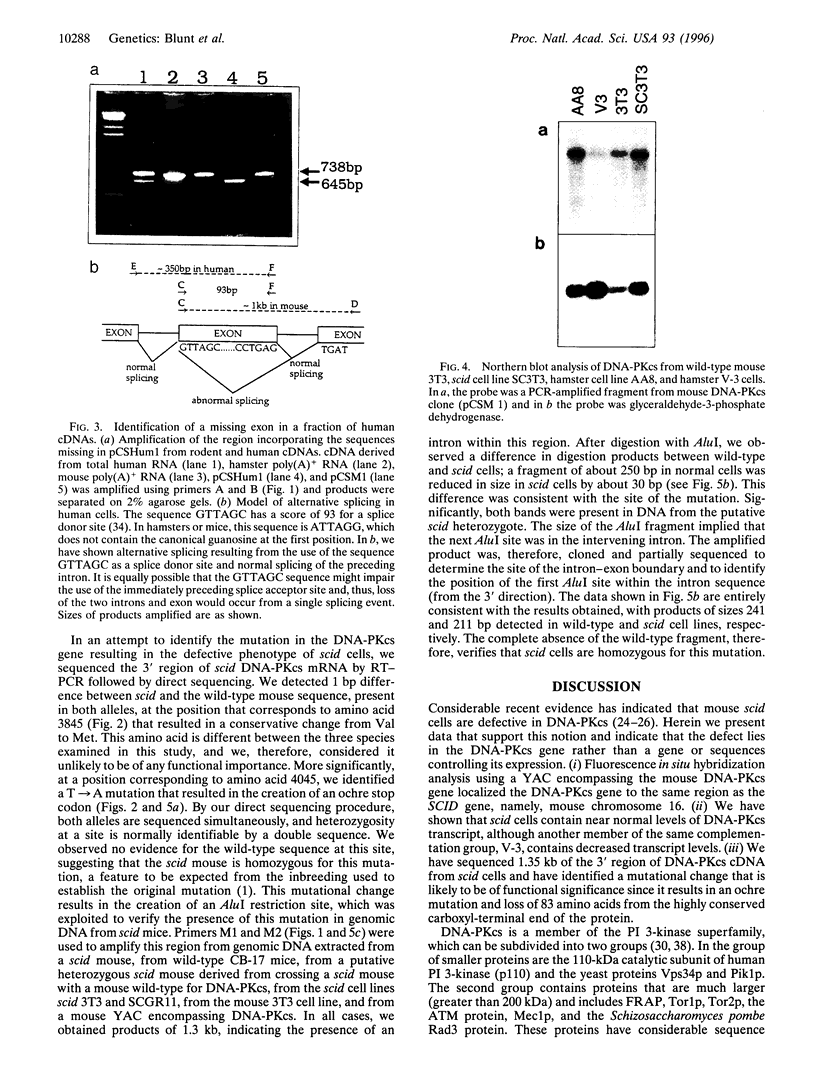
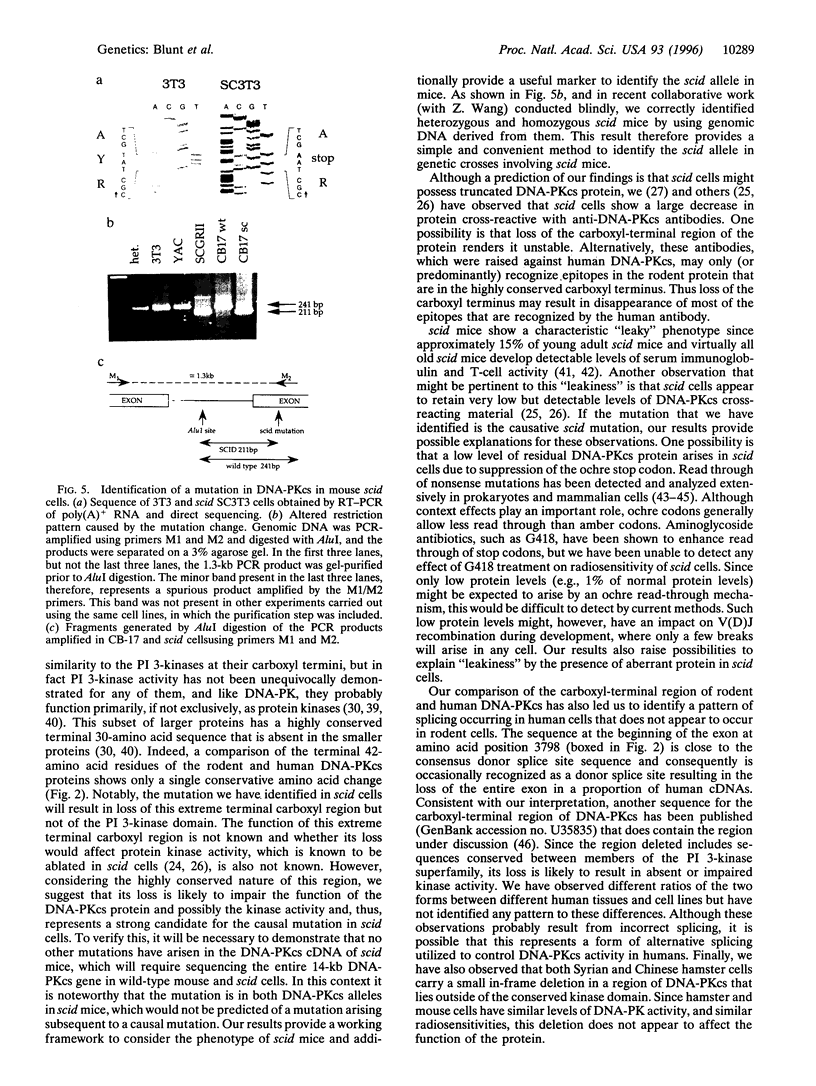
Abstract
DNA-dependent protein kinase (DNA-PK) consists of a heterodimeric protein (Ku) and a large catalytic subunit (DNA-PKcs). The Ku protein has double-stranded DNA end-binding activity that serves to recruit the complex to DNA ends. Despite having serine/threonine protein kinase activity, DNA-PKcs falls into the phosphatidylinositol 3-kinase superfamily. DNA-PK functions in DNA double-strand break repair and V(D)J recombination, and recent evidence has shown that mouse scid cells are defective in DNA-PKcs. In this study we have cloned the cDNA for the carboxyl-terminal region of DNA-PKcs in rodent cells and identified the existence of two differently spliced products in human cells. We show that DNA-PKcs maps to the same chromosomal region as the mouse scid gene. scid cells contain approximately wild-type levels of DNA-PKcs transcripts, whereas the V-3 cell line, which is also defective in DNA-PKcs, contains very reduced transcript levels. Sequence comparison of the carboxyl-terminal region of scid and wild-type mouse cells enabled us to identify a nonsense mutation within a highly conserved region of the gene in mouse scid cells. This represents a strong candidate for the inactivating mutation in DNA-PKcs in the scid mouse.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Oltz E. M., Young F., Gorman J., Taccioli G., Chen J. VDJ recombination. Immunol Today. 1992 Aug;13(8):306–314. doi: 10.1016/0167-5699(92)90043-7. [DOI] [PubMed] [Google Scholar]
- Biedermann K. A., Sun J. R., Giaccia A. J., Tosto L. M., Brown J. M. scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1394–1397. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Malynn B. A., Pollock R. R., Ferrier P., Covey L. R., Fulop G. M., Phillips R. A., Yancopoulos G. D., Alt F. W. Isolation of scid pre-B cells that rearrange kappa light chain genes: formation of normal signal and abnormal coding joins. EMBO J. 1989 Mar;8(3):735–742. doi: 10.1002/j.1460-2075.1989.tb03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunt T., Finnie N. J., Taccioli G. E., Smith G. C., Demengeot J., Gottlieb T. M., Mizuta R., Varghese A. J., Alt F. W., Jeggo P. A. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell. 1995 Mar 10;80(5):813–823. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Davisson M. T., Ruetsch N. R., Sweet H. O., Shultz L. D., Bosma M. J. The mouse mutation severe combined immune deficiency (scid) is on chromosome 16. Immunogenetics. 1989;29(1):54–57. doi: 10.1007/BF02341614. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Fried M., Custer R. P., Carroll A., Gibson D. M., Bosma M. J. Evidence of functional lymphocytes in some (leaky) scid mice. J Exp Med. 1988 Mar 1;167(3):1016–1033. doi: 10.1084/jem.167.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma M. J., Carroll A. M. