Abstract
Stress urinary incontinence (SUI), the most common type of incontinence in women, is a frequent and costly ailment responsible for an alteration in the quality of life. Although medical treatment gives some rather deceiving results, surgical techniques that include colposuspension or tension-free vaginal tape, employed in cases of urethral support defect, give a 5-year cure rate of more than 80%. However, these techniques could lead to complications or recurrence of symptoms. Recently, the initiation of urethral cell therapy has been undertaken by doctors and researchers. One principal source of autologous adult stem cells is generally used: muscle precursor cells (MPCs) which are the progenitors of skeletal muscle cells. Recently, a few research groups have shown interest in the MPCs and their potential for the treatment of urinary incontinence. However, using MPCs or fibroblasts isolated from a striated muscle biopsy could be questionable on several points. One of them is the in vitro cultivation of cells, which raises issues over the potential cost of the technique. Besides, numerous studies have shown the multipotent or even the pluripotent nature of stromal vascular fraction (SVF) or adipose-derived stem cells (ASCs) from adipose tissue. These cells are capable of acquiring in vitro many different phenotypes. Furthermore, recent animal studies have highlighted the potential interest of SVF cells or ASCs in cell therapy, in particular for mesodermal tissue repair and revascularization. Moreover, the potential interest of SVF cells or ASCs for the treatment of urinary incontinence in women is supported by many other characteristics of these cells that are discussed here. Because access to these cells via lipoaspiration is simple, and because they are found in very large numbers in adipose tissue, their future potential as a stem cell reservoir for use in urethral or other types of cell therapy is enormous.
Keywords: stress urinary incontinence, stem cells, adipose tissue
Introduction
Urinary incontinence is a frequent and costly ailment responsible for an alteration in the quality of life, as well as being a handicap [1]. In Europe 35% of women are affected [2]. After 65 years of age, its impact on quality of life exceeds that of other chronic pathologies such as arterial hypertension, angina pectoris or diabetes [3]. Its direct cost exceeds even that of breast cancer in the United States [4]. Stress urinary incontinence (SUI) is the most common type of incontinence in women (more than 80%) [5]. Its two principal causes are abnormal urethral mobility, because of a support defect, and a sphincteric defect resulting in sphincteric insufficiency.
Results obtained with medical treatment are rather deceiving [6, 7]. However, perineal re-education, for example, improves incontinence by reducing the number of leaks and thus improves quality of life [8]. However, despite an initial cure rate of 60%, improvements gained from re-education disappear after a number of years [9]. Injections of inert material (glutaraldehyde cross-linked collagen, silicone, carbon-coated zirconium beads or hyaluronic acid/dextran copolymer) give mediocre results with less than a 50% success rate at the cost of some minor morbidity [10–12]. Surgical techniques that include colposuspension or tension-free vaginal tape, employed in cases of urethral support defect give a 5-year cure rate of more than 80%[13, 14]. However, these surgical techniques are not exempt from complications or recurrence of symptoms [15]. In fact, sphincteric insufficiency in women is a significant factor in the failure of surgical treatment for SUI [16–18]. Thus, the treatment of persistent or recurrent SUI, in spite of good surgical practice, remains problematic and can, in certain cases, necessitate the insertion of an artificial sphincter.
The object of our work is to review the pathophysiology of SUI in women, as well as to investigate the perspectives offered by the use of stem cells in the treatment of this pathology.
The sphincteric complex
The urethral mucosa is surrounded by a sub-mucosa composed of longitudinal collagen and elastin fibres, and occupied by a vascular plexus. Surrounding this, there are longitudinal smooth muscle fibres, then circular smooth muscle fibres, all of which are surrounded by the striated sphincter that is composed of circular fibres to which are attached the muscles of the urogenital diaphragm [19–22]. The sphincter is attached to the pubis by the pubo-urethral ligaments and supported by the anterior vaginal fascia, attached to the tendinous arch of the pelvic fascia and the levator ani. Resting continence is assured by the circular smooth muscle component, the slow fibres of the striated urethral sphincter, and by the sub-mucosal vascular plexus [16–23]. During effort, there is passive transmission of intra-abdominal pressure linked to urethral support, as well as active reflex contraction of the urethral sphincter and the pelvic floor [24–27]. Continence, therefore, requires that there is maintenance of urethral support, mucosal, submucosal and muscular trophicity, as well as neurological control (Fig. 1).
Fig 1.
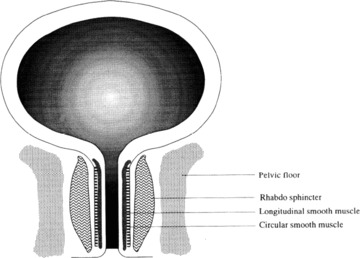
Schematic representation of the peri- and intra-urethral structure in the human female (from [22]).
