Abstract
Sinorhizobium meliloti requires exopolysaccharides in order to form a successful nitrogen-fixing symbiosis with Medicago species. Additionally, during early stages of symbiosis, S. meliloti is presented with an oxidative burst that must be overcome. Levels of production of the exopolysaccharides succinoglycan (EPS-I) and galactoglucan (EPS-II) were found to correlate positively with survival in hydrogen peroxide (H2O2). H2O2 damage is dependent on the presence of iron and is mitigated when EPS-I and EPS-II mutants are cocultured with cells expressing either exopolysaccharide. Purified EPS-I is able to decrease in vitro levels of H2O2, and this activity is specific to the symbiotically active low-molecular-weight form of EPS-I. This suggests a potential protective function of exopolysaccharides against H2O2 during early symbiosis.
INTRODUCTION
All aerobically growing organisms are exposed to reactive oxygen species (ROS) produced by univalent reduction of oxygen within the cell. Autoxidation of enzymes and leakage from the electron transport chain are two sources of internally produced ROS (1). Extracellular sources of ROS that may enter bacterial cells include the oxidation of extracellular compounds and the secretion of redox-cycling compounds by neighboring organisms (2). In particular, hydrogen peroxide (H2O2) has high membrane permeation; this means that the intracellular level of H2O2 in Escherichia coli is equivalent to the environmental level under most culture conditions (1).
ROS damage major biomolecules in cells: superoxide damages iron-sulfur clusters, inactivating the corresponding proteins; hydroxyl radicals damage DNA, causing cell death; and H2O2, at high concentrations, may damage lipid membranes (1). Natural production of ROS in aerobically growing organisms and environmental exposure to ROS necessitate the production of antioxidants. Superoxide dismutases, which convert superoxide to H2O2, and catalases, which convert H2O2 to water and oxygen, are enzymatic examples of various antioxidants. Additionally, small-molecule antioxidants, such as ascorbate and glutathione, can scavenge ROS (3).
As an obligate aerobe, the nitrogen-fixing plant symbiont Sinorhizobium meliloti must encode mechanisms for preventing ROS-related damage. These mechanisms include 3 catalases and 2 superoxide dismutases as enzymatic protection, as well as small molecules (4).
S. meliloti cells encounter new ROS not only in the soil but also during the establishment of symbiosis with their plant hosts, such as Medicago sativa and Medicago truncatula. This symbiosis begins with complex signal exchanges between plant and bacterial partners. Plant-produced flavonoids initiate the bacterial transcription of nod genes and the consequent bacterial production of Nod factor, which stimulates root nodule morphogenesis. In addition to Nod factor, S. meliloti produces an exopolysaccharide, succinoglycan (EPS-I), that is required for successful bacterial invasion of host tissue through plant-derived infection threads. These are invaginations of the plant cell wall within which bacteria replicate and penetrate into deeper plant cell layers. As the infection threads reach newly divided plant cells in the emerging nodule, bacteria are released from infection threads into the plant cytoplasm, where they terminally differentiate and fix nitrogen (5). The nodule provides the proper environment for nitrogen fixation, including low free-oxygen levels to protect the oxygen-sensitive nitrogenase.
Superoxide and H2O2 are present in infection threads and in fully developed 6-week-old nodules (6). These ROS are likely formed primarily by plant NADPH oxidase (7). While H2O2 can be damaging, it appears to be required for successful infection: reduction of H2O2 levels by overexpression of bacterial catalase results in decreased efficiency of symbiosis and has negative effects on the formation of infection threads (8).
ROS may act in more than one way during nodulation. In the first 2 min of bacterium-host interaction, the levels of ROS in the plant increase rapidly and transiently (9). However, after 5 min, the presence of bacteria or Nod factor has an inhibitory effect on ROS flux (10). Transient changes in the levels of ROS (induced by chemical inhibitors of plant NADPH oxidase) are able to mimic the initial loss and subsequent reinitiation of root hair polar growth that characterizes early symbiosis (11). All of this evidence points to some positive roles for ROS in the S. meliloti– M. truncatula symbiosis.