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- Burke J. F., Mogg A. E. Suppression of a nonsense mutation in mammalian cells in vivo by the aminoglycoside antibiotics G-418 and paromomycin. Nucleic Acids Res. 1985 Sep 11;13(17):6265–6272. doi: 10.1093/nar/13.17.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. M., Hardy R. R., Bosma M. J. Occurrence of mature B (IgM+, B220+) and T (CD3+) lymphocytes in scid mice. J Immunol. 1989 Aug 15;143(4):1087–1093. [PubMed] [Google Scholar]
- Dvir A., Peterson S. R., Knuth M. W., Lu H., Dynan W. S. Ku autoantigen is the regulatory component of a template-associated protein kinase that phosphorylates RNA polymerase II. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11920–11924. doi: 10.1073/pnas.89.24.11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnie N. J., Gottlieb T. M., Blunt T., Jeggo P. A., Jackson S. P. DNA-dependent protein kinase defects are linked to deficiencies in DNA repair and V(D)J recombination. Philos Trans R Soc Lond B Biol Sci. 1996 Feb 29;351(1336):173–179. doi: 10.1098/rstb.1996.0014. [DOI] [PubMed] [Google Scholar]
- Fulop G. M., Phillips R. A. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990 Oct 4;347(6292):479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- Gellert M. Molecular analysis of V(D)J recombination. Annu Rev Genet. 1992;26:425–446. doi: 10.1146/annurev.ge.26.120192.002233. [DOI] [PubMed] [Google Scholar]
- Getts R. C., Stamato T. D. Absence of a Ku-like DNA end binding activity in the xrs double-strand DNA repair-deficient mutant. J Biol Chem. 1994 Jun 10;269(23):15981–15984. [PubMed] [Google Scholar]
- Gottlieb T. M., Jackson S. P. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993 Jan 15;72(1):131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- Hartley K. O., Gell D., Smith G. C., Zhang H., Divecha N., Connelly M. A., Admon A., Lees-Miller S. P., Anderson C. W., Jackson S. P. DNA-dependent protein kinase catalytic subunit: a relative of phosphatidylinositol 3-kinase and the ataxia telangiectasia gene product. Cell. 1995 Sep 8;82(5):849–856. doi: 10.1016/0092-8674(95)90482-4. [DOI] [PubMed] [Google Scholar]
- Hendrickson E. A., Qin X. Q., Bump E. A., Schatz D. G., Oettinger M., Weaver D. T. A link between double-strand break-related repair and V(D)J recombination: the scid mutation. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4061–4065. doi: 10.1073/pnas.88.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. When is a lipid kinase not a lipid kinase? When it is a protein kinase. Cell. 1995 Oct 6;83(1):1–4. doi: 10.1016/0092-8674(95)90225-2. [DOI] [PubMed] [Google Scholar]
- Jeggo P. A. Studies on mammalian mutants defective in rejoining double-strand breaks in DNA. Mutat Res. 1990 Jul;239(1):1–16. doi: 10.1016/0165-1110(90)90028-a. [DOI] [PubMed] [Google Scholar]
