Abstract
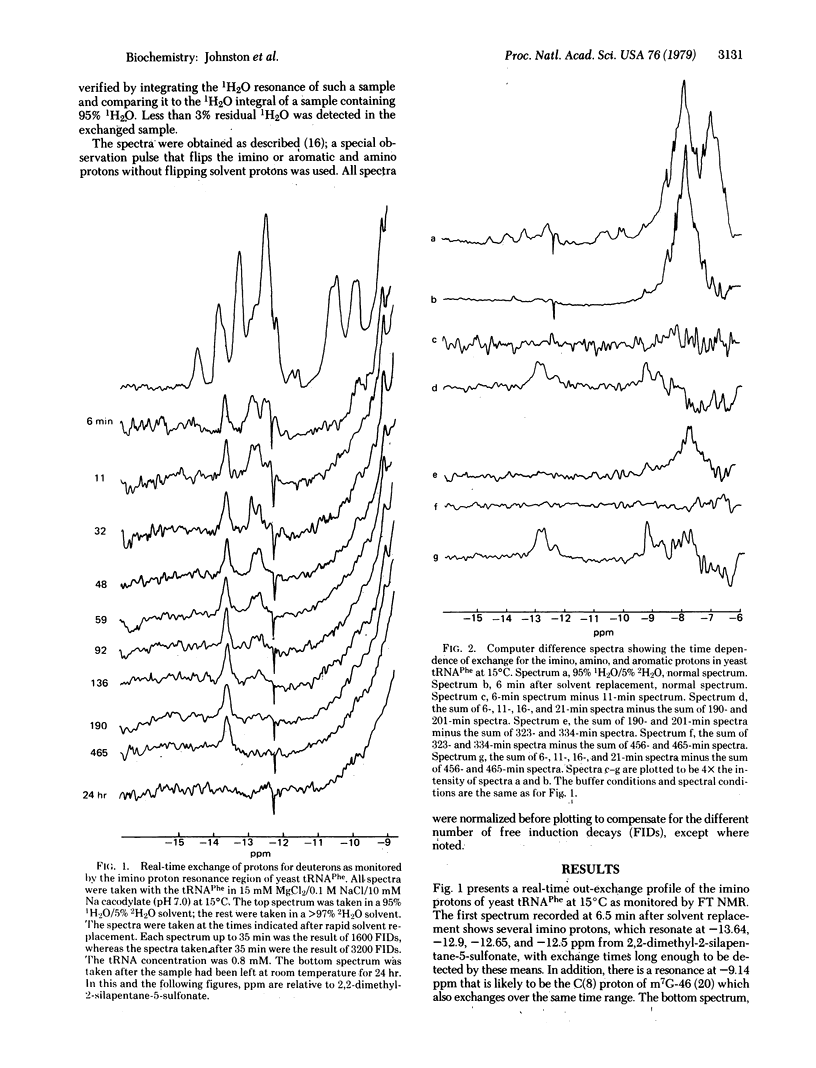
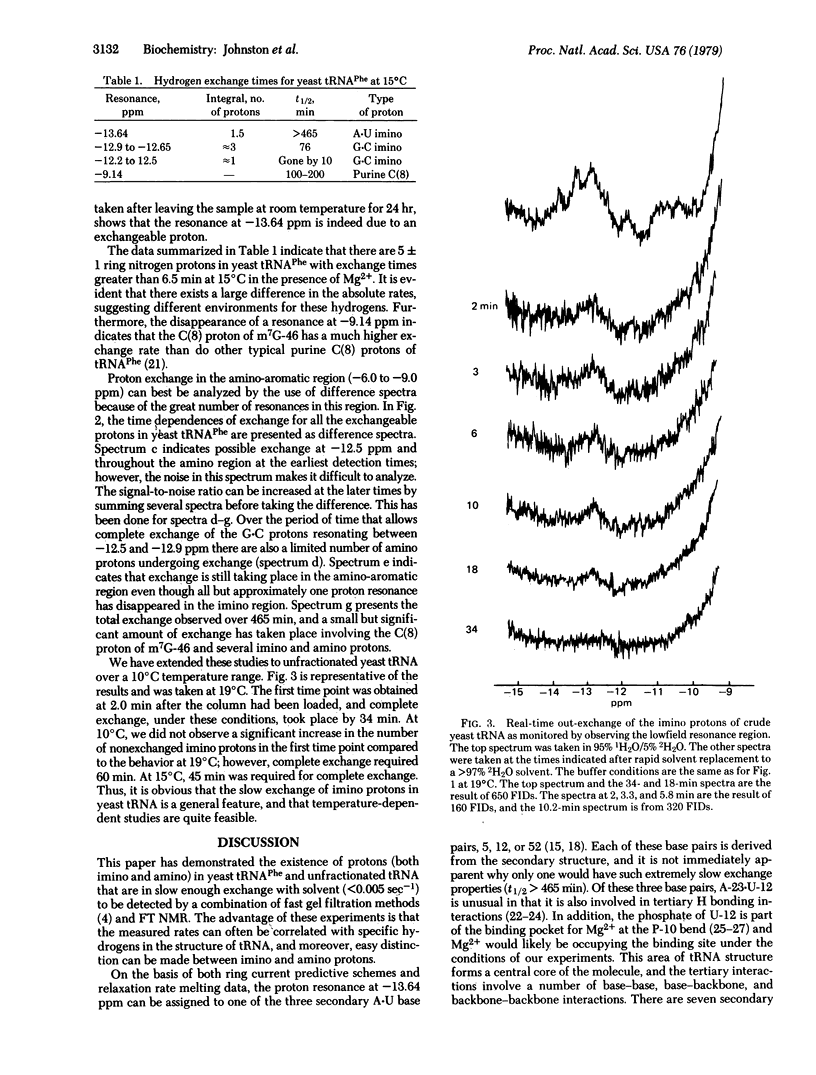
Real-time solvent exchange measurements using Fourier transform NMR at 270 MHz are presented. By means of the fast gel filtration column techniques originally developed for tritium exchange experiments, we were able to replace the solvent of a tRNA sample from an 1H2O to an 2H2O buffer and obtain a useful spectrum in 2-5 min. At 15 degrees C, there are 5 +/- 1 lowfield (-11 to -15 ppm relative to 2,2-dimethyl-2-silapentane-5-sulfonate) imino protons with exchange half times of minutes to hours. In addition, the m7G-46 C(8) proton and several amino protons are observed to exchange with similar rates. Analogous studies on unfractionated yeast tRNA suggest that such a class of slowly exchanging imino protons is present in several tRNAs, and that the activation energy for exchange is small [[approximatley 5 kcal/mol (21 kJ/mol)]. We speculate that these imino resonances arise from D-stem protons and that their slow exchange reflects stabilization by the numerous tertiary interactions involving this stem and the Mg2+ bound at the P-10 bend.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cross D. G. Hydrogen exchange in nucleosides and nucleotides. Measurement of hydrogen exchange by stopped-flow and ultraviolet difference spectroscopy. Biochemistry. 1975 Jan 28;14(2):357–362. doi: 10.1021/bi00673a023. [DOI] [PubMed] [Google Scholar]
- Englander J. J., Kallenbach N. R., Englander S. W. Hydrogen exchange study of some polynucleotides and transfer RNA. J Mol Biol. 1972 Jan 14;63(1):153–169. doi: 10.1016/0022-2836(72)90527-x. [DOI] [PubMed] [Google Scholar]
- Englander J. J., Von Hippel P. H. Slow exchange of the "outside" amino hydrogens of DNA. J Mol Biol. 1972 Jan 14;63(1):171–177. doi: 10.1016/0022-2836(72)90528-1. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Downer N. W., Teitelbaum H. Hydrogen exchange. Annu Rev Biochem. 1972;41:903–924. doi: 10.1146/annurev.bi.41.070172.004351. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Englander J. J. HYDROGEN EXCHANGE STUDIES OF sRNA. Proc Natl Acad Sci U S A. 1965 Feb;53(2):370–378. doi: 10.1073/pnas.53.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander S. W., Englander J. J. Hydrogen--tritium exchange. Methods Enzymol. 1978;49:24–39. doi: 10.1016/s0076-6879(78)49005-6. [DOI] [PubMed] [Google Scholar]