Exopolysaccharides have been associated with protection against H2O2. Pseudomonas syringae cells devoid of exopolysaccharides are sensitive to ROS (12). In a study of S. meliloti, Davies and Walker (13) carried out a two-part screen for mutants sensitive to H2O2 that were also defective in forming productive nitrogen-fixing nodules on alfalfa ( Medicago sativa). Among the mutants that were both symbiotically deficient and sensitive to H2O2, one-third of the mutated genes (3 out of 9) were involved in the production of EPS-I; additionally, a mutant defective in EPS-I production (the exoY mutant) was sensitive to H2O2 (13). These studies point to a possible connection between ROS and EPS-I in S. meliloti.
The structure of S. meliloti EPS-I consists of repeating units of octasaccharides, each carrying three nonsugar modifications (succinyl, acetyl, and pyruvyl). This EPS-I is synthesized in both a high-molecular-weight (HMW) (hundreds of octasaccharide subunits) and a low-molecular-weight (LMW) (octasaccharide monomers, dimers, and trimers) form (14). The production of these two forms appears to be specified by separate biosynthesis genes (exoQ for the HMW form and exoT for the LMW form), each of which acts in conjunction with an additional gene, exoP (15). Additionally, the LMW form of EPS-I can be produced from the HMW form by the glycanase ExoK (16).
EPS-I production is controlled by noncarbon nutrient limitation (e.g., limitation of nitrogen or phosphorus) (17) and some environmental stresses (18–20). Transcriptional regulators of EPS-I biosynthetic genes include the two-component system ExoS/ChvI (21) and the regulators SyrA and SyrM (22). Little is known about how environmental cues influence the action of these or other EPS-I regulators.
S. meliloti EPS-I mutants cannot form nitrogen-fixing nodules. Cheng and Walker (21) observed that these mutants fail to initiate and elongate infection threads on alfalfa ( Medicago sativa). The symbiotic defect of EPS-I mutants can be reversed by the addition of exogenous EPS-I. Specifically, the LMW fraction is reported to be the active fraction necessary for symbiosis (23, 24).
S. meliloti also has the cryptic ability to produce a second exopolysaccharide, galactoglucan (EPS-II). EPS-II is a polymer of repeating galactose and glucose disaccharides with pyruvyl and acetyl modifications (25). Some commonly used lab strains do not produce EPS-II, due to disruption by a native insertion element in the regulatory gene expR (26). Biofilm formation and alfalfa root surface colonization are improved with the production of EPS-II (27). In the absence of EPS-I, EPS-II is sufficient for the nodulation of alfalfa but not for that of other host plants tested (26). Purified LMW EPS-II has also been reported to suppress the nodulation defects of a completely exopolysaccharide deficient S. meliloti strain, similarly to EPS-I (25).
In this work, we aimed to investigate the roles of EPS-I and EPS-II in the protection of S. meliloti against H2O2. We evaluated the ability of exopolysaccharides to provide protection against ROS-dependent death and the mechanism of that protection.
MATERIALS AND METHODS
Growth conditions.
The bacterial strains and plasmids used in this study are shown in Table 1. Luria broth (LB) medium was used for bacterial growth (28), and 1.5% agar was added for solid media. Antibiotics were used at the following concentrations: streptomycin (Sm), 500 μg/ml; neomycin (Nm), 50 μg/ml; spectinomycin (Sp), 50 μg/ml; tetracycline (Tc), 10 μg/ml; hygromycin (Hy), 50 μg/ml. Nmr of Rm7210 (exoY) was replaced with Spr as described previously (29).
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype | Source or reference |
|---|---|---|
| S. meliloti strains | ||
| Rm1021 | Wild type (Smr) | 46 |
| Rm7017 | exoD17::Tn5 (Smr Nmr) | 17 |
| Rm7031 | exoA31::Tn5 (Smr Nmr) | 17 |
| Rm7210 | exoY210::Tn5 (Smr Nmr) | 17 |
| Rm7210Sp | exoY210::Tn5-233 (Smr Spr Gmr) | This study |
| Rm8332 | exoQ332::Tn5 (Smr Spr) | 47 |
| Rm8530 | expR101 (Smr) | 48 |
| Rm8532 | expR101 exoA31::Tn5 (Smr Spr) | 48 |
| MB801 | exoX::Tn5 (Smr Nmr) | M. Barnett |
| APL41 | katA::uidA pAPL10 integrated into Rm1021 | This study |
| APL42 | katA::uidA pAPL10 integrated into Rm7210 | This study |
| APL43 | katA::uidA pAPL10 integrated into MB801 | This study |
| Plasmids | ||
| pRK607 | pRK2013Ω::Tn5-233 (Spr Gmr) | 29 |
| pRF771 | Ptrp overexpression vector, pTE3 with extended polylinker (Tcr) | 49 |
| pE65 | nodD3 in pTE3 (Tcr) | 50 |
| pMB89 | syrA in pTE3 (Tcr) | 51 |
| pS73 | syrM in pTE3 (Tcr) | 32 |
| pDW33 | Terminator and polylinker preceding uidA (Apr Hyr) | 52 |
| pAPL10 | PkatA in pDW33 | This study |
H2O2 sensitivity assay.