Deterioration of the urethral sphincter
The urodynamic measure of urethral closure pressure is used to assess the quality of the urethral sphincter at rest. Urethral closure pressure decreases with age and has been directly linked to the rarefaction of striated urethral muscle cells that occurs with ageing [28, 29]. There also exists an inverse correlation between the urethral vascular index, measured by Doppler ultrasound, the urethral closure pressure and age [30]. Thus it would appear that urethral vascularization is less extensive during urinary incontinence [31]. Compared to continent women, sphincteric volume measured by ultrasonography is also weaker in women who suffer with SUI, particularly when sphincteric insufficiency is also present [32, 33].
Biochemical studies show that there is a modification in the metabolism of collagen and elastin fibres in the peri-urethral tissue of incontinent women [34–40]. SUI in women is also accompanied by signs of denervation of the urethral sphincter [41].
Taking all of this into consideration, the ideal treatment for SUI in women would consist, at the urethral level, of:
-
1
Increasing the number of smooth and striated muscle cells,
-
2
Increasing vascularization,
-
3
Increasing the secretion of collagen and elastin in peripheral tissue AND
-
4
Increasing neurological innervations.
In recent years, the medical profession has moved in a new therapeutic direction, which could satisfy all of the above criteria, through the use of autologous adult stem cells.
Stem cells and their use in the treatment of urinary incontinence
Autologous adult stem cells are increasingly used for the treatment of a number of pathologies. This trend began several years ago with the use of bone marrow stem cells for haematopoietic regeneration following chemotherapy, and is continuing with a large number of clinical trials currently underway for the treatment of various cancers (http://www.clinicaltrials.gov). These cells are also being used in several clinical trials, which include the regeneration of cardiac muscle, lower limb revascularization, as well as many other examples (http://www.clinicaltrials.gov).
More recently, stem cells from striated muscle have been used primarily for the regeneration of cardiac muscle, but with mitigated results [42, 43] and a few research groups have shown interest in the muscle precursor cells (MPCs) and their potential for the treatment of urinary incontinence.
One of the first studies was carried out by Yokoyama, et al. in 2001, in which injections of collagen were compared to those of autologous rat MPCs that had been cultured beforehand in order to amplify the number of cells. Results showed that collagen almost completely disappeared at the end of a month, even though the MPC cells were still present [44]. In 2002, Yiou, et al. confirmed these positive MPC results, by their use in mice to repair the urethral sphincter muscle [45]. The injection of non amplified cells made this an original procedure. Indeed, the cells were extracted from striated muscle, harvested from the legs of mice, labelled and then re-injected directly into the urethral sphincter, which had undergone prior treatment with a myotoxic substance. In 2003, Chancellor continued these studies in a rat model, via the use of an allogenic graft of progenitor muscle cells in which the urethra had been denervated, thus leading to its atrophy [46]. The study demonstrated that MPC cells were capable of restoring muscular contraction 2 weeks after injection into the urethra. Given that the denervation model does not reflect the real clinical picture, a year later the same team demonstrated in a rat model of urethral sphincter insufficiency, this time provoked by cauterization, that the same injected cells brought about an improvement in the sphincter leak pressure. Moreover, two different labelling techniques allowed the identification of cells injected into urethral striated muscle 2 months after injection [47]. The same year, Peyromaure, et al. demonstrated in rats, that 90 days after injection, MPCs can be identified in the striated muscle layer of the sphincter, but not in the smooth muscle layer [48]. More recently, a study was carried out, again in rats, to demonstrate the distinct effects of the injection of MPCs, fibroblasts, or both types of cells together [49]. This work clearly demonstrated that the injection of MPCs improves the leak pressure as well as the amplitude of sphincter contraction. Conversely, a single injection of fibroblasts only improved the leak point pressure. Furthermore, the injection of a large quantity of fibroblasts alone (1 × 107 cells) caused obstruction of the sphincter, leading to urinary retention.
The first clinical study in this field was carried out by Strasser, et al. in 2004 [50], followed by publication of the results recently in a famous international review [51]. Of 63 women with urinary incontinence, 42 of them received an ultrasound guided injection of a mixture of fibroblasts and autologous myoblasts, whereas 21 of them received a collagen injection. After being followed for a year, no side effects were reported in either of the two groups. The thickness of the rhabdo-sphincter was greatly increased in women who had received the injection of cells, compared to the group that received the collagen injection (a difference of more than 1 mm). The same thing applied when measuring the contractility of the sphincter. Moreover, at the end of a year, 38 of the 42 woman that received the injection of cells were considered to be continent.
However, following serious breach of ethical rules, as well as illegal conduction of the clinical trials, the Strasser’s publication has been retracted by the Lancet editor [52]. It is now difficult not to question the entire results of the group concerning this particular protocol.
Nevertheless, a recently published clinical trial from Chancellor, et al. seems to confirm Strasser’s results, proving that MPCs are efficient for the treatment of SUI [53].
Anyway, independent of the results coming from Strasser or Chancellor, the opportunity of using MPCs or fibroblasts isolated from a striated muscle biopsy is probably suboptimal for a number of other reasons.
