FIGURE 11.
Proposed scheme for the metabolism of provitamin A carotenoids. β-Carotene is converted by BCO1 in the cytoplasm. β-Cryptoxanthin and other asymmetric carotenoids are transported to mitochondria. In mitochondria, BCO2 converts β-cryptoxanthin by oxidative cleavage at the C9,C10 double bond yielding β-apo-10′-carotenal and 3-hydroxy-β-ionone. β-Apo-10′-carotenal is reduced to the corresponding alcohol and transported to the cytoplasm by yet to be identified proteins as indicated by the question marks. β-Apo-10′-carotenol can then be esterified by LRAT and/or converted by BCO1 by oxidative cleavage to all-trans-retinal.
Aside from different substrate preferences, the two carotenoid oxygenases also display a different cellular localization. BCO1 localizes to cytoplasm, whereas BCO2 is a mitochondrial protein. This different localization suggests that carotenoid metabolism is compartmentalized (Fig. 11). In this model, BC is metabolized in the cytoplasm by BCO1, whereas other carotenoids are metabolized by BCDO2 in mitochondria. This compartmentalization elegantly prevents competition of the two carotenoid oxygenases for BC, which can be a limited nutrient in natural environments. However, BCO2 might also contribute to BC catabolism when it is provided in excessive doses. As we previously showed in cell lines, BCO2 protects against carotenoid-induced mitochondrial damage (29, 54). For BC, it remains to be investigated whether this pathway plays a role in the in vivo situation and whether it involves subsequent action of both BCOs. The compartmentalization of carotenoid metabolism as well as the shuttling of carotenoids and their apocarotenoid derivatives between different cellular compartments are the focus of future investigations of our laboratories.
Acknowledgments
We thank Dr. Hansgeorg Ernst (BASF, Germany) for the gift of apocarotenoid standards and Dr. Leslie Webster for critical comments on the manuscript. We thank Darwin Babino for BCO1 enzyme production. We also thank Maryanne Pendergast and the Neurosciences Imaging Center for assistance with confocal microscopy.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1EY019641 and RO1EY020551 from the NEI.
This article was selected as a Paper of the Week.
- ROL
- all-trans-retinol
- APO10ol
- β-apo-10′-carotenol
- BC
- β-carotene
- BCO1
- β-carotene-15,15′-monooxygenase 1
- BCO2
- β-carotene-9′,10′-dioxygenase
- ko/ko
- Bco1−/−; Bco2−/− double mutant mice
- RA
- all-trans-retinoic acid
- RBP
- retinol-binding protein
- hRBP
- human RBP
- RE
- retinyl ester
- RPE
- retinal pigment epithelium
- LRAT
- lecithin:retinol acyltransferase
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- Bistris propane
- 1,3-bis[tris(hydroxymethyl)methylamino]propane
- RAR
- retinoic acid receptor
- VAD
- vitamin A-deficient
- VAS
- vitamin A-sufficient
- TLCK
- tosyl-lysine chloromethyl ketone
- qRT
- quantitative RT.