- Jeggo P. A., Tesmer J., Chen D. J. Genetic analysis of ionising radiation sensitive mutants of cultured mammalian cell lines. Mutat Res. 1991 Mar;254(2):125–133. doi: 10.1016/0921-8777(91)90003-8. [DOI] [PubMed] [Google Scholar]
- Kapeller R., Cantley L. C. Phosphatidylinositol 3-kinase. Bioessays. 1994 Aug;16(8):565–576. doi: 10.1002/bies.950160810. [DOI] [PubMed] [Google Scholar]
- Keith C. T., Schreiber S. L. PIK-related kinases: DNA repair, recombination, and cell cycle checkpoints. Science. 1995 Oct 6;270(5233):50–51. doi: 10.1126/science.270.5233.50. [DOI] [PubMed] [Google Scholar]
- Kirchgessner C. U., Patil C. K., Evans J. W., Cuomo C. A., Fried L. M., Carter T., Oettinger M. A., Brown J. M. DNA-dependent kinase (p350) as a candidate gene for the murine SCID defect. Science. 1995 Feb 24;267(5201):1178–1183. doi: 10.1126/science.7855601. [DOI] [PubMed] [Google Scholar]
- Kovalic D., Kwak J. H., Weisblum B. General method for direct cloning of DNA fragments generated by the polymerase chain reaction. Nucleic Acids Res. 1991 Aug 25;19(16):4560–4560. doi: 10.1093/nar/19.16.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge O., Nishijima M., Akamatsu Y. A Chinese hamster cDNA encoding a protein essential for phosphatidylserine synthase I activity. J Biol Chem. 1991 Dec 15;266(35):24184–24189. [PubMed] [Google Scholar]
- Lewis S. M. The mechanism of V(D)J joining: lessons from molecular, immunological, and comparative analyses. Adv Immunol. 1994;56:27–150. doi: 10.1016/s0065-2776(08)60450-2. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Lewis S., Bosma G. C., Rosenberg N., Mizuuchi K., Bosma M. J., Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988 Oct 7;55(1):7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Blackwell T. K., Fulop G. M., Rathbun G. A., Furley A. J., Ferrier P., Heinke L. B., Phillips R. A., Yancopoulos G. D., Alt F. W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988 Aug 12;54(4):453–460. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- Martin R., Mogg A. E., Heywood L. A., Nitschke L., Burke J. F. Aminoglycoside suppression at UAG, UAA and UGA codons in Escherichia coli and human tissue culture cells. Mol Gen Genet. 1989 Jun;217(2-3):411–418. doi: 10.1007/BF02464911. [DOI] [PubMed] [Google Scholar]
- Miller R. D., Hogg J., Ozaki J. H., Gell D., Jackson S. P., Riblet R. Gene for the catalytic subunit of mouse DNA-dependent protein kinase maps to the scid locus. Proc Natl Acad Sci U S A. 1995 Nov 7;92(23):10792–10795. doi: 10.1073/pnas.92.23.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola F., Zdzienicka M. Z., Lieber M. R. V(D)J recombination in mammalian cell mutants defective in DNA double-strand break repair. Mol Cell Biol. 1993 Jun;13(6):3464–3471. doi: 10.1128/mcb.13.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson S. R., Kurimasa A., Oshimura M., Dynan W. S., Bradbury E. M., Chen D. J. Loss of the catalytic subunit of the DNA-dependent protein kinase in DNA double-strand-break-repair mutant mammalian cells. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3171–3174. doi: 10.1073/pnas.92.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Jones M. K., Hill L. S., Atkinson J., Martin R. Context effects on misreading and suppression at UAG codons in human cells. Mol Cell Biol. 1995 Dec;15(12):6593–6600. doi: 10.1128/mcb.15.12.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltoratsky V. P., Shi X., York J. D., Lieber M. R., Carter T. H. Human DNA-activated protein kinase (DNA-PK) is homologous to phosphatidylinositol kinases. J Immunol. 1995 Nov 15;155(10):4529–4533. [PubMed] [Google Scholar]
- Rathmell W. K., Chu G. Involvement of the Ku autoantigen in the cellular response to DNA double-strand breaks. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7623–7627. doi: 10.1073/pnas.91.16.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipley J. D., Menninger J. C., Hartley K. O., Ward D. C., Jackson S. P., Anderson C. W. Gene for the catalytic subunit of the human DNA-activated protein kinase maps to the site of the XRCC7 gene on chromosome 8. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7515–7519. doi: 10.1073/pnas.92.16.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smider V., Rathmell W. K., Lieber M. R., Chu G. Restoration of X-ray resistance and V(D)J recombination in mutant cells by Ku cDNA. Science. 1994 Oct 14;266(5183):288–291. doi: 10.1126/science.7939667. [DOI] [PubMed] [Google Scholar]
- Taccioli G. E., Cheng H. L., Varghese A. J., Whitmore G., Alt F. W. A DNA repair defect in Chinese hamster ovary cells affects V(D)J recombination similarly to the murine scid mutation. J Biol Chem. 1994 Mar 11;269(10):7439–7442. [PubMed] [Google Scholar]
- Taccioli G. E., Gottlieb T. M., Blunt T., Priestley A., Demengeot J., Mizuta R., Lehmann A. R., Alt F. W., Jackson S. P., Jeggo P. A. Ku80: product of the XRCC5 gene and its role in DNA repair and V(D)J recombination. Science. 1994 Sep 2;265(5177):1442–1445. doi: 10.1126/science.8073286. [DOI] [PubMed] [Google Scholar]
- Taccioli G. E., Rathbun G., Oltz E., Stamato T., Jeggo P. A., Alt F. W. Impairment of V(D)J recombination in double-strand break repair mutants. Science. 1993 Apr 9;260(5105):207–210. doi: 10.1126/science.8469973. [DOI] [PubMed] [Google Scholar]
- Thacker J., Wilkinson R. E. The genetic basis of resistance to ionising radiation damage in cultured mammalian cells. Mutat Res. 1991 Mar;254(2):135–142. doi: 10.1016/0921-8777(91)90004-9. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Jeggo P. A. Nomenclature of human genes involved in ionizing radiation sensitivity. Mutat Res. 1995 Sep;337(2):131–134. doi: 10.1016/0921-8777(95)00018-f. [DOI] [PubMed] [Google Scholar]
- Whitmore G. F., Varghese A. J., Gulyas S. Cell cycle responses of two X-ray sensitive mutants defective in DNA repair. Int J Radiat Biol. 1989 Nov;56(5):657–665. doi: 10.1080/09553008914551881. [DOI] [PubMed] [Google Scholar]