On the one hand, the collection of cells generally requires a large biopsy of skeletal muscle (between 5 and 30 g), an additional operation, and leaving the patient with a fairly significant scar. On the other hand, the use of these cells only partly satisfies the criteria stated earlier for the use of autologous adult stem cells. In fact, if these cells are indeed capable of increasing the quantity of striated muscle as well as the secretion of collagen (from fibroblasts) at the urethral level, it would appear that they might have difficulty in also restoring the activity of longitudinal and circular smooth muscle fibres, in creating a satisfactory level of revascularization or in improving neurological innervation.
Finally, and this is without doubt the crucial point of the problem, the small quantity of stem cells harvested does not allow immediate re-injection of these cells. They require cultivation in vitro, which, apart from the practical aspects and the absolute necessity for sterility, raises issues over the potential cost of the technique. Moreover, even if, in his studies, Strasser used these stem cells with autologous serum for cultivation, the passage number of the cells has not been clearly stated, although this is certainly significantly high because the cells are sometimes cultured for up to 8 weeks! In the same way, Chancellor’s team recommend about 10 days of culture, with five to six serial platings, to reach a sufficient amount of muscle cells for re-injection [53]. This then raises the question as to the potential changes that could appear within these cellular populations, as well as the safety implications of their injection, particularly, for example, in relation to the teratogenic risks [54].
It would therefore seem no more risky given our actual level of understanding of stem cells to carry out extemporaneous autologous injections, with cells being harvested and re-injected during the same operation.
Stem cells from human adipose tissue
White fat is the most important energy reserve in the body. This energy is stored as triglycerides by white adipocytes.
White fat adipocytes (the most predominant type of fat in adult human beings) are spherical cells, with a diameter of around 100 μm, but which can be sometimes more than 200 μm. Their cytoplasm encloses a bulky, single lipid vacuole (triglycerides), surrounded by a thin cytoplasmic ring containing a Golgi apparatus, rough endoplasmic reticulum, smooth endoplasmic reticulum, mitochondria and a flattened nucleus at the periphery of the cell. White adipocytes exist as isolated cells within loose conjunctive tissue and bone marrow or as a collection of cells grouped together to form white adipose tissue.
White adipose tissue represents around 15% to 20% of the weight of non-obese adults and up to 50% in obese individuals. In this tissue the adipocytes packed one against the other take on a polyhedral form. They are separated by reticulin fibres and an extensive capillary network as well as by amyelinic, sympathetic noradrenergic nerve fibres. Adipocytes are grouped in small lobules, visible to the naked eye, separated by fine conjunctive partitions containing fibroblasts, macrophages, mastocytes and collagen fibrils.
Adipose tissue contains only 40% to 60% mature adipocytes, but is also composed of the stromal vascular fraction (SVF). This consists of fibroblasts, macrophages, mastocytes, endothelial cells, haematopoietic cells and preadipocytes. This last cell type is considered to be the precursor of mature adipocytes. Once cultured, these cells are called adipose-derived stem cells (ASCs).
Numerous studies have shown the multipotent nature (capable of differentiating into cells of the same germ layer), or even the pluripotent nature (capable of differentiating into cells of another germ layer) of SVF or ASCs cells, capable of acquiring in vitro, muscular, osseous, chondrocyte, neuronal, epithelial, macrophagic, smooth muscle and even hepatic phenotypes [55–62]. Moreover, a recent study showed that the SVF is not made up of unipotent progenitors, but instead is composed of multipotent cells, containing adipogenic, osteogenic, chondrogenic and neurogenic phenotypes [63]. Furthermore, recent animal studies have highlighted the potential interest of SVF or ASCs cells in cell therapy, in particular for mesodermal tissue repair and revascularization [64–67].
The potential interest of SVF cells for the treatment of urinary incontinence in women is further supported by many other characteristics of these cells that are discussed below.
Firstly, cells from the SVF contain a large number of fibroblasts capable of secreting collagen [68]. Moreover, the capacity of ASCs cells to secrete and to organize an endogenous extracellular matrix with some quite basic stimulation has already been highlighted [69]. Lastly, in the absence of adipogenic factors, these cells do not differentiate into adipocytes and possess quite a strong potential for making conjunctive tissue [69].
On the other hand, several studies have clearly shown that mesenchymatous cells from adipose tissue have the potential to differentiate into smooth or striated muscle cells.
As far as smooth muscle is concerned, a very convincing study by Rodriguez, et al. showed that in addition to the expression of classic markers of smooth muscle cells (smooth muscle cell-specific α actin, smoothelin, calponin, caldesmon, MHC and SM22), ASCs are able to produce functional cells, capable of contracting under stimulation with carbachol, followed by relaxation of the cells after around 10 min. [59]. A study in mice had already demonstrated the existence of this potential [70].
As far as striated muscle is concerned, several studies have reiterated the potential of ASCs. They can express specific striated muscle markers (desmin, myod1, myogenin, myogenic regulatory factor 6 and myosin heavy chain) and form multinucleated cells characteristic of myotubules [58, 62]. Moreover, in rabbit, the injection of cells from adipose tissue into damaged muscle enables the restoration of volume as well as the force of muscular contraction, in a way that is as efficient as when muscle satellite cells are injected [55].