REFERENCES
- 1. Hall J. A., Grainger J. R., Spencer S. P., Belkaid Y. (2011) The role of retinoic acid in tolerance and immunity. Immunity 35, 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. von Lintig J., Kiser P. D., Golczak M., Palczewski K. (2010) The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem. Sci. 35, 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rhinn M., Dollé P. (2012) Retinoic acid signalling during development. Development 139, 843–858 [DOI] [PubMed] [Google Scholar]
- 4. Noy N. (2010) Between death and survival: retinoic acid in regulation of apoptosis. Annu. Rev. Nutr. 30, 201–217 [DOI] [PubMed] [Google Scholar]
- 5. Chambon P. (1996) A decade of molecular biology of retinoic acid receptors. FASEB J. 10, 940–954 [PubMed] [Google Scholar]
- 6. Palczewski K. (2006) G protein-coupled receptor rhodopsin. Annu. Rev. Biochem. 75, 743–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sommer A., Vyas K. S. (2012) A global clinical view on vitamin A and carotenoids. Am. J. Clin. Nutr. 96, 1204S–1206S [DOI] [PubMed] [Google Scholar]
- 8. Grune T., Lietz G., Palou A., Ross A. C., Stahl W., Tang G., Thurnham D., Yin S. A., Biesalski H. K. (2010) β-Carotene is an important vitamin A source for humans. J. Nutr. 140, 2268S-2285S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. von Lintig J. (2010) Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu. Rev. Nutr. 30, 35–56 [DOI] [PubMed] [Google Scholar]
- 10. Lietz G., Lange J., Rimbach G. (2010) Molecular and dietary regulation of β,β-carotene 15,15′-monooxygenase 1 (BCMO1). Arch. Biochem. Biophys. 502, 8–16 [DOI] [PubMed] [Google Scholar]
- 11. Olson J. A., Hayaishi O. (1965) The enzymatic cleavage of β-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc. Natl. Acad. Sci. U.S.A. 54, 1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodman D. S., Huang H. S. (1965) Biosynthesis of vitamin A with rat intestinal enzymes. Science 149, 879–880 [DOI] [PubMed] [Google Scholar]
- 13. von Lintig J., Vogt K. (2000) Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving β-carotene to retinal. J. Biol. Chem. 275, 11915–11920 [DOI] [PubMed] [Google Scholar]
- 14. Wyss A., Wirtz G., Woggon W., Brugger R., Wyss M., Friedlein A., Bachmann H., Hunziker W. (2000) Cloning and expression of β,β-carotene 15,15′-dioxygenase. Biochem. Biophys. Res. Commun. 271, 334–336 [DOI] [PubMed] [Google Scholar]
- 15. Redmond T. M., Gentleman S., Duncan T., Yu S., Wiggert B., Gantt E., Cunningham F. X., Jr. (2001) Identification, expression, and substrate specificity of a mammalian β-carotene 15,15′-dioxygenase. J. Biol. Chem. 276, 6560–6565 [DOI] [PubMed] [Google Scholar]
- 16. Paik J., During A., Harrison E. H., Mendelsohn C. L., Lai K., Blaner W. S. (2001) Expression and characterization of a murine enzyme able to cleave β-carotene. The formation of retinoids. J. Biol. Chem. 276, 32160–32168 [DOI] [PubMed] [Google Scholar]
- 17. Lindqvist A., Andersson S. (2002) Biochemical properties of purified recombinant human β-carotene 15,15′-monooxygenase. J. Biol. Chem. 277, 23942–23948 [DOI] [PubMed] [Google Scholar]
- 18. O'Byrne S. M., Wongsiriroj N., Libien J., Vogel S., Goldberg I. J., Baehr W., Palczewski K., Blaner W. S. (2005) Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT). J. Biol. Chem. 280, 35647–35657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. D'Ambrosio D. N., Clugston R. D., Blaner W. S. (2011) Vitamin A metabolism: an update. Nutrients. 3, 63–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quadro L., Blaner W. S., Salchow D. J., Vogel S., Piantedosi R., Gouras P., Freeman S., Cosma M. P., Colantuoni V., Gottesman M. E. (1999) Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO J. 18, 4633–4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawaguchi R., Yu J., Honda J., Hu J., Whitelegge J., Ping P., Wiita P., Bok D., Sun H. (2007) A membrane receptor for retinol-binding protein mediates cellular uptake of vitamin A. Science 315, 820–825 [DOI] [PubMed] [Google Scholar]
- 22. Wang X. D., Tang G. W., Fox J. G., Krinsky N. I., Russell R. M. (1991) Enzymatic conversion of β-carotene into β-apo-carotenals and retinoids by human, monkey, ferret, and rat tissues. Arch. Biochem. Biophys. 285, 8–16 [DOI] [PubMed] [Google Scholar]
