Abstract
The multistep cleavage of carotenoids in Mucorales during the sexual phase results in a cocktail of trisporic acid (C18) sex pheromones. We hypothesized that the C18 trisporoid intermediates have a specific regulatory function for sex pheromone production and carotenogenesis that varies with genus/species and vegetative and sexual phases of their life cycles. Real-time quantitative PCR kinetics determined for Blakeslea trispora displayed a very high transcript turnover in the gene for carotenoid cleavage dioxygenase, tsp3, during the sexual phase. An in vivo enzyme assay and chromatographic analysis led to the identification of β-apo-12′-carotenal as the first apocarotenoid involved in trisporic acid biosynthesis in B. trispora. Supplementation of C18 trisporoids, namely D'orenone, methyl trisporate C, and trisporin C, increased tsp3 transcripts in the plus compared to minus partners. Interestingly, the tsp1 gene, which is involved in trisporic acid biosynthesis, was downregulated compared to tsp3 irrespective of asexual or sexual phase. Only the minus partners of both B. trispora and Mucor mucedo had enhanced β-carotene production after treatment with C20 apocarotenoids, 15 different trisporoids, and their analogues. We conclude that the apocarotenoids and trisporoids influence gene transcription and metabolite production, depending upon the fungal strain, corresponding genus, and developmental phase, representing a “chemical dialect” during sexual communication.
INTRODUCTION
Mucorales fungi belonging to the subphylum incertae sedis Mucoromycotina (formerly classified in the class Zygomycetes) comprise 9 families, 51 genera, and around 205 species (1). These basal fungi are fast-growing soil saprotrophs that feed on dead and decaying organic matter. The asexual phase predominates their life cycle, with multinucleated haploid sporangiospores that germinate to mycelia of either the plus or minus mating type in heterothallic strains. Adversities, like environmental stresses or nutrient depletion, especially of nitrogen and phosphorus (2), lead to the sexual phase, in which complementary mating partners in close proximity exchange metabolites and form special aerial hyphae known as zygophores. The fusion of zygophores results in thousands of nuclei from both parents in the morphologically modified structure known as the progametangia. During further structural modifications and dormancy, which may extend from months to a year, most of the nuclei undergo degradation. Finally, gametangia bearing two nuclei from opposite partners fuse to form a thick-walled dikaryotic sexual spore known as the zygospore. Mitosis followed by meiosis results in four haploid products in a sporangium that develops from the zygospore (3). One Mucorales species in particular, Blakeslea trispora, has been commercially exploited for its potential to produce an excess of carotenoids, like β-carotene and lycopene, during its sexual phase of the life cycle (4, 5). According to a new report by Global Industrial Analysts, Inc., the worldwide market for carotenoids is projected to reach $1.3 billion by 2017. Hence, microbial carotenoids have been in the limelight as an ecofriendly sustainable alternative source for synthetics. Mucoralean carotenoids are not only under investigation for their biotechnological applications but also to help in understanding how they influence the sexual phase in those fungi.
Apocarotenoids are the unsaturated nonpolar isoprenoids formed by the oxidative cleavage of carotenoids (6). The sex hormone trisporic acid and its trisporoid precursors are C18 apocarotenoids that regulate the sexual phase in plus and minus mating partners among heterothallic species, such as Mucor mucedo, B. trispora, Mucor circinelloides, and Phycomyces blakesleeanus (7–9). They were first discovered as stimulants of carotenogenesis-inducing positive feedback regulation, rather than as sex hormones. Both carotenoids and trisporoids were detected in quantifiable amounts during the sexual phase under dark conditions in M. mucedo and B. trispora, while P. blakesleeanus induced carotenogenesis upon exposure to blue light (10). However, trisporic acids extracted from B. trispora mated cultures grown in the dark exhibited reduced carotenogenic activity on exposure to light (11). Hence, we chose B. trispora and M. mucedo, which prefers darkness for enhanced carotene production, as the best-suited organisms for studies on the carotenoid catabolic pathway among Mucorales fungi.
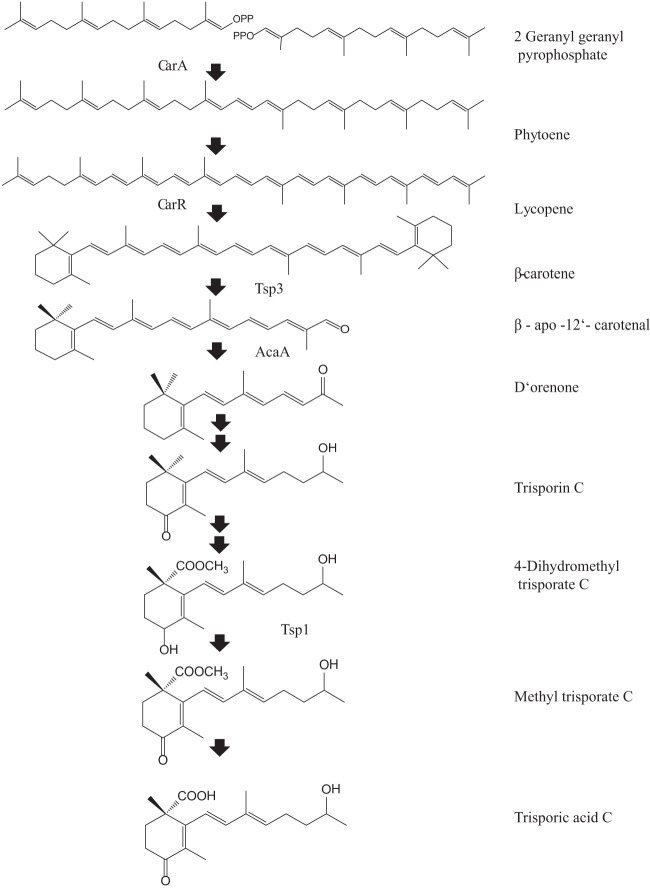
In fungi, carotenogenesis takes place via the mevalonate pathway. Phytoene synthase and lycopene cyclase (CarRA), along with phytoene dehydrogenase (CarB), convert the first colorless linear C40 carotenoid, phytoene (Fig. 1), to yellow-pigmented β-carotene (12, 13). Little is known about the physiological functions of enzymes or the products of β-carotene cleavage and trisporic acid biogenesis within the Mucorales. The hypothetical biosynthetic pathway in P. blakesleeanus commences with oxidative cleavage of β-carotene at the C11′—C12′ as the caroteneoid cleavage dioxygenase (CarS) releases β-apo-12′-carotenal (C25), which further cleaves to the first C18 trisporoid (β-apo-13-carotenone) D'orenone at the C13—C14 bond by apocarotenoid cleavage oxygenase (AcaA) (14). CarS mutants in Phycomyces fail to produce those apocarotenoids present in the wild type (15). No functional characterization has been reported for the CarS homologue in Blakeslea trispora, designated TSP3 (16). All of the C18 apocarotenoids (Fig. 1) from D'orenone (β-apo-13-carotenone) to methyl trisporate in the trisporic acid biosynthetic pathway are known as trisporoids (17–19). Few enzymes are known so far, such as 4-dihydromethyltrisporate dehydrogenase (TSP1), 4-dihydrotrisporin dehydrogenase (TSP2) (20–22), and a putative esterase enzyme from the minus partner that converts methyl trisporate to trisporic acids in both homothallic Mucorales (Zygorhynchus moelleri) and heterothallic Mucorales (23). The high-mobility group (HMG) transcription factor genes sexM and sexP are associated with the minus and plus mating-type loci in P. blakesleeanus and M. mucedo (24, 25). Technically, it is difficult to carry out functional gene analyses via classical genetic approaches in either B. trispora or M. mucedo without sequenced genome data. Moreover, these two organisms possess multinucleated spores, and no reports are available on successful generation of mitotically stable transformants.