This last point could be considered as being highly significant as far as the use of these cells in the treatment of urinary incontinence is concerned. Furthermore, it should be noted that, in our experimental procedure of isolation, at least 70% to 90% of SVF cells are positive for the myoblastic marker, CD56 (Fig. 2), and 60% to 80% of these cells are CD34+ (Fig. 2). These percentages represent probably an important argument that could accentuate the potentiality of these cells for the urethral repair.
Fig 2.
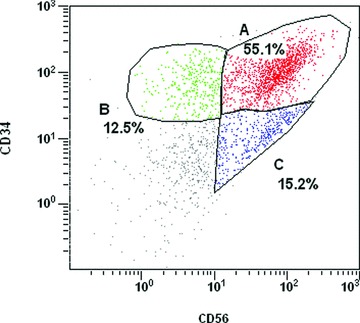
Flow cytometric analysis of SVF from subcutaneous human adipose tissue. Cells from a representative individual donor were stained with monoclonal antibodies directed against CD56 coupled to FITC or against CD34 (Beckman Coulter, QBEnd10) coupled to phycoerythrin. Experiments have been conducted on four different samples from four different patients which have produced the following ranges of percentages: 70–90% (CD56+), 40–70% (CD56+/CD34+). SVF cells were obtained by digestion of liposucted tissue samples, for 30 min. at 37°C in Ringer-Lactate buffer containing 1.5 mg/ml collagenase (NB5, SERVA, Heidelberg, Germany, PZ activity 0.175 U/mg). Digested tissue was centrifuged at 3000 rpm for 3 min. The cell pellet harvested after centrifugation was resuspended and incubated twice for 10 min. in blood lysis buffer to eliminate red blood cells. Cells were then centrifuged at 3000 rpm for 3 min. and the pellet was resuspended in ringer lactate and filtered through Steriflip 100 > m (Millipore, Molsheim, France). After centrifugation at 3000 rpm for 3 min., cells were resuspended in Ringer lactate buffer.
In addition, certain adipose tissue cells possess angiogenic potential. This is supported primarily by the existence of an important CD34+ population (between 40% and 70%) within the SVF [64, 71]; this marker being present on stem cells, but also on endothelial cells and their precursors. This is largely confirmed by more recent studies that highlight certain endothelial phenotypic characteristics of cells from the SVF. Results from our laboratory, presented here, demonstrated that a significant proportion of CD34+ cells express the endothelial marker, CD31 (from our experiments, 65% to 90%, Fig. 3). It should be noted that these proportions are not found by all groups working on the subject, without doubt a result of the cellular extraction protocol and of the enzyme used. Moreover, the intravenous injection of mice with CD34+, CD31− cells, 24 hrs after ligature of the femoral artery (induced ischemia), led to an increase in blood flux and capillary density in these mice compared to the controls [64]. Identical results were obtained by other groups in different animal models [65–67, 72]. As we could find 10% to 30% of CD31− cells in the CD34+ population (Fig. 3), it is highly probable that the injection of these cells will lead to a increase of the vascularization in the injected area.
Fig 3.
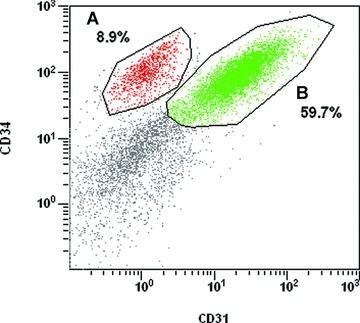
Flow cytometric analysis of SVF from subcutaneous human adipose tissue. Cells from a representative individual donor were stained with monoclonal antibodies directed against CD31 coupled to FITC or against CD34 (Beckman Coulter, QBEnd10) coupled to phycoerythrin. Experiments have been conducted on four different samples from four different patients which have produced the following ranges of percentages: 60–80% (CD34+), 40–60% (CD31+/ CD34+), 10–30% (CD34+/CD31–). SVF cells were obtained by digestion of liposucted tissue samples, as described in Fig. 2.
Finally, some studies also show that ASCs could differentiate into neurons. Since 2002, Safford, et al. has shown that cells from mice could express markers such as the glial fibrillary acidic protein (GFAP), nestin and NeuronalNuclei (NeuN) [60]. The existence of these types of markers in human beings was confirmed by Zuk, who demonstrated the expression of neuron-specific enolase, NeuN, and microtubule-associated protein-2 [62]. On the other hand, Zuk’s group did not detect GFAP, which may be because this marker is specific to murine cells. Indeed, the results of a recent study by Lin, et al. would seem to confirm this thinking as their results seem to point to the same conclusions as the results presented by Safford [70].