S. meliloti strains were inoculated into 3 ml LB with appropriate antibiotics and were incubated at 30°C overnight. Cultures were diluted to an optical density at 600 nm (OD600) of 0.1 in LB medium without antibiotics and were grown to mid-exponential phase (OD600, 0.3 to 0.4) at 30°C. Each culture was then diluted 1:100 in LB and was split in half; H2O2 was added, to a final concentration of 1 mM, to one-half of the cultures. H2O2-treated and untreated cultures were grown for 30 additional minutes at 30°C and were diluted to 1 × 10−5 to 2.5 × 10−6 of the starting volume. A 100-μl aliquot of each dilution was plated onto LB plates containing selective antibiotics. Plates were incubated at 30°C. Colonies were counted after 3 to 4 days, and CFU counts per ml were back-calculated to determine the percentage of survival of treated versus untreated cultures.
H2O2 at a range of concentrations (0.3 to 10 mM) was tested on wild-type Rm1021. At 1 mM, H2O2 gave a midrange response allowing for the detection of both increased and decreased survival relative to that of the wild type. Similarly, we tested a range of time points (10 to 120 min) and chose 30 min as optimal. For coculture experiments, the strains were mixed in a 1:1 ratio and were grown together for 3 h prior to the addition of 1 mM H2O2.
For assays of iron-dependent ROS damage, the membrane-permeant iron chelator 2,2′-dipyridyl (30) (final concentration, 1 mM; dissolved in ethanol) or an equal volume of ethanol was added to 1:100 dilutions of mid-exponential-phase cultures. Cells were grown at 30°C for 15 min before splitting and H2O2 addition. Survival was determined as described above.
Assays were repeated at least three times for each strain. The average survival of the exoY strain was 56% that of the wild type, while the survival of the exoX strain was 163% that of the wild type. Significance within any one experiment was determined by Student's t test.
Zone-of-inhibition assay.
The zone-of-inhibition assay was performed as described previously (31). Overnight cultures were diluted to an OD600 of 0.2, and 100 μl was added to 3 ml soft LB agar (0.7% agar) and was poured onto plain LB agar plates. After the soft agar had solidified, a filter paper disk (diameter, 5 mm) was placed on the center of the plate, and 5 μl 30% H2O2 was added to the disk. Plates were incubated at 30°C for 2 days, and the diameter of the zone of clearing was measured. Assays were performed at least in triplicate.
Total-catalase-activity assay.
Overnight cultures of S. meliloti were diluted to an OD600 of 0.1 in LB medium without antibiotics. Cultures were grown at 30°C to mid-exponential phase (OD600, 0.3 to 0.4); then they were split, and 1 mM H2O2 was added to one set. Cultures were returned to 30°C for 30 min. Portions (1 ml each) of treated and untreated cultures were harvested by centrifugation for 1 min. Cell pellets were resuspended in 1 ml lysis buffer (50 mM sodium phosphate [pH 7] and 0.1% Triton X-100) and were diluted 5- to 10-fold in 1× Amplex Red reaction buffer.
Twenty-five microliters of diluted cell lysates was incubated with 20 μM (final concentration) H2O2 in a 96-well microtiter plate (Microfluor 2 Black; Thermo Labsystems) in a final volume of 50 μl. Plates were incubated for 30 min at room temperature. Amplex Red reagent (final concentration, 50 μM) and horseradish peroxidase (final concentration, 0.2 U/ml) in 1× reaction buffer were added to cell lysates to a final volume of 100 μl, and the mixture was incubated at 37°C for 30 min in the dark. Plates were read as described previously (10). A standard curve of catalase was used to determine equivalent catalase units in each S. meliloti sample. Catalase activity was normalized to total-cell protein as determined by a modified Bradford assay (Bio-Rad protein assay). All assays were performed at least in triplicate.