Fig 1.
Schematic diagram of the putative biosynthetic pathway and genes encoding respective proteins involved in β-carotene metabolism. The enzymes depicted left of the arrows lead to β-carotene biosynthesis, while those to the right of the arrows are involved in trisporic acid biogenesis. CarRA is a bifunctional enzyme having 2 domains, CarR and CarA, that lead to biogenesis of β-carotene. The first apocarotenoid formed by carotenoid cleavage oxygenase (TSP3) is further cleaved down to C18 trisporoid compounds. A double black arrow indicates more than one step is involved in formation of the subsequent metabolite.
The collaborative biogenesis of trisporic acids and induction of carotenogenesis during the sexual phase takes place by the exchange of trisporoids between complementary mating partners. The role of individual trisporoids as stimulators of the feedback loop are not known. Hence, we explored biochemically how the synthetic intermediates and natural trisporoids differentially modulate β-carotene production in plus and minus mating types among M. mucedo (Mucoraceae) and B. trispora (Choanephoraceae), which belong to 2 different families (26). Functional characterization of the carotenoid cleavage dioxygenase (TSP3) was performed to identify the first apocarotenoid product formed in B. trispora. We conducted time series transcriptional analyses of carRA, tsp3, and tsp1 in B. trispora, with these genes treated independently with 3 trisporoids in plus and minus partners. The data were then compared to those for untreated cultures, which naturally produce trisporoids, to better understand the chemical dialect and synergy of pheromones involved in sexual communication.
MATERIALS AND METHODS
Strains and culture conditions.
FSU 331 (+) and FSU 332 (−) were the strains of B. trispora used, while FSU 621 (+) and FSU 620 (−) strains of M. mucedo were obtained from the Jena Microbial Resource Collection (JMRC) at the Friedrich Schiller University and Hans Knoell Institute. Preinoculum cultures were prepared on solid agar plates (9 mm) of induction medium (IM) (27) by plating a single disc of fungal mycelium (1-mm diameter) and growing the individual strains for 80 h. Spores were collected in distilled water and counted by using a hemocytometer. Carotene induction assays were carried out with 103 spores ml−1 on solid IM agar plates for both species. For transcript analysis, Blakeslea strains of each mating type were inoculated with 108 spores ml−1 for faster growth in 50 ml IM, at 23 to 24°C, in the dark, with a shaker speed of 220 rpm for 144 h. For mated culture experiments, a 1:1 ratio of spore inocula was used.
Carotene induction assay.
Three independent solid agar cultures of each of the mating types of B. trispora and M. mucedo were treated with 20 different stimulants suspended in 200 ml liter−1 ethanol by using an aerosol spray flask. Three solvent control cultures treated with ethanol were maintained for each strain. The cultures were further incubated for 44 h at 22°C in the dark before extraction. The fresh weight of the biomass was determined, and samples treated with 300 μl of chloroform were sonicated for 10 min at 38°C. The chloroform phase was then completely removed, and 20 μl per sample was analyzed by reverse-phase high-performance liquid chromatography (HPLC; pump 525, autosampler 560, diode array detector [DAD] 440; software from Kroma System 2000 and Kontron Instruments). Separation was carried out using a CC 250/4 Nucleosil 120-5 C18 column (Macherey-Nagel, Düren, Germany) with acetone-water (70:30) at a flow rate of 1 ml min−1 at 22°C. The elution profile was monitored with a DAD 440 (Kontron Systems) with an absorbance maximum for β-carotene in acetone of 453 nm. Concentrations were calculated based on calibration against a β-carotene standard. The recovery of β-carotene was determined from mycelial extracts to which known amounts of β-carotene (Sigma) and β-apo-8′-carotenal (Sigma) as the internal standard had been added before extraction. The average recovery was approximately 90% of the initial amount added. The procedure was carried out under dim light.
cDNA cloning.
The in vivo carotenoid cleavage assays were carried out in Escherichia coli strain JM109, as it possesses a stable genotype pβ-carotene plasmid engineered with a gene cluster of 4 enzymes of Erwinia carotovora, i.e., CrtB (geranyl geranyl diphosphate), CrtE (phytoene synthase), CrtI (phytoene desaturase), and CrtY (lycopene cyclase) (28–30). The pβ-carotene plasmid is a pACYC177 low-copy-number plasmid with constitutive expression and includes a kanamycin resistance marker (14, 31). The cDNA of B. trispora was amplified with the full-length tsp3 primers (see Table S1 in the supplemental material) by using Accuprime Taq polymerase (Invitrogen) and subjected to 3′A-overhang addition on postamplified product by following the manufacturer's instructions. The product was further ligated into the pBAD/Topo Thio vector (Invitrogen), which has an ampicillin resistance marker. The integrity of the product was verified by sequencing.
In vivo enzyme assay.
The pβ-carotene plasmid was cotransformed with pBAD-TSP3 in E. coli JM109 chemically competent cells. Overnight cultures were grown at 28°C with a shaker speed of 250 rpm in Luria-Bertani (LB) broth and induced with 0.08%, 0.2%, or 2% arabinose (Sigma) at an optical density at 600 nm of 0.5. Samples were collected at hour 0, 4, 16, and 24 after arabinose induction. A positive control without the pβ-carotene plasmid and with 2% arabinose induction and a negative control without TSP3 were maintained along with a no-arabinose control to check for leaky expression (see Fig. S2 in the supplemental material). Cell pellets were obtained by centrifugation at 2,599 × g (rotor radius, 92.79 mm) at 4°C for 20 min and resuspended in HPLC-grade acetone. After centrifugation, the supernatant was dried and dissolved in the HPLC solvent mixture, followed by HPLC and LC-mass spectrometry (MS) analysis.
Chromatography and mass spectrometry.
HPLC was conducted on an HP1100 system equipped with a photodiode array detector and an automatic sample injector. The separations were carried out by using a Bischoff C30 reverse-phase column (250 mm by 4.6 mm by 3 μm) with methanol (A) and methyl tertiary butyl ether (B) as the solvents (32). The column was developed at a flow rate of 1 ml/min with 20% B initially for up to 5 min. A gradient was maintained within 5 min to 90% B, with a 1-min hold time, and then switched to the initial 20% B until the end of the run time. The standard compound β-carotene was purchased from Sigma-Aldrich (Seelze, Germany), and β-apo-12′-carotenal was obtained from Carotenature (Lupsingen, Switzerland). Both standards and samples were treated with the same solvent mixture with an injection volume of 15 μl and monitored at three wavelengths, 420, 450, and 461.4 nm. The chromatographic spectra were acquired by using the Chemstation software package. Mass spectrometry was carried out with an LCQ mass spectrometer with an APCI interface (Finnigan MAT, Bremen, Germany). The capillary temperature was set at 160°C, and the vaporizer temperature was 450°C.