- Gamble R. C., Schoemaker J. P. Rate of tritium labeling of specific purines in relation to nucleic acid and particularly transfer RNA conformation. Biochemistry. 1976 Jun 29;15(13):2791–2799. doi: 10.1021/bi00658a014. [DOI] [PubMed] [Google Scholar]
- Goldstein R. N., Stefanovic S., Kallenbach N. R. On the conformation of transfer RNA in solution: dependence of denaturation temperature and structural parameters of mixed and formylmethionyl Escherichia coli transfer RNA on sodium ion concentration. J Mol Biol. 1972 Aug 21;69(2):217–236. doi: 10.1016/0022-2836(72)90227-6. [DOI] [PubMed] [Google Scholar]
- Hanson C. V. A study of rapid hydrogen exchange in nucleic acids. J Mol Biol. 1971 Jun 28;58(3):847–863. [PubMed] [Google Scholar]
- Hingerty B., Brown R. S., Jack A. Further refinement of the structure of yeast tRNAPhe. J Mol Biol. 1978 Sep 25;124(3):523–534. doi: 10.1016/0022-2836(78)90185-7. [DOI] [PubMed] [Google Scholar]
- Holbrook S. R., Sussman J. L., Warrant R. W., Church G. M., Kim S. H. RNA-ligant interactions. (I) Magnesium binding sites in yeast tRNAPhe. Nucleic Acids Res. 1977 Aug;4(8):2811–2820. doi: 10.1093/nar/4.8.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook S. R., Sussman J. L., Warrant R. W., Kim S. H. Crystal structure of yeast phenylalanine transfer RNA. II. Structural features and functional implications. J Mol Biol. 1978 Aug 25;123(4):631–660. doi: 10.1016/0022-2836(78)90210-3. [DOI] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Klug A. Crystallographic refinement of yeast phenylalanine transfer RNA at 2-5A resolution. J Mol Biol. 1976 Dec 25;108(4):619–649. doi: 10.1016/s0022-2836(76)80109-x. [DOI] [PubMed] [Google Scholar]
- Johnston P. D., Redfield A. G. An NMR study of the exchange rates for protons involved in the secondary and tertiary structure of yeast tRNA Phe. Nucleic Acids Res. 1977 Oct;4(10):3599–3615. doi: 10.1093/nar/4.10.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P. D., Redfield A. G. Pulsed FT-NMR double resonance studies of yeast tRNAPhe: specific nuclear Overhauser effects and reinterpretation of low temperature relaxation data. Nucleic Acids Res. 1978 Oct;5(10):3913–3927. doi: 10.1093/nar/5.10.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Penman S. Association by hydrogen bonding of free nucleosides in non-aqueous solution. J Mol Biol. 1966 Jan;15(1):220–231. doi: 10.1016/s0022-2836(66)80222-x. [DOI] [PubMed] [Google Scholar]