To date, few studies have been carried out on the use of adipose tissue stem cells for urinary incontinence [73–75]. The originality of the first study by Rodriguez, et al. rests upon the fact that they used human cells (from lipoaspiration), for re-injection in mice and rats. Moreover, the cells injected into the bladder and the urethra were a mixed population of different cell types that were cultivated for few days but not selected beforehand. The authors showed that the human cells (labelled with a fluorescent marker) were still present 12 weeks after injection. The cells possessed a morphology and phenotype (actin-α) characteristic of smooth muscle. Moreover, recent results from the same team [74] show that ASCs, cultivated in a smooth muscle inductive media and seeded in a poly-lactic-glycolic acid scaffold can increase smooth muscle mass and are able to contract. Lastly, another team have demonstrated that injection of human ASCs increased the leak point pressure of urethrolysis-rats [75]. In fact, these results are very encouraging for the development of the use of these cells, because in theory they would be capable of reconstituting the smooth muscle of the sphincter, leading to the acquisition of a contraction function, something that has never been demonstrated with the MPCs.
Conclusion
In summary, it would seem that the SVF cells or ASCs of human adipose tissue are completely adapted to the treatment of SUI in women, because they seem to satisfy all of the decisive criteria for this type of treatment. Indeed, their injection, in principle, could bring about an increase in vascularization and in the number of smooth and striated muscle cells of the urethra, an increase in the secretion of collagen and elastin in peripheral tissue, and perhaps an improvement in neurological innervation, because certain cells could differentiate into neurons. Moreover, as with other progenitor cells, SVF cells and ASCs are a source of many growth factors that could significantly improve urethral function. At present it is unlikely that all of these characteristics could be shown by cells derived from skeletal muscle.
Furthermore, several other elements could act in favour of the use of SVF cells or ASCs in the treatment of urinary incontinence. Firstly, there is the fact that they are very easily accessible via liposuction, without this posing any particular risk to the patient, as liposuction is probably one of the safest surgical procedures available [76].
Then, concerning SVF cells, there is the fact that they can be injected directly after being sorted. Indeed, this avoids cell culture, and successive passages, which reduces considerably the risk from contamination and mutations, as well as the cost of the technique. The use of SVF cells is all the more acceptable as the number of cells that can be extracted from adipose tissue is relatively great. It is also true that few people in today’s society are lacking in this tissue! In relation to this, it is important to underline that the technique for the isolation of SVF cells from adipose tissue can have important repercussions on the component cells of the SVF after purification. Thus, our technique, derived from classical techniques for SVF cell isolation, in theory, makes it possible to isolate most of the cells required for the treatment of incontinence, such as fibroblasts [68], myoblasts (CD56), endothelial cells (CD31 and CD133) and probably precursors of smooth muscle cells (CD141). Other groups, for example do not isolate all of these cellular types [66]. The fact that tools exist that allow, in a reproducible manner, the isolation of the same cellular types from standardized samples, will enable without any doubt the exponential development of the use of these cells in the years to come.
References
- 1.Saadoun K, Ringa V, Fritel X, et al. Negative impact of urinary incontinence on quality of life, a cross-sectional study among women aged 49–61 years enrolled in the GAZEL cohort. Neurourol Urodyn. 2006;25:696–702. doi: 10.1002/nau.20245. [DOI] [PubMed] [Google Scholar]
- 2.Hunskaar S, Lose G, Sykes D, et al. The prevalence of urinary incontinence in women in four European countries. BJU Int. 2004;93:324–30. doi: 10.1111/j.1464-410x.2003.04609.x. [DOI] [PubMed] [Google Scholar]
- 3.Ko Y, Lin SJ, Salmon JW, et al. The impact of urinary incontinence on quality of life of the elderly. Am J Manag Care. 2005;11:S103–11. [PubMed] [Google Scholar]
- 4.Wilson L, Brown JS, Shin GP, et al. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398–406. doi: 10.1016/s0029-7844(01)01464-8. [DOI] [PubMed] [Google Scholar]
- 5.Hannestad YS, Rortveit G, Sandvik H, et al. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of incontinence in the County of Nord-Trondelag. J Clin Epidemiol. 2000;53:1150–7. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 6.Norton PA, Zinner NR, Yalcin I, et al. Duloxetine versus placebo in the treatment of stress urinary incontinence. Am J Obstet Gynecol. 2002;187:40–8. doi: 10.1067/mob.2002.124840. [DOI] [PubMed] [Google Scholar]