Transcript analysis.
Three independent liquid culture experiments with plus, minus, and mated (+/−) cultures of Blakeslea trispora were carried out for the transcript analysis of genes involved in carotenoid metabolism. RNA was extracted from about 100 mg of the stored frozen sample by using TRIzol reagent (Invitrogen). Genomic DNA contamination was avoided by including a Turbo DNase treatment (Ambion). The quality and integrity of RNA samples were ensured by including a formaldehyde–Tris-acetate-EDTA (TAE) agarose gel run prior to cDNA synthesis with SuperScript III reverse transcriptase (Invitrogen) (33, 34). The target genes were carRA (phytoene synthase and lycopene cyclase), the carotenoid cleavage gene tsp3 (carotenoid cleavage oxygenase), and tsp1 (4-dihydromethyltrisporate dehydrogenase). Among the four housekeeping genes, gpd (glyceraldehyde phosphate dehydrogenase), EF-1α (α subunit of translation elongation factor 1), pyrG (orotidine-5′-monophosphate decarboxylase), and act-1 (actin) were selected for normalization and relative quantification of data. act-1 had the most stable gene expression and hence was chosen as the internal standard for data analysis, based on the Minimum Information for Publication of Quantitative Real-Time PCR Experiments guidelines (35). Moreover, we performed transcript analysis of carRA in mated cultures of B. trispora to choose the optimal growth phase as the basal time point for determining the relative fold change in gene expression (data not shown) (36). Primers with amplicon sizes ranging between 91 and 165 bp were designed using Primer-BLAST software from NCBI. Vector NTI software was used to select the best available primer pair with the least number of potential secondary structures in the amplicon and with a GC content of 46 to 70%. In order to estimate PCR efficiency, all selected primers (synthesized by Eurofins [Ebersberg, Germany]) were run with pooled samples in 5-fold dilution series over five points (data not shown) (37). Built-in software from Stratagene (Mx 3000P) was used to construct a standard curve for each primer pair, and the efficiency was determined. Quantitative PCR (qPCR) assays were always carried out using three technical replicates for each of the three independent biological replicates, and a no-reverse transcriptase control (NRT) was included for every sample along with a no-template control (NTC) for every primer pair used in each run, using the Brilliant II SYBR green qPCR kit (Agilent). A high annealing temperature of 60°C with 35 cycles was optimized as the run condition in order to minimize nonspecific binding and competition for the substrate. The details for the primers used for the real-time analysis are provided in Table S1 of the supplemental material.
RESULTS
Trisporoids and probable intermediates (synthetics) differentially influence β-carotene production in B. trispora and M. mucedo.
The individual mating partners of B. trispora secrete trisporoids along with a mixture of trisporic acids that stimulate carotenogenesis with the minus partner and hardly influence the plus partner (38). This finding motivated us to perform carotene induction assays using individual apocarotenoids on heterothallic B. trispora and M. mucedo to assess whether the “chemical dialect” enforced by those metabolites are unique among species that seem to have similar mechanisms of trisporoid synthesis in the dark.
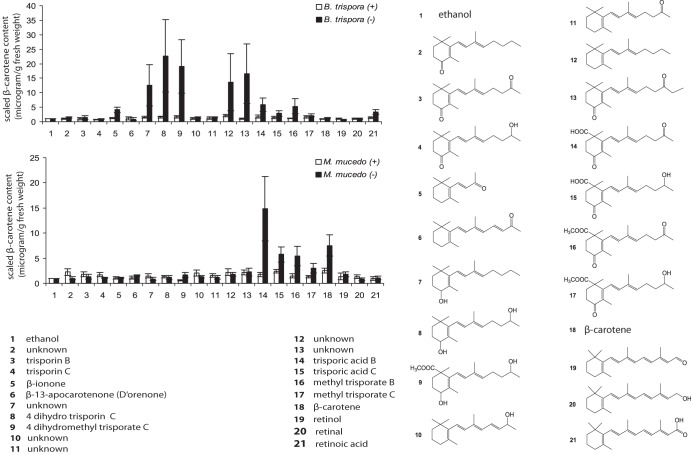
Among the 21 test compounds, including a control solvent of ethanol (compound 1), trisporic acid B (TSAB) (compound 14) (Fig 2) was the most active inducer of carotenogenesis in M. mucedo (−), even at the low concentration of 14 μg ml−1 in solid agar plates of fungal cultures (data not shown). Therefore, preincubated (for 90 h) individual mating partners were treated with the stimulants at 14 μg ml−1 per plate, incubated for an additional 44 h, and extracted to quantitatively analyze β-carotene production. All of those compounds enlisted, compounds 2, 7, 10, 11, 12, and 13, were synthesized in our laboratory (39). We presumed they are the probable trisporoid intermediates, based on our synthetic approach, as none of them had yet been isolated or reported as a natural product from any mucoralean members, suggesting their low abundance, instability, or quick biotransformations to sex hormones. Hence, supplementing those synthetic trisporoids at an arbitrary concentration was the only choice to gain an understanding of their impact on physiological traits in fungi. Likewise, trisporin B (compound 3) and C (compound 4), β-apo-13-carotenone (D'orenone) (compound 6), and compound 11 had no significant impact on carotene production in the investigated strains. Meanwhile, chain elongation by one carbon generated the synthetic analogue (compound 13) of trisporin B (compound 3) as an efficient enhancer of carotenogenesis in B. trispora (−). Unknown compounds 7, 12, and 13, 4-dihydrotrisporin C (compound 8), and its methyl derivative, 4-dihydromethyl trisporic acid C (compound 9), enhanced the metabolite level only in B. trispora (−).
Fig 2.
β-Carotene production in B. trispora and M. mucedo treated with (14 μg/plate) apocarotenoids and trisporoids. Graphs were plotted based on mean values from 3 replicates, and error bars indicate standard errors of the means. Among the 21 stimulants, the compounds that are synthetics and have never been reported as natural products were not given names.
Trisporic acid B (compound 14) made a stronger impact than trisporic acid C (TSAC) (compound 15) on β-carotene production in minus partners of M. mucedo and B. trispora. The plus mating partners did not respond to the methyl esters (compounds 16 and 17) in either Mucor or Blakeslea, respectively, while the carotenoid levels were enhanced in the minus mating partners of both organisms. Interestingly, in Mucor the effects of the free acid TSAC (compound 15) on carotene production were comparable to those of methyl TSAB (compound 16). Externally added β-carotene (compound 18) had no effect on B. trispora but stimulated carotenogenesis in M. mucedo (−). The C20 apocarotenoids, namely, retinal (compound 19), retinol (compound 20), and retinoic acid (compound 21), had no impact on carotenogenesis in either Mucorales species. This finding correlated with the results of supplementation experiments in Mucor rouxii in which retinal and retinol were used (40). β-Ionone (compound 5) triggered a weak stimulation of carotenogenesis only for B. trispora (−) and had no influence on M. mucedo.
β-apo-12′-Carotenal (C25) as the first apocarotenoid cleavage product in B. trispora.