Construction of uidA transcriptional fusion and GUS assay.
A katA–β-glucuronidase (GUS) transcriptional fusion plasmid was constructed by PCR amplifying the region from 22 bp upstream through the first 281 bp of the katA open reading frame (ORF) by using primers with SpeI/XhoI sites. Ligation of the fragment into pDW33 generated pAPL10. Conjugation into Rm1021, Rm7210, or MB801 resulted in the integration of the fusion plasmid into the S. meliloti genome via a single-crossover event and duplicated the region of the katA ORF. GUS assays were performed in triplicate as described previously (32).
EPS purification.
S. meliloti exopolysaccharides were recovered from culture supernatants by cetrimide precipitation as described previously (33), followed by precipitation with 3 volumes of acetone. Pellets were resuspended in water and were incubated at 37°C for 1 h. Exopolysaccharide fractions were dialyzed against water for final purification. Levels of reducing sugars were calculated by using the neocuproine assay as described previously (34). The total carbohydrate content was determined by the anthrone-sulfuric acid method as described previously (33).
EPS fractionation.
EPS-I preparations from culture supernatants of wild type or exoX (EPS-I-overproducing) strains were purified as described above and were separated on a Sephadex G-75 column (2.5 by 40 cm) equilibrated with a solution of 50 mM sodium phosphate (pH 7) and 100 mM sodium chloride (14). Eighty 3.4-ml fractions were eluted with the same sodium phosphate–sodium chloride buffer. Low-molecular-weight fractions were concentrated 5-fold in a SpeedVac concentrator prior to use. The total carbohydrate contents of fractions were determined by the anthrone-sulfuric acid method (33).
Measurement of iron concentrations.
Fifty micrograms of purified EPS-I was added to 2-fold dilutions (ranging from 0 to 100 μM) of ferrous sulfate (FeSO4) or ferric chloride (FeCl3) dissolved in 0.5 M hydrochloric acid, and the mixtures were incubated at either room temperature or 30°C for 10 to 20 min. The iron concentration was measured using a modified (35) ferrozine assay (36). Assays of EPS-I-treated and untreated samples were performed in triplicate.
Measurement of hydrogen peroxide concentrations.
Dilutions of H2O2 were added to purified exopolysaccharides, dialyzed exoY cell culture supernatants, or water in a 96-well microtiter plate (Microfluor 2 Black; Thermo Labsystems) in a final volume of 50 μl. Plates were incubated for 15 min at room temperature. The Amplex Red assay was performed as described previously (10). All assays were performed at least in triplicate.
RESULTS
EPS production improves survival in H2O2.
To determine whether EPS-I has an effect on the survival of S. meliloti in H2O2, we challenged three strains expressing different levels of EPS-I (wild-type, exoY, and exoX strains) with 1 mM H2O2 for various times and determined the CFU/ml (Fig. 1A). Preliminary experiments with different concentrations of H2O2 showed that 1 mM provided a midrange response in the wild-type strain (Rm1021), allowing us to distinguish both increased and decreased survival (data not shown). As little as 10 min of exposure to H2O2 decreased viability in all strains (ranging from approximately 50% to 80% survival). After 30 min in H2O2, significant differences among EPS-I− (exoY), EPS-I-overexpressing (exoX), and wild-type strains were consistently seen (Fig. 1A). Based on these results, we chose the 30-min time point for further experiments. The EPS-I-overexpressing (exoX) strain showed no significant decrease in survival between 10 and 30 min in H2O2. This indicates that increased EPS-I production correlates with increased S. meliloti survival in H2O2.
Fig 1.