- 23. Tang G. W., Wang X. D., Russell R. M., Krinsky N. I. (1991) Characterization of β-apo-13-carotenone and β-apo-14′-carotenal as enzymatic products of the excentric cleavage of β-carotene. Biochemistry 30, 9829–9834β [DOI] [PubMed] [Google Scholar]
- 24. Eroglu A., Harrison E. H. (2013) Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids. J. Lipid Res. 54, 1719–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X. D., Russell R. M., Liu C., Stickel F., Smith D. E., Krinsky N. I. (1996) β-Oxidation in rabbit liver in vitro and in the perfused ferret liver contributes to retinoic acid biosynthesis from β-apocarotenoic acids. J. Biol. Chem. 271, 26490–26498 [PubMed] [Google Scholar]
- 26. Eroglu A., Hruszkewycz D. P., dela Sena C., Narayanasamy S., Riedl K. M., Kopec R. E., Schwartz S. J., Curley R. W., Jr., Harrison E. H. (2012) Naturally occurring eccentric cleavage products of provitamin A β-carotene function as antagonists of retinoic acid receptors. J. Biol. Chem. 287, 15886–15895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eroglu A., Hruszkewycz D. P., Curley R. W., Jr., Harrison E. H. (2010) The eccentric cleavage product of β-carotene, β-apo-13-carotenone, functions as an antagonist of RXRα. Arch. Biochem. Biophys. 504, 11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kiefer C., Hessel S., Lampert J. M., Vogt K., Lederer M. O., Breithaupt D. E., von Lintig J. (2001) Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J. Biol. Chem. 276, 14110–14116 [DOI] [PubMed] [Google Scholar]
- 29. Amengual J., Lobo G. P., Golczak M., Li H. N., Klimova T., Hoppel C. L., Wyss A., Palczewski K., von Lintig J. (2011) A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 25, 948–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mein J. R., Dolnikowski G. G., Ernst H., Russell R. M., Wang X. D. (2011) Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin, and β-cryptoxanthin by ferret carotene-9′,10′-monooxygenase. Arch. Biochem. Biophys. 506, 109–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu K. Q., Liu C., Ernst H., Krinsky N. I., Russell R. M., Wang X. D. (2006) The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J. Biol. Chem. 281, 19327–19338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hessel S., Eichinger A., Isken A., Amengual J., Hunzelmann S., Hoeller U., Elste V., Hunziker W., Goralczyk R., Oberhauser V., von Lintig J., Wyss A. (2007) CMO1 deficiency abolishes vitamin A production from β-carotene and alters lipid metabolism in mice. J. Biol. Chem. 282, 33553–33561 [DOI] [PubMed] [Google Scholar]
- 33. Batten M. L., Imanishi Y., Maeda T., Tu D. C., Moise A. R., Bronson D., Possin D., Van Gelder R. N., Baehr W., Palczewski K. (2004) Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J. Biol. Chem. 279, 10422–10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shmarakov I., Fleshman M. K., D'Ambrosio D. N., Piantedosi R., Riedl K. M., Schwartz S. J., Curley R. W., Jr., von Lintig J., Rubin L. P., Harrison E. H., Blaner W. S. (2010) Hepatic stellate cells are an important cellular site for β-carotene conversion to retinoid. Arch. Biochem. Biophys. 504, 3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amengual J., Gouranton E., van Helden Y. G., Hessel S., Ribot J., Kramer E., Kiec-Wilk B., Razny U., Lietz G., Wyss A., Dembinska-Kiec A., Palou A., Keijer J., Landrier J. F., Bonet M. L., von Lintig J. (2011) β-Carotene reduces body adiposity of mice via BCMO1. PLoS One 6, e20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kane M. A., Folias A. E., Wang C., Napoli J. L. (2008) Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal. Chem. 80, 1702–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Isken A., Golczak M., Oberhauser V., Hunzelmann S., Driever W., Imanishi Y., Palczewski K., von Lintig J. (2008) RBP4 disrupts vitamin A uptake homeostasis in a STRA6-deficient animal model for Matthew-Wood syndrome. Cell Metab. 