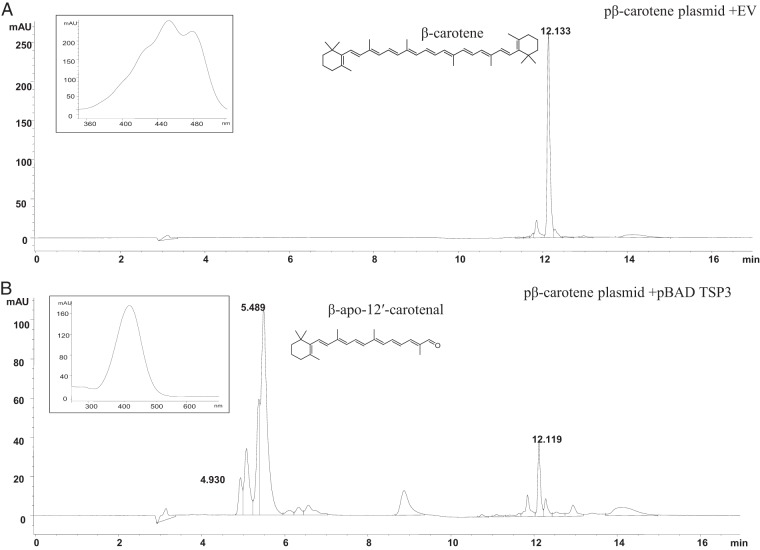
In vivo enzyme assays were carried out in E. coli cells with the β-carotene-overproducing pβ-carotene plasmid coexpressed with pBAD-TSP3. Among the coexpression E. coli broth cultures, 0.08% and 0.2% arabinose induction indicated notable bleaching effects in the pelleted cells (see Fig. S1 in the supplemental material). LC-MS analysis of cells harvested and resuspended in acetone at 0 and 4 h did not indicate the presence of β-carotene or any other apocarotenoid, while after 24 h, the acetone extracts of pelleted cells showed the presence of the C25 compound β-12′-apocarotenal, along with the initial substrate, β-carotene, by HPLC (Fig. 3). The maximum absorption wavelength (λmax) was 422 nm for β-apo-12′-carotenal and 450 nm for β-carotene. The compounds were identified using authentic standards. β-apo-12′-Carotenal showed the characteristic UV absorption and the expected molecular ion (M+H+) at m/z 351.10 (see Fig. S2 in the supplemental material).
Fig 3.
HPLC chromatogram from the in vivo enzyme assay with TSP3 coexpressed along with the β-carotene-producing plasmid after 24 h and the EV-empty pBAD vector. The inset show the UV-visible spectra for β-carotene substrate (A) and β-apo-12′-carotenal (B).
Time series analyses of carotenoid metabolic gene expression in Blakeslea trispora.
Unlike M. mucedo, B. trispora produces 1,000 times more trisporic acids, and a putative nonheme carotenoid cleavage oxygenase (TSP3) was reported as the first enzyme involved in sex hormone synthesis (16). Hence, we performed transcript analysis in B. trispora for carRA, tsp3, and tsp1, genes involved in three different stages of carotenoid metabolism, and to identify how the developmental phases induce temporal trends in gene expression. The fold change in target gene expression normalized to that of the internal standard, β-actin, relative to expression at time zero (12 h after incubation) was carried out for the time series analysis for up to 144 h with a 24-h interval, as per the 2−ΔCT method (41). carRA (Fig. 4A) was significantly upregulated in B. trispora (−), with a 160-fold increase (P < 0.001) after 144 h of incubation, while low and constitutive transcript levels up to 25-fold higher (P = 0.002) were observed with the plus strain. There was no statistically significant difference in carRA transcript levels beyond 48 h of growth in mated (+/−) or minus cultures. The expression of tsp3 was 16,000-fold higher at 48 h in (+/−) cultures and declined to 7,000-fold after 144 h of incubation (Fig. 4B). In plus cultures, the gene tsp3 was constitutively upregulated for up to 72 h and showed a progressive increase, with a maximum 400-fold increase at 120 h, declining to 200-fold at 144 h. A statistically significant difference was observed for the 6 time points under investigation and among the 3 culture types, i.e., plus, minus, and mated cultures, based on their 3 independent biological replicates in tsp3 transcripts (n = 18; P = 0.028). Curiously, the mRNA level of tsp1, the penultimate enzyme proposed in trisporic acid biogenesis, exhibited downregulation irrespective of asexual or sexual phase in B. trispora (Fig. 4C). Our results indicated that carRA and tsp3 transcripts vary during the asexual and sexual developmental phases of fungal growth in submerged liquid induction medium, based on their diverse physiological functions.
Fig 4.
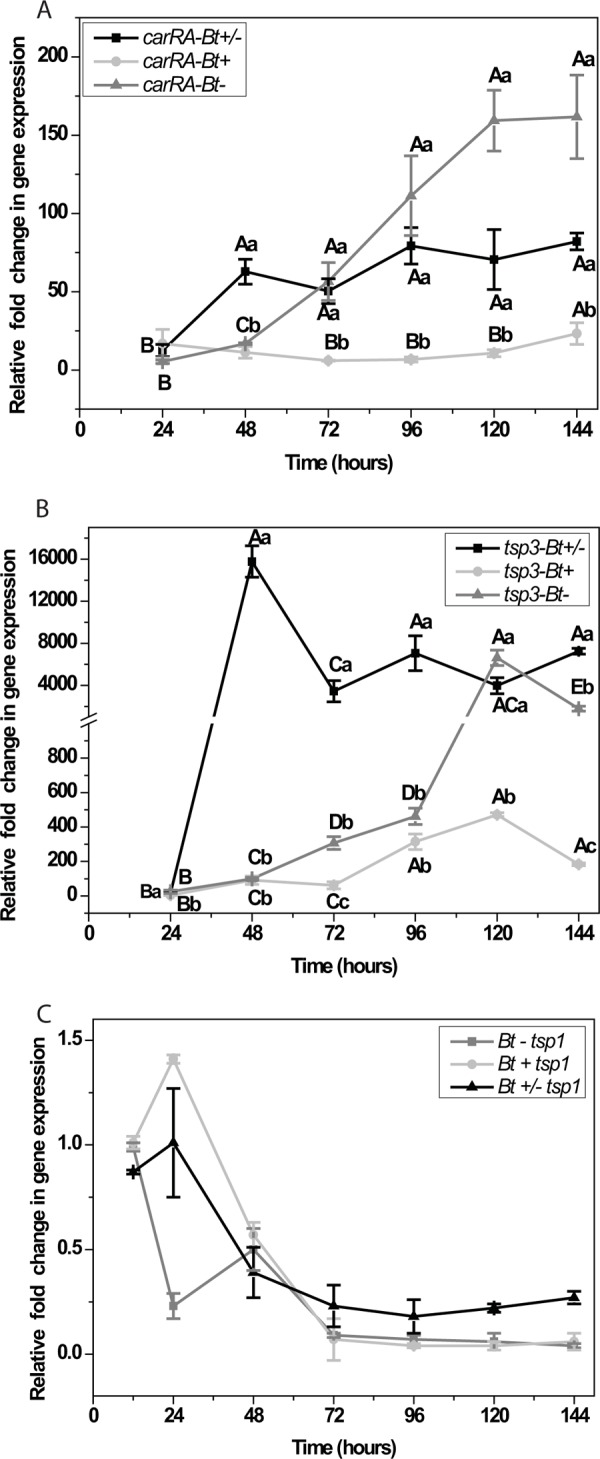
Real-time qRT-PCR analyses of the dynamics of carRA (A), tsp3 (B), and tsp1 (C) in Blakeslea trispora plus (Bt+), minus (Bt−), and mated (Bt+/−) cultures. The 2−ΔCT method was used for the relative quantification of gene expression, using actin as the internal standard. Graphs are plotted with the mean values ± standard errors of the means from 3 biological replicates. Statistical analysis was conducted by using a 2-way ANOVA with 2 independent factors, time and mating type, along with the dependent variable, the log-transformed (log 10) values of the relative fold change. The small letters indicate significant differences (P < 0.05) based on Tukey's test (post hoc) at each time point among the 3 mating types. Capital letters indicate significant differences (P < 0.05) between different time points for a single mating type. No significant difference was observed in tsp1, irrespective of time and mating type.