- 7.Radley SC, Chapple CR, Bryan NP, et al. Effect of methoxamine on maximum urethral pressure in women with genuine stress incontinence: a placebo-controlled, double-blind crossover study. Neurourol Urodyn. 2001;20:43–52. doi: 10.1002/1520-6777(2001)20:1<43::aid-nau6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Balmforth JR, Mantle J, Bidmead J, et al. A prospective observational trial of pelvic floor muscle training for female stress urinary incontinence. BJU Int. 2006;98:811–7. doi: 10.1111/j.1464-410X.2006.06393.x. [DOI] [PubMed] [Google Scholar]
- 9.Bo K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 years. Obstet Gynecol. 2005;105:999–1005. doi: 10.1097/01.AOG.0000157207.95680.6d. [DOI] [PubMed] [Google Scholar]
- 10.Barranger E, Fritel X, Kadoch O, et al. Results of transurethral injection of silicone micro-implants for females with intrinsic sphincter deficiency. J Urol. 2000;164:1619–22. [PubMed] [Google Scholar]
- 11.Ghoniem G, Corcos J, Comiter C, et al. Cross-linked polydimethylsiloxane injection for female stress urinary incontinence: results of a multicenter, randomized, controlled, single-blind study. J Urol. 2009;181:204–10. doi: 10.1016/j.juro.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Schulz JA, Nager CW, Stanton SL, et al. Bulking agents for stress urinary incontinence: short-term results and complications in a randomized comparison of periurethral and transurethral injections. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15:261–5. doi: 10.1007/s00192-004-1148-6. [DOI] [PubMed] [Google Scholar]
- 13.Jelovsek JE, Barber MD, Karram MM, et al. Randomised trial of laparoscopic Burch colposuspension versus tension-free vaginal tape: long-term follow up. Bjog. 2008;115:219–25. doi: 10.1111/j.1471-0528.2007.01592.x. ; discussion 225. [DOI] [PubMed] [Google Scholar]
- 14.Ward KL, Hilton P. Tension-free vaginal tape versus colposuspension for primary urodynamic stress incontinence: 5-year follow up. Bjog. 2008;115:226–33. doi: 10.1111/j.1471-0528.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 15.Novara G, Galfano A, Boscolo-Berto R, et al. Complication rates of tension-free midurethral slings in the treatment of female stress urinary incontinence: a systematic review and meta-analysis of randomized controlled trials comparing tension-free midurethral tapes to other surgical procedures and different devices. Eur Urol. 2008;53:288–308. doi: 10.1016/j.eururo.2007.10.073. [DOI] [PubMed] [Google Scholar]
- 16.Dainer M, Hall CD, Choe J, et al. The Burch procedure: a comprehensive review. Obstet Gynecol Surv. 1999;54:49–60. doi: 10.1097/00006254-199901000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Paick JS, Ku JH, Shin JW, et al. Tension-free vaginal tape procedure for urinary incontinence with low Valsalva leak point pressure. J Urol. 2004;172:1370–3. doi: 10.1097/01.ju.0000139882.57216.45. [DOI] [PubMed] [Google Scholar]
- 18.Rezapour M, Falconer C, Ulmsten U. Tension-Free vaginal tape (TVT) in stress incontinent women with intrinsic sphincter deficiency (ISD)–a long-term follow-up. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:S12–4. doi: 10.1007/s001920170005. [DOI] [PubMed] [Google Scholar]
- 19.Berkow SG. The corpus spongeosum of the urethra: its possible role in urinary control and stress incontinence in women. Am J Obstet Gynecol. 1953;65:346–51. doi: 10.1016/0002-9378(53)90437-2. [DOI] [PubMed] [Google Scholar]
- 20.Delancey JO, Ashton-Miller JA. Pathophysiology of adult urinary incontinence. Gastroenterology. 2004;126:S23–32. doi: 10.1053/j.gastro.2003.10.080. [DOI] [PubMed] [Google Scholar]
- 21.Hickey DS, Phillips JI, Hukins DW. Arrangements of collagen fibrils and muscle fibres in the female urethra and their implications for the control of micturition. Br J Urol. 1982;54:556–61. doi: 10.1111/j.1464-410x.1982.tb13590.x. [DOI] [PubMed] [Google Scholar]
- 22.Thind P. The significance of smooth and striated muscles in the sphincter function of the urethra in healthy women. Neurourol Urodyn. 1995;14:585–618. doi: 10.1002/nau.1930140602. [DOI] [PubMed] [Google Scholar]
- 23.Rud T, Andersson KE, Asmussen M, et al. Factors maintaining the intraurethral pressure in women. Invest Urol. 1980;17:343–7. [PubMed] [Google Scholar]
- 24.DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994;170:1713–20. doi: 10.1016/s0002-9378(94)70346-9. [DOI] [PubMed] [Google Scholar]
- 25.Fauconnier A, Delmas V, Lassau JP, et al. Ventral tethering of the vagina and its role in the kinetics of urethra and bladder-neck straining. Surg Radiol Anat. 1996;18:81–7. doi: 10.1007/BF01795224. [DOI] [PubMed] [Google Scholar]
- 26.Lose G. Urethral pressure and power generation during coughing and voluntary contraction of the pelvic floor in females with genuine stress incontinence. Br J Urol. 1991;67:580–5. doi: 10.1111/j.1464-410x.1991.tb15219.x. [DOI] [PubMed] [Google Scholar]
- 27.Thind P, Lose G, Colstrup H. Initial urethral pressure increase during stress episodes in genuine stress incontinent women. Br J Urol. 1992;69:137–40. doi: 10.1111/j.1464-410x.1992.tb15483.x. [DOI] [PubMed] [Google Scholar]