- Nakanishi M., Tsuboi M. Two channels of hydrogen exchange in a double-helical nucleic acid. J Mol Biol. 1978 Sep 5;124(1):61–71. doi: 10.1016/0022-2836(78)90147-x. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Rich A. Structural domains of transfer RNA molecules. Science. 1976 Nov 19;194(4267):796–806. doi: 10.1126/science.790568. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Teeter M. M., Rich A. Structural analysis of spermine and magnesium ion binding to yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):64–68. doi: 10.1073/pnas.75.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. Initial stages of the thermal unfolding of yeast phenylalanine transfer RNA as studied by chemical modification: the effect of magnesium. Eur J Biochem. 1977 Nov 15;81(1):91–101. doi: 10.1111/j.1432-1033.1977.tb11930.x. [DOI] [PubMed] [Google Scholar]
- Rordorf B. F., Kearns D. R. NMR investigation of proton exchange in transfer RNA by high resolution NMR. Biochem Biophys Res Commun. 1975 Aug 4;65(3):857–862. doi: 10.1016/s0006-291x(75)80464-5. [DOI] [PubMed] [Google Scholar]
- Teitelbaum H., Englander S. W. Open states in native polynucleotides. I. Hydrogen-exchange study of adenine-containing double helices. J Mol Biol. 1975 Feb 15;92(1):55–78. doi: 10.1016/0022-2836(75)90091-1. [DOI] [PubMed] [Google Scholar]
- Teitelbaum H., Englander S. W. Open states in native polynucleotides. II. Hydrogen-exchange study of cytosine-containing double helices. J Mol Biol. 1975 Feb 15;92(1):79–92. doi: 10.1016/0022-2836(75)90092-3. [DOI] [PubMed] [Google Scholar]
- Tomasz M. Extreme lability of the C-8 proton: a consequence of 7-methylation of guanine residues in model compounds and in DNA and its analytical application. Biochim Biophys Acta. 1970 Jan 21;199(1):18–28. doi: 10.1016/0005-2787(70)90690-8. [DOI] [PubMed] [Google Scholar]
- Webb P. K., Fresco J. R. Tritium exchange studies of transfer RNA in native and denaturated conformations. J Mol Biol. 1973 Mar 5;74(3):387–402. doi: 10.1016/0022-2836(73)90379-3. [DOI] [PubMed] [Google Scholar]