Individual trisporoids differentially regulate gene expression in B. trispora mating partners.
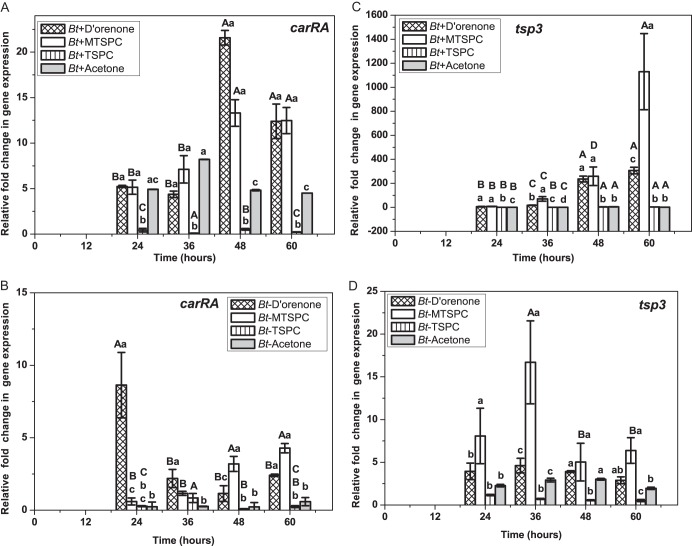
Until now, all research on biological functions of trisporic acids and trisporins was limited either to carotenogenesis or the potential for the development of sexual structures known as zygophores in M. mucedo or P. blakesleeanus. We speculated that the prominent fluctuations of tsp3 transcripts in B. trispora during the sexual phase imply the synergistic effects of de novo trisporoids regulating a positive feedback metabolic loop (Fig. 4B). In order to understand the genetic potential of individual trisporoids as sexual stimulants, real-time PCR transcript analysis of carRA and tsp3 were conducted in plus and minus strains after treating them with methyl trisporate C (compound 17), D'orenone (compound 6), and trisporin C (compound 4), which are formed at early and late stages of trisporic acid biogenesis (Fig. 5). The trisporoids were treated at 12 h after spore incubation, and temporal trends for the relative fold change in gene expression were determined for up to 60 h of incubation as mentioned above, but here the time interval of sample collection was shortened to 12 h.
Fig 5.
Transcript data analysis for carRA (A and B) and tsp3 (C and D) after treatment with trisporoids in plus and minus B. trispora cultures. Graphs were plotted as means ± standard errors of the means for 3 independent biological replicates. A statistically significant difference was observed among the 4 trisporoids treatments at the 4 different time points only with B. trispora (−) tsp3 (D) gene expression (ANOVA; P = 0.018; n = 4 × 4 × 3). Student -Newman-Keul's post hoc comparison showed a significant difference for D'orenone with MTSPC (P = 0.044), MTSPC with TSPC (P = 0.015), and MTSPC with acetone (P = 0.037). All treatments showed statistically significant differences (P < 0.001; n = 4 × 3) among each other at the 4 designated time points (in small letters); capital letters indicate statistically significant differences for each treatment (P = 0.002; n = 4 × 3) at 24 h, 48 h, and 60 h).
Ethanol, methanol, and isopropanol are active solvent stimulants of microbial carotenogenesis (4). Therefore, the sparingly water-soluble trisporoids were used in acetone solution (50 μM, final concentration) on both mating partners grown independently in 50 ml liquid induction medium at 12 h after incubation. The three tested trisporoids induced statistically significant differences in transcripts (Fig. 5A and C) at each of the 4 given time points in the plus partners (P < 0.001; n = 4 × 3, or 12), whereas in minus partners, transcript levels differed significantly among the three treatments at 24 h (P = 0.002), 48 h (P = 0.001), and 60 h (P = 0.007). carRA gene expression varied with each of the trisporoid treatments among the 4 time points (n = 4 × 3, or 12) with D'orenone (P = 0.016), methyl trisporate C (MTSPC; P < 0.001), trisporin C (TSPC; P = 0.043) in minus strains and D'orenone (P < 0.001), MTSPC (P = 0.003), and TSPC (P = 0.033) in plus strains (Fig. 5A and B). However, MTSPC and D'orenone imposed unique temporal trends (Fig. 5B) of differential expression in minus strains. The first C18 trisporoid, D'orenone, and methyl trisporate C induced carotene cleavage in the plus mating type (Fig. 5C) but had no impact on carotenogenesis (Fig. 2). The impact of trisporins (compounds 3 and 4) were negligible in both mating partners, in comparison to that of D'orenone or β-apo-13-carotenone (compound 6).
At 48 h after supplementation (60 h after incubation) with D'orenone, the tsp3 transcript levels in B. trispora (+) rose 325-fold (Fig. 5C). All treatments induced statistically significant differences (P = 0.009; n = 4 × 3) after 24 h, 36 h (P = 0.003), 36 h (P = 0.027), and 48 h (P = 0.029). The influence of each trisporoid on the B. trispora (+) tsp3 transcript levels differed significantly among the different time points (n = 4 × 3), i.e., D‘orenone (P = 0.003), MTSPC (P = 0.001), and TSPC (P < 0.001). Contrary to its impact on carRA, MTSPC was the major activator of tsp3 expression in both mating types, with a maximum 1,200-fold increase in plus partners at 60 h after incubation (Fig. 5C). The maximum expression in B. trispora (−) was at 36 h by both MTSPC and D'orenone, with 17.5-fold and 4.8-fold increases, respectively (Fig. 5D). tsp3 gene expression significantly varied among the treatments, including the control-only treatment for B. trispora (−), based on consideration of the different time points as a single variable (analysis of variance [ANOVA], P = 0.018; n = 4 × 4 × 3).
DISCUSSION
The cocktail of trisporic acids, designated A, B, C, D, E based on the substitution pattern at C15 and functional groups attached to the ring (C4), was discovered in the 1960s, and since then this cocktail has been extensively studied as the exclusive sex hormones in mucoralean fungi. These C18 apocarotenoid hormones regulate the sexual phase and positive feedback metabolism of β-carotene. Except for the zygophore-inducing effects of trisporins in P. blakesleeanus, no information was available about the function of individual trisporoid metabolites among Mucorales. We adopted the chemical approach by treating 4 different genotypes with diverse trisporoids and probable intermediates involved in the pathway to assess their potentials in feedback regulation. Our data support the hypothesis that the mode of action of each trisporoid varies among genotypes and growth phases, and this in turn led to the concept of a “chemical dialect.”