7, 258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Golczak M., Maeda A., Bereta G., Maeda T., Kiser P. D., Hunzelmann S., von Lintig J., Blaner W. S., Palczewski K. (2008) Metabolic basis of visual cycle inhibition by retinoid and nonretinoid compounds in the vertebrate retina. J. Biol. Chem. 283, 9543–9554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kowatz T., Babino D., Kiser P., Palczewski K., von Lintig J. (2013) Characterization of human β,β-carotene-15,15′-monooxygenase (BCMO1) as a soluble monomeric enzyme. Arch. Biochem. Biophys. 10.1016/j.abb.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Golczak M., Palczewski K. (2010) An acyl-covalent enzyme intermediate of lecithin:retinol acyltransferase. J. Biol. Chem. 285, 29217–29222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Griscavage J. M., Wilk S., Ignarro L. J. (1995) Serine and cysteine proteinase inhibitors prevent nitric oxide production by activated macrophages by interfering with transcription of the inducible NO synthase gene. Biochem. Biophys. Res. Commun. 215, 721–729 [DOI] [PubMed] [Google Scholar]
- 42. Golczak M., Kiser P. D., Sears A. E., Lodowski D. T., Blaner W. S., Palczewski K. (2012) Structural basis for the acyltransferase activity of lecithin:retinol acyltransferase-like proteins. J. Biol. Chem. 287, 23790–23807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amengual J., Golczak M., Palczewski K., von Lintig J. (2012) Lecithin:retinol acyltransferase is critical for cellular uptake of vitamin A from serum retinol-binding protein. J. Biol. Chem. 287, 24216–24227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu L., Tang X. H., Gudas L. J. (2008) Homeostasis of retinol in lecithin:retinol acyltransferase gene knockout mice fed a high retinol diet. Biochem. Pharmacol. 75, 2316–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wolf G. (1995) The enzymatic cleavage of β-carotene: still controversial. Nutr. Rev. 53, 134–137 [DOI] [PubMed] [Google Scholar]
- 46. Sharma R. V., Mathur S. N., Dmitrovskii A. A., Das R. C., Ganguly J. (1976) Studies on the metabolism of β-carotene and apo-β-carotenoids in rats and chickens. Biochim. Biophys. Acta 486, 183–194 [DOI] [PubMed] [Google Scholar]
- 47. Sharma R. V., Mathur S. N., Ganguly J. (1976) Studies on the relative biopotencies and intestinal absorption of different apo-β-carotenoids in rats and chickens. Biochem. J. 158, 377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fleshman M. K., Lester G. E., Riedl K. M., Kopec R. E., Narayanasamy S., Curley R. W., Jr., Schwartz S. J., Harrison E. H. (2011) Carotene and novel apocarotenoid concentrations in orange-fleshed Cucumis melo melons: determinations of β-carotene bioaccessibility and bioavailability. J. Agric. Food Chem. 59, 4448–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Handelman G. J., van Kuijk F. J., Chatterjee A., Krinsky N. I. (1991) Characterization of products formed during the autoxidation of β-carotene. Free Radic. Biol. Med. 10, 427–437 [DOI] [PubMed] [Google Scholar]
- 50. van Helden Y. G., Heil S. G., van Schooten F. J., Kramer E., Hessel S., Amengual J., Ribot J., Teerds K., Wyss A., Lietz G., Bonet M. L., von Lintig J., Godschalk R. W., Keijer J. (2010) Knockout of the Bcmo1 gene results in an inflammatory response in female lung, which is suppressed by dietary β-carotene. Cell. Mol. Life Sci. 67, 2039–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Messing S. A., Gabelli S. B., Echeverria I., Vogel J. T., Guan J. C., Tan B. C., Klee H. J., McCarty D. R., Amzel L. M. (2010) Structural insights into maize viviparous14, a key enzyme in the biosynthesis of the phytohormone abscisic acid. Plant Cell 22, 2970–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schwartz S. H., Tan B. C., Gage D. A., Zeevaart J. A., McCarty D. R. (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276, 1872–1874 [DOI] [PubMed] [Google Scholar]
- 53. Oberhauser V., Voolstra O., Bangert A., von Lintig J., Vogt K. (2008) NinaB combines carotenoid oxygenase and retinoid isomerase activity in a single polypeptide. Proc. Natl. Acad. Sci. U.S.A. 105, 19000–19005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lobo G. P., Isken A., Hoff S., Babino D., von Lintig J. (2012) BCDO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway. Development 139, 2966–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]