- 28.Perucchini D, DeLancey JO, Ashton-Miller JA, et al. Age effects on urethral striated muscle. I. Changes in number and diameter of striated muscle fibers in the ventral urethra. Am J Obstet Gynecol. 2002;186:351–5. doi: 10.1067/mob.2002.121089. [DOI] [PubMed] [Google Scholar]
- 29.Strasser H, Tiefenthaler M, Steinlechner M, et al. Age dependent apoptosis and loss of rhabdosphincter cells. J Urol. 2000;164:1781–5. [PubMed] [Google Scholar]
- 30.Yang JM, Yang SH, Huang WC. Functional correlates of Doppler flow study of the female urethral vasculature. Ultrasound Obstet Gynecol. 2006;28:96–102. doi: 10.1002/uog.2809. [DOI] [PubMed] [Google Scholar]
- 31.Liang CC, Chang SD, Chang YL, et al. Three-dimensional power Doppler measurement of perfusion of the periurethral tissue in incontinent women–a preliminary report. Acta Obstet Gynecol Scand. 2006;85:608–13. doi: 10.1080/00016340500342920. [DOI] [PubMed] [Google Scholar]
- 32.Athanasiou S, Khullar V, Boos K, et al. Imaging the urethral sphincter with three-dimensional ultrasound. Obstet Gynecol. 1999;94:295–301. doi: 10.1016/s0029-7844(99)00247-1. [DOI] [PubMed] [Google Scholar]
- 33.Kondo Y, Homma Y, Takahashi S, et al. Transvaginal ultrasound of urethral sphincter at the mid urethra in continent and incontinent women. J Urol. 2001;165:149–52. doi: 10.1097/00005392-200101000-00036. [DOI] [PubMed] [Google Scholar]
- 34.Chen B, Wen Y, Wang H, Polan ML. Differences in estrogen modulation of tissue inhibitor of matrix metalloproteinase-1 and matrix metalloproteinase-1 expression in cultured fibroblasts from continent and incontinent women. Am J Obstet Gynecol. 2003;189:59–65. doi: 10.1067/mob.2003.378. [DOI] [PubMed] [Google Scholar]
- 35.Chen B, Wen Y, Yu X, Polan ML. The role of neutrophil elastase in elastin metabolism of pelvic tissues from women with stress urinary incontinence. Neurourol Urodyn. 2007;26:274–9. doi: 10.1002/nau.20347. [DOI] [PubMed] [Google Scholar]
- 36.Edwall L, Carlstrom K, Jonasson AF. Markers of collagen synthesis and degradation in urogenital tissue from women with and without stress urinary incontinence. Neurourol Urodyn. 2005;24:319–24. doi: 10.1002/nau.20142. [DOI] [PubMed] [Google Scholar]
- 37.Goepel C, Thomssen C. Changes in the extracellular matrix in periurethral tissue of women with stress urinary incontinence. Acta Histochem. 2006;108:441–5. doi: 10.1016/j.acthis.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Kushner L, Mathrubutham M, Burney T, et al. Excretion of collagen derived peptides is increased in women with stress urinary incontinence. Neurourol Urodyn. 2004;23:198–203. doi: 10.1002/nau.10174. [DOI] [PubMed] [Google Scholar]
- 39.Trabucco E, Soderberg M, Cobellis L, et al. Role of proteoglycans in the organization of periurethral connective tissue in women with stress urinary incontinence. Maturitas. 2007;58:395–405. doi: 10.1016/j.maturitas.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Wen Y, Zhao YY, Li S, et al. Differences in mRNA and protein expression of small proteoglycans in vaginal wall tissue from women with and without stress urinary incontinence. Hum Reprod. 2007;22:1718–24. doi: 10.1093/humrep/dem039. [DOI] [PubMed] [Google Scholar]
- 41.Hale DS, Benson JT, Brubaker L, et al. Histologic analysis of needle biopsy of urethral sphincter from women with normal and stress incontinence with comparison of electromyographic findings. Am J Obstet Gynecol. 1999;180:342–8. doi: 10.1016/s0002-9378(99)70211-5. [DOI] [PubMed] [Google Scholar]
- 42.Herreros J, Prosper F, Perez A, et al. Autologous intramyocardial injection of cultured skeletal muscle-derived stem cells in patients with non-acute myocardial infarction. Eur Heart J. 2003;24:2012–20. doi: 10.1016/j.ehj.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Menasche P, Hagege AA, Vilquin JT, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–83. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 44.Yokoyama T, Yoshimura N, Dhir R, et al. Persistence and survival of autologous muscle derived cells versus bovine collagen as potential treatment of stress urinary incontinence. J Urol. 2001;165:271–6. doi: 10.1097/00005392-200101000-00077. [DOI] [PubMed] [Google Scholar]
- 45.Yiou R, Dreyfus P, Chopin DK, et al. Muscle precursor cell autografting in a murine model of urethral sphincter injury. BJU Int. 2002;89:298–302. doi: 10.1046/j.1464-4096.2001.01618.x. [DOI] [PubMed] [Google Scholar]
- 46.Cannon TW, Lee JY, Somogyi G, et al. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003;62:958–63. doi: 10.1016/s0090-4295(03)00679-4. [DOI] [PubMed] [Google Scholar]