Apocarotenoid precursors as signaling molecules in Mucorales.
Peptide hormones act as signaling molecules in Ascomycota and Basidiomycota, while nonpeptide apocarotenoids known as trisporoids are the exclusive sex pheromones within the order Mucorales of the subphylum Muoromycotina (42). No information is yet available about their receptors of pheromone perception and hence signals transduction pathways. Among the enlisted stimulants used for carotene induction assay, trisporins, methyl trisporates, and dihydromethyl trisporates were the natural pheromones isolated from Phycomyces, M. mucedo, and Blakeslea. The addition of excess amounts of trisporin B (10−4 M) triggered the early phases of mating in P. blakesleeanus but failed to produce mature zygospores (43). Methyl trisporates extracted from B. trispora were highly active in bioassays for zygophore induction on the minus but not on the plus partners in M. mucedo and P. blakesleeanus (44). Synthetic trisporoids possessing a polar hydroxyl group at C4, i.e., compounds 7, 8, and 9, were the most active stimulators of carotenogenesis in Blakeslea. Why those compounds did not activate M. mucedo is so far unclear (Fig. 2), and experiments should be done on additional Mucorales members to figure out the mechanism of action. The induction effect of synthetic analogues (12, 13) in B. trispora is curious, and further studies on the biological functions of these metabolites are essential to make valid conclusions. The inefficiency of stimulants on β-carotene production in plus strains of both Blakeslea and M. mucedo (Fig. 2) supports the hypothesis that a suppressor gene (44) is associated with the plus mating partners. All compounds represented a mixture of E/Z isomers and were of 95% purity. In natural trisporoid extracts, both isomers were detected. Therefore, the isomer mixture of each compound mimics the natural situation but this may not be true for the concentrations.
The trisporic acid biosynthetic pathway is quite complex because of the diverse metabolites (Fig. 1) along with the morphological and physiological changes that take place among the mating partners. Molecular genetics approaches were limited to early steps in the mevalonate pathway due to the inadequacy of protein and DNA database information on downstream processes. Transcript-level analysis and relative quantification were previously carried out for the carotenogenic genes carRA and carB in individual mating partners and mated cultures of B. trispora incubated for up to 3 days, with a basal time point of 24 h after inoculation for data analysis (45, 46). Hence, we focused upon an extended time series transcript analyses of the 3 functional genes in B. trispora, one involved in β-carotene production (carRA), and 2 others in trisporic acid biogenesis (tsp3 and tsp1) for testing the null hypothesis, that trisporoids generated by mating partners do not differentially regulate gene transcription during the asexual and sexual developmental phases (Fig. 4A, B, and C). A statistically significant difference in carRA gene upregulation was observed only at 48 h among B. trispora (−) and mated cultures, and this may be because of activation of molecular signaling cascades involved in early steps of the TSA pathway in the sexual phase. Constitutive gene expression beyond 72 h in the mated phase supports the ongoing trisporoid-regulating feedback loop, while it is reasonable to have higher transcript levels for minus strains that produce more β-carotene and negligible trisporic acids compared to the plus strain or mated culture in B. trispora. The plus strain known to produce 0.1% of trisporic acids compared to mated cultures (100%) in B. trispora had progressive transcript turnover in carRA on a temporal trend, and this supports the hypothesis that trisporoids synergistically enhances carotenogenesis at the genetic level (Fig. 4A).
The carotenoid cleavage oxygenase (tsp3) of B. trispora had transcript turnover when plus and minus strains were grown in the dark upon treatment with 14 μg ml−1 trisporic acid B (16). In our time series experiments without any stimulation, tsp3 transcripts showed significant upregulation over the growth phases, extending up to 144 h or 6 days in plus and minus strains. The striking increase of 16,000-fold at 48 h (Fig. 4B) exclusively in mated cultures and the further decline to 4,000-fold by 72 h exemplifies the transcriptional bursts associated with dynamic biological networks controlled by molecular signals in eukaryotes (47). A stable state of equilibrium might have been acquired through the activity of functional proteins and metabolites involved in the TSA pathway beyond 72 h, thereby maintaining a constitutive transcript level. Through in vivo enzyme assays we identified β-apo-12′-carotenal, formed by TSP3 activity, which suggested that the specificity of β-carotene cleavage at C11′—C12′ (see Fig. S2 in the supplemental material) is conserved among the 2 members in the order Mucorales, namely, B. trispora and P. blakesleeanus. Since both species are not closely related, even belonging to different families (Choanephoraceae and Phycomycetaceae), it can be presumed that this specific carotene cleavage is conserved throughout the whole order.
Both mating partners of M. mucedo expressed equal transcript levels and translation of TSP1, but a slightly induced activity was only associated with the M. mucedo (−) partner after 60 to 80 min of trisporic acid treatment, suggesting a role for posttranslational gene regulation (21, 48). TSP1 transcript turnover was constitutive in both B. trispora (Fig. 4C) and P. blakesleeanus (Kerstin et al., unpublished data) irrespective of sexual or asexual phases. However, we did not observe any significant changes in transcript levels of B. trispora tsp1, even with developmental phases during sexual stages (Fig. 4C), suggesting posttranscriptional modifications. Hence, we did not perform further transcript analysis of tsp1 after trisporoid treatment.
Trisporins (compounds 3 and 4) activate zygophore development at millimolar concentrations (43), and methyl trisporates (compounds 16 and 17) enhance carotenogenesis even at micromolar levels (49); these are the exclusive morphogenetic factors in the mucoralean sexual phase, while β-apo-13-carotenone (D'orenone) (compound 6) is an unexplored compound. The latter compound did not stimulate carotenogenesis in solid cultures of either Blakeslea or Mucor treated with a lower concentration of 14 μg ml−1 (Fig. 2), but at 50 μM it induced transcript levels of carRA and tsp3 in Blakeslea (Fig. 5). Such discrepancies with transcript levels and secondary metabolites are known in other members of the Mucorales (50). The biological function of D'orenone is known to be as an apocarotenoid that inhibits root elongation in Arabidopsis and is a member of the strigolactone biosynthetic pathway (51). The inefficiency of trisporin C, either in carotenogenesis at 14 μg ml−1 (Fig. 2) or in transcription at an even higher concentration of 50 μM (Fig. 5) throws light on its differential effects and dose dependency, which varies with genus, species, or strain, as proposed in the pheromone-action-unitary theory (44). It is interesting that the early trisporoid triggered carRA transcripts while late trisporoid MTSPC influenced tsp3. In general, the higher transcript levels in mated cultures without trisporoid stimulation (Fig. 4A and B) for the corresponding genes explain the synergistic effects of these metabolite cocktails that are naturally present in fungal partners. Trisporoids and apocarotenoids as signaling molecules determine trends in the transcription of genes associated with the carotenoid metabolic network throughout the developmental phase of B. trispora.
Fungal communication plays a vital role in growth, development, morphogenesis, mating, activation of virulence factors, and pathogenesis. The heterothallic Mucorales-like Rhizopus stolonifer and Mucor piriformis are causal agents for postharvest diseases in fruits and vegetables (52). Mucor sp., Rhizopus oryzae, and Lichtheimia sp. are potential pathogens that cause rapidly emerging mucoromycosis in immunocompromised humans in tropical and temperate areas (53–55). Hence, it is important to unravel the molecular mechanisms of trisporoid signaling in Mucorales in order to determine the potential role of sexual phase in pathogenesis and survival within the host. The current study has demonstrated a broad diversity of dynamics and biological effects of the early and late trisporoids on gene expression and secondary metabolism in plus, minus, and mated cultures of the two mucoralean representatives. The elucidation of similarities and differences in the expression of genes involved in carotenoid degradation and the participation of their gene products in sexual and asexual interactions are additional essential elements of the complex language between different zygomycetes (see Table S2 in the supplemental material). For the future, it is necessary to establish the genetic basis of differences between the plus and minus strains in response to individual trisporoids. M. mucedo treated with trisporoid stimulants had higher transcript levels of sexM than of sexP (25). Parallel studies in B. trispora and other members (including homothallic ones) of the order Mucorales will promote the elucidation of genetic variations and contrasts among partners that are otherwise morphologically indistinguishable.
Supplementary Material
ACKNOWLEDGMENTS
We are thankful to A. David for maintaining fungal cultures, G. H. Jiménez-Alemán for repurifying the trisporoids, M. Reichelt, K. Ploss, and M. Kunert for technical support for chromatographic analyses, S. A. Babili (Albert Ludwig University, Freiburg, Germany) for the pβ-carotene plasmid. We also acknowledge E. Wheeler (Boston, MA) for editing the manuscript.
We acknowledge the Max Planck Gesselschaft for financial support.
Footnotes
Published ahead of print 20 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02096-13.
REFERENCES
- 1.Kirk PM, Cannon PF, Minter DW, Stalpers JA. 2008. Ainsworth & Bisby's dictionary of the fungi, 10th ed. CAB International, Wallingford, United Kingdom [Google Scholar]
- 2.Schachtschabel D, Menzel K, Krauter G, David A, Roth M, Horn U, Boland W, Wöstemeyer J, Schimek C. 2010. Production and derivate composition of trisporoids in extended fermentation of Blakeslea trispora. Appl. Microbiol. Biotechnol. 88:241–249 [DOI] [PubMed] [Google Scholar]
- 3.Chaudhary S, Polaino S, Shakya VPS, Idnurm A. 2013. A new genetic linkage map of the zygomycete fungus Phycomyces blakesleeanus. PLoS One 8(3):e58931. 10.1371/journal.pone.0058931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhosale P. 2004. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl. Microbiol. Biotechnol. 63:351–361 [DOI] [PubMed] [Google Scholar]
- 5.López-Nieto MJ, Costa J, Peiro E, Mendez E, Rodríguez-Sáiz M, de la Fuente JL, Cabri W, Barredo JL. 2004. Biotechnological lycopene production by mated fermentation of Blakeslea trispora. Appl. Environ. Microbiol. 66:153–159 [DOI] [PubMed] [Google Scholar]
- 6.Kloer DP, Schulz GE. 2006. Structural and biological aspects of carotenoid cleavage. Cell. Mol. Life Sci. 63:2291–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bu'lock JD, Winstanley DJ, Drake D. 1972. Specificity and transformations of trisporic acid series of fungal sex-hormones. Phytochemistry 11:2011–2018 [Google Scholar]
- 8.van den Ende H, van den Briel M, Werkman BA. 1972. Trisporic acid synthesis in mated cultures of fungus Blakeslea trispora. Arch. Mikrobiol. 86:175–179 [DOI] [PubMed] [Google Scholar]
- 9.Feofilova EP. 2006. Heterothallism of mucoraceous fungi: a review of biological implications and uses in biotechnology. Appl. Biochem. Microbiol. 42:439–454 [PubMed] [Google Scholar]
- 10.Caglioti L, Cainelli G, Camerino B, Mondelli R, Prieto A, Quilico A, Salvator T, Selva A. 1966. The structure of trisporic C acid. Tetrahedron 22:175–1875927157 [Google Scholar]
- 11.Thomas DM, Goodwin TW. 1967. Studies on carotenogenesis in Blakeslea trispora. I. General observations on synthesis in mated and unmated strains. Phytochemistry 6:355–360 [Google Scholar]
- 12.Sieiro C, Poza M, de Miguel T, Villa TG. 2003. Genetic basis of microbial carotenogenesis. Int. Microbiol. 6:11–16 [DOI] [PubMed] [Google Scholar]
- 13.Velayos A, Blasco JL, Alvarez MI, Iturriaga EA, Eslava AP. 2000. Blue-light regulation of phytoene dehydrogenase (carB) gene expression in Mucor circinelloides. Planta 210:938–946 [DOI] [PubMed] [Google Scholar]
- 14.Medina HR, Cerdá-Olmedo E, Al-Babili S. 2011. Cleavage oxygenases for the biosynthesis of trisporoids and other apocarotenoids in Phycomyces. Mol. Microbiol. 82:199–208 [DOI] [PubMed] [Google Scholar]
- 15.Tagua VG, Medina HR, Martin-Domingúez R, Eslava AP, Corrochano LM, Cerdá-Olmedo E, Idnurm A. 2012. A gene for carotene cleavage required for pheromone biosynthesis and carotene regulation in the fungus Phycomyces blakesleeanus. Fungal Genet. Biol. 49:398–404 [DOI] [PubMed] [Google Scholar]
- 16.Burmester A, Richter M, Schultze K, Voelz K, Schachtschabel D, Boland W, Wöstemeyer J, Schimek C. 2007. Cleavage of beta-carotene as the first step in sexual hormone synthesis in zygomycetes is mediated by a trisporic acid regulated beta-carotene oxygenase. Fungal Genet. Biol. 44:1096–1108 [DOI] [PubMed] [Google Scholar]
- 17.Sutter RP, Capage DA, Harrison TL, Keen WA. 1973. Trisporic acid biosynthesis in separate plus and minus cultures of Blakeslea trispora: identification by Mucor assay of two mating-type-specific components. J. Bacteriol. 114:1074–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polaino S, Herrador MM, Cerdá-Olmedo E, Barrero AF. 2010. Splitting of beta-carotene in the sexual interaction of Phycomyces. Org. Biomol. Chem. 8:4229–4231 [DOI] [PubMed] [Google Scholar]
- 19.Schachtschabel D, David A, Menzel KD, Schimek C, Wöstemeyer J, Boland W. 2008. Cooperative biosynthesis of trisporoids by the (+) and (−) mating types of the zygomycete Blakeslea trispora. ChemBioChem 9:3004–3012 [DOI] [PubMed] [Google Scholar]
- 20.Czempinski K, Kruft V, Wöstemeyer J, Burmester A. 1996. 4-Dihydramethyltrisporate dehydrogenase from Mucor mucedo, an enzyme of the sexual hormone pathway: purification, and cloning of the corresponding gene. Microbiology 142:2647–2654 [DOI] [PubMed] [Google Scholar]
- 21.Schimek C, Petzold A, Schultze K, Wetzel J, Wolschendorf F, Burmester A, Wöstemeyer J. 2005. 4-Dihydromethyltrisporate dehydrogenase, an enzyme of the sex hormone pathway in Mucor mucedo, is constitutively transcribed but its activity is differently regulated in (+) and (−) mating types. Fungal Genet. Biol. 42:804–812 [DOI] [PubMed] [Google Scholar]