- 47.Chermansky CJ, Tarin T, Kwon DD, et al. Intraurethral muscle-derived cell injections increase leak point pressure in a rat model of intrinsic sphincter deficiency. Urology. 2004;63:780–5. doi: 10.1016/j.urology.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 48.Peyromaure M, Sebe P, Praud C, et al. Fate of implanted syngenic muscle precursor cells in striated urethral sphincter of female rats: perspectives for treatment of urinary incontinence. Urology. 2004;64:1037–41. doi: 10.1016/j.urology.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 49.Kwon D, Kim Y, Pruchnic R, et al. Periurethral cellular injection: comparison of muscle-derived progenitor cells and fibroblasts with regard to efficacy and tissue contractility in an animal model of stress urinary incontinence. Urology. 2006;68:449–54. doi: 10.1016/j.urology.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 50.Strasser H, Berjukow S, Marksteiner R, et al. Stem cell therapy for urinary stress incontinence. Exp Gerontol. 2004;39:1259–65. doi: 10.1016/j.exger.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Strasser H, Marksteiner R, Margreiter E, et al. Autologous myoblasts and fibroblasts versus collagen for treatment of stress urinary incontinence in women: a randomised controlled trial. Lancet. 2007;369:2179–86. doi: 10.1016/S0140-6736(07)61014-9. [DOI] [PubMed] [Google Scholar]
- 52.Kleinert S, Horton R. Retraction–autologous myoblasts and fibroblasts versus collagen [corrected] for treatment of stress urinary incontinence in women: a [corrected] randomised controlled trial. Lancet. 2008;372:789–90. doi: 10.1016/S0140-6736(08)61320-3. [DOI] [PubMed] [Google Scholar]
- 53.Carr LK, Steele D, Steele S, et al. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:881–3. doi: 10.1007/s00192-007-0553-z. [DOI] [PubMed] [Google Scholar]
- 54.Rubio D, Garcia-Castro J, Martin MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–9. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 55.Bacou F, El Andalousi RB, Daussin PA, et al. Transplantation of adipose tissue-derived stromal cells increases mass and functional capacity of damaged skeletal muscle. Cell Transplant. 2004;13:103–11. [PubMed] [Google Scholar]
- 56.Charriere G, Cousin B, Arnaud E, et al. Preadipocyte conversion to macrophage. Evidence of plasticity. J Biol Chem. 2003;278:9850–5. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- 57.Erickson GR, Gimble JM, Franklin DM, et al. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;290:763–9. doi: 10.1006/bbrc.2001.6270. [DOI] [PubMed] [Google Scholar]
- 58.Mizuno H, Zuk PA, Zhu M, et al. Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg. 2002;109:199–209. doi: 10.1097/00006534-200201000-00030. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez LV, Alfonso Z, Zhang R, et al. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:12167–72. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Safford KM, Hicok KC, Safford SD, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002;294:371–9. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 61.Seo MJ, Suh SY, Bae YC, et al. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258–64. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- 62.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guilak F, Lott KE, Awad HA, et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229–37. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 64.Miranville A, Heeschen C, Sengenes C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–55. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 65.Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–65. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 66.Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–63. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 67.Valina C, Pinkernell K, Song YH, et al. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–77. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 68.Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–85. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 69.Vermette M, Trottier V, Menard V, et al. Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials. 2007;28:2850–60. doi: 10.1016/j.biomaterials.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 70.Lin Y, Chen X, Yan Z, et al. Multilineage differentiation of adipose-derived stromal cells from GFP transgenic mice. Mol Cell Biochem. 2006;285:69–78. doi: 10.1007/s11010-005-9056-8. [DOI] [PubMed] [Google Scholar]
- 71.Festy F, Hoareau L, Bes-Houtmann S, et al. Surface protein expression between human adipose tissue-derived stromal cells and mature adipocytes. Histochem Cell Biol. 2005;124:113–21. doi: 10.1007/s00418-005-0014-z. [DOI] [PubMed] [Google Scholar]
- 72.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–8. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 73.Jack GS, Almeida FG, Zhang R, et al. Processed lipoaspirate cells for tissue engineering of the lower urinary tract: implications for the treatment of stress urinary incontinence and bladder reconstruction. J Urol. 2005;174:2041–5. doi: 10.1097/01.ju.0000176489.96993.84. [DOI] [PubMed] [Google Scholar]
- 74.Jack GS, Zhang R, Lee M, et al. Urinary bladder smooth muscle engineered from adipose stem cells and a three dimensional synthetic composite. Biomaterials. 2009;30:3259–70. doi: 10.1016/j.biomaterials.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim KH, Jung H, Yoon SJ, et al. Effect of human adipose tissue-derived stem cells on stress urinary incontinence in rats. J Eur Urol Suppl. 2008;7:317. [Google Scholar]
- 76.Housman TS, Lawrence N, Mellen BG, et al. The safety of liposuction: results of a national survey. Dermatol Surg. 2002;28:971–8. doi: 10.1046/j.1524-4725.2002.02081.x. [DOI] [PubMed] [Google Scholar]