- 22.Wetzel J, Scheibner O, Burmester A, Schimek C, Wöstemeyer J. 2009. 4-Dihydrotrisporin-dehydrogenase, an enzyme of the sex hormone pathway of Mucor mucedo: purification, cloning of the corresponding gene, and developmental expression. Eukaryot. Cell 8:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werkman BA. 1976. Localisation and partial characterisation of a sex-specific enzyme in homothallic and heterothallic mucorales. Arch. Microbiol. 109:209–213 [Google Scholar]
- 24.Idnurm A, Walton FJ, Floyd A, Heitman J. 2008. Identification of the sex genes in an early diverged fungus. Nature 451:193–198 [DOI] [PubMed] [Google Scholar]
- 25.Wetzel J, Burmester A, Kolbe M, Wöstemeyer J. 2012. The mating-related loci sexM and sexP of the zygomycetous fungus Mucor mucedo and their transcriptional regulation by trisporoid pheromones. Microbiology 158:1016–1023 [DOI] [PubMed] [Google Scholar]
- 26.Voigt K. 2012. Zygomycota, p 130–162 In Frey W. (ed), Syllabus of plant families—A. Engler's Syllabus der Pflanzenfamilien. Part 1/1: blue-green algae, Myxomycetes and Myxomycete-like organisms, phytoparasitic protists, heterotrophic heterokontobionta and fungi p.p. Borntraeger Verlag, Stuttgart, Germany [Google Scholar]
- 27.Schimek C, Kleppe K, Saleem AR, Voigt K, Burmester A, Wöstemeyer J. 2003. Sexual reactions in Mortierellales are mediated by the trisporic acid system. Mycol. Res. 107:736–747 [DOI] [PubMed] [Google Scholar]
- 28.von Lintig J, Vogt K. 2000. Filling the gap in vitamin A research: molecular identification of an enzyme cleaving beta-carotene to retinal. J. Biol. Chem. 275:11915–11920 [DOI] [PubMed] [Google Scholar]
- 29.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55:611–622 [DOI] [PubMed] [Google Scholar]
- 30.Campos-Takaki GM, Dietrich SMC. 2009. Characterization of cell walls from mucoralean fungi by biochemical composition, transmission electron microscopy and X-ray microanalysis. [Google Scholar]
- 31.Misawa N, Nakagawa M, Kobayashi K, Yamano S, Izawa Y, Nakamura K, Harashima K. 1990. Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli J. Bacteriol. 172:6704–6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacker T, Strohschein S, Albert K. 1999. Separation and identification of various carotenoids by C30 reversed-phase high-performance liquid chromatography coupled to UV and atmospheric pressure chemical ionization mass spectrometric detection. J. Chromatogr. 854:37–44 [DOI] [PubMed] [Google Scholar]
- 33.Masek T, Vopalensky V, Suchomelova P, Pospisek M. 2005. Denaturing RNA electrophoresis in TAE agarose gels. Anal. Biochem. 336:46–50 [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed, vol 3 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 35.Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M. 2010. A practical approach to RT-qPCR: publishing data that conform to the MIQE guidelines. Methods 50:S1–S5 [DOI] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 37.Nolan T, Hands RE, Bustin SA. 2006. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 1:1559–1582 [DOI] [PubMed] [Google Scholar]
- 38.Thomas DM, Harris RC, Kirk JTO, Goodwin TW. 1967. Studies on carotenogenesis in Blakeslea trispora. 2. Mode of action of trisporic acid. Phytochemistry 6:361–366 [Google Scholar]
- 39.Schachtschabel D, Boland W. 2007. Efficient generation of a trisporoid library by combination of synthesis and biotransformation. J. Org. Chem. 72:1366–1372 [DOI] [PubMed] [Google Scholar]
- 40.Mosqueda-Cano G, Gutierrez-Corona J. 1995. Environmental and developmental regulation of carotenogenesis in the dimophic fungus Mucor rouxii. Curr. Microbiol. 31:141–145 [Google Scholar]
- 41.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polaino S, Idnurm A. 2012. Sexual pheromones in the fungi, p 171–188 In Witzany G. (ed), Biocommunication of fungi. Springer, Houten, Netherlands [Google Scholar]
- 43.Ootaki T, Yamazaki Y, Noshita T, Takahashi S. 1996. Excess carotenoids disturb prospective cell-cell recognition system in mating responses of Phycomyces blakesleeanus. Mycoscience 37:427–435 [Google Scholar]
- 44.Sutter RP, Whitaker JP. 1981. Zygophore-stimulating precursors (pheromones) of trisporic acids active in (−) Phycomyces blakesleeanus acid-catalysed anhydro derivatives of methyl 4-dihydrotrisporate-C. J. Biol. Chem. 256:2334–2341 [PubMed] [Google Scholar]
- 45.Schmidt AD, Heinekamp T, Matuschek M, Liebmann B, Bollschweiler C, Brakhage AA. 2005. Analysis of mating-dependent transcription of Blakeslea trispora carotenoid biosynthesis genes carB and carRA by quantitative real-time PCR. Appl. Microbiol. Biotechnol. 67:549–555 [DOI] [PubMed] [Google Scholar]
- 46.Kuzina V, Ramirez-Medina H, Visser H, van Ooyen AJ, Cerdá-Olmedo E, van den Berg JA. 2008. Genes involved in carotene synthesis and mating in Blakeslea trispora. Curr. Genet. 54:143–152 [DOI] [PubMed] [Google Scholar]
- 47.Raj A, van Oudenaarden A. 2008. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135:216–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heitman J, Kronstad JW, Taylor JW, Casselton LA. 2007. Mating in the Chytridiomycota and Zygomycota, p 405–418 In Heitmann J. (ed), Sex in fungi: molecular determination and evolutionary implications. ASM Press, Washington, DC [Google Scholar]
- 49.Rao S, Modi VV. 1977. Carotenogenesis: possible mechanism of action of trisporic acid in Blakeslea trispora. Experientia 33:31–33 [Google Scholar]
- 50.Quiles-Rosillo MD, Ruiz-Vázquez RM, Torres-Martínez S, Garre V. 2005. Light induction of the carotenoid biosynthesis pathway in Blakeslea trispora. Fungal Genet. Biol. 42:141–153 [DOI] [PubMed] [Google Scholar]
- 51.Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagés V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, Bouwmeester H, Bécard G, Beveridge CA, Rameau C, Rochange SF. 2008. Strigolactone inhibition of shoot branching. Nature 455:189–194 [DOI] [PubMed] [Google Scholar]
- 52.Reyes AA. 1990. Pathogenicity, growth and sporulation of Mucor mucedo and Botrytis cinerea in cold or CA storage. HortScience 25:549–552 [Google Scholar]
- 53.Li CH, Cervantes M, Springer DJ, Boekhout T, Ruiz-Vázquez RM, Torres-Martinez SR, Heitman J, Lee SC. 2011. Sporangiospore size dimorphism is linked to virulence of Mucor circinelloides. PLoS Pathog. 7(6):e1002086. 10.1371/journal.ppat.1002086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomes MZR, Lewis RE, Kontoyiannis DP. 2011. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin. Microbiol. Rev. 24:411–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun HY, Singh N. 2011. Mucormycosis: its contemporary face and management strategies. Lancet Infect. Dis. 11:301–311 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.