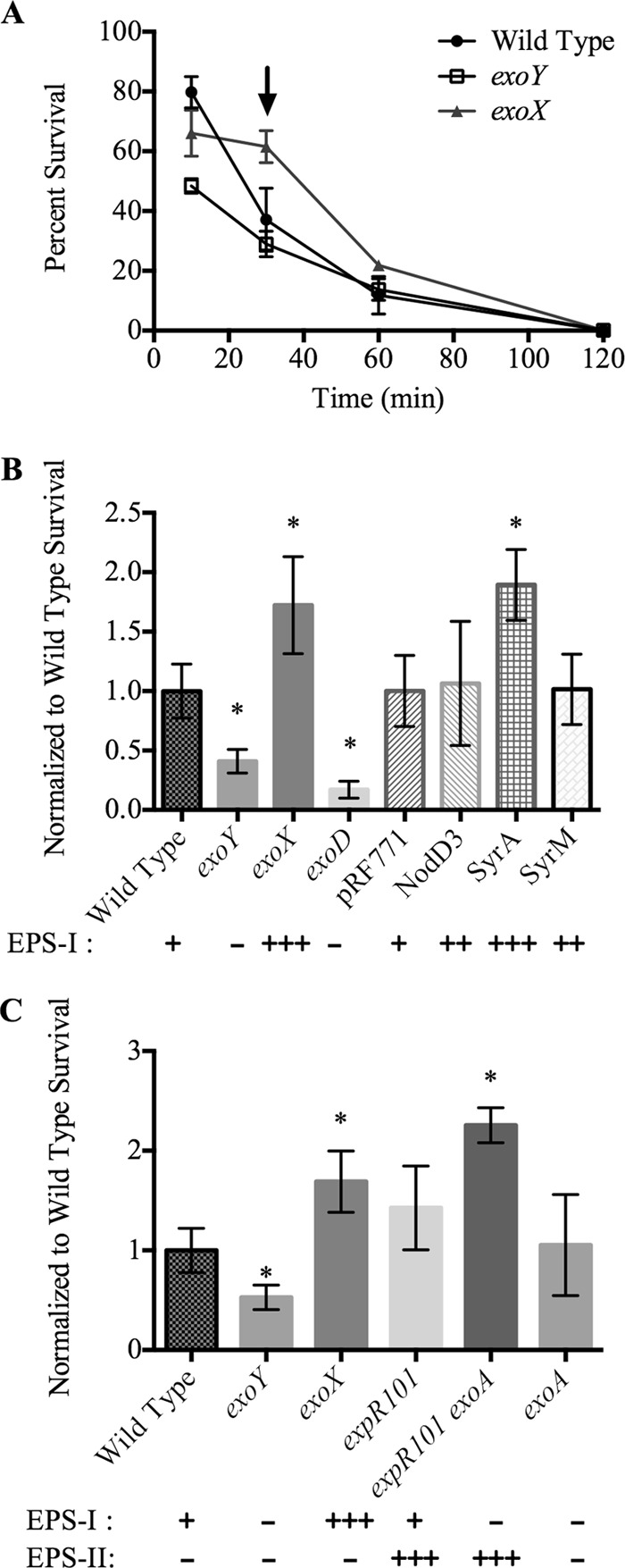
Effects of H2O2 on the survival of S. meliloti strains. (A) S. meliloti strains were tested for survival in 1 mM H2O2 at various time points. The arrow indicates the 30-min time point chosen for subsequent assays. (B) Survival of several S. meliloti strains expressing different levels of EPS-I after treatment with 1 mM H2O2. Survival was normalized to that of the wild-type strain Rm1021 (33% ± 7.4%) or to that of pRF771 (37% ± 11%). (C) Survival of S. meliloti strains expressing different levels of EPS-I or EPS-II after treatment with H2O2. Survival was normalized to the average survival of the wild type (23% ± 5.3%). Qualitative levels of EPS-I or EPS-II expression are denoted by −, +, ++, and +++. Error bars indicate standard deviations. Asterisks indicate significant differences (P, <0.05) from the wild type.
S. meliloti strains expressing different levels of EPS-I due to Tn5 insertion mutations (exoY, exoX, exoD) or to overexpression of EPS-I and nod gene regulators (nodD3, syrA, syrM) were tested for survival in 1 mM H2O2 for 30 min (Fig. 1B). Given the day-to-day variability of the overall percentage of survival, we normalized the data for each strain within an experiment to the average for the wild type. Relative survival was the same if strains were normalized to a strain carrying neutral Tn5 or to the wild-type strain (data not shown). Strains overexpressing EPS-I (e.g., the exoX strain or the syrA-overexpressing strain) consistently showed levels of survival higher than that of the wild type, while strains with decreased EPS-I expression (e.g., the exoY and exoD strains) showed lower levels of survival in H2O2.
S. meliloti can also produce a second acidic extracellular polysaccharide: EPS-II (26). To determine whether EPS-II can protect cells against H2O2 damage, we tested strains producing EPS-I, EPS-II, or both for viability in 1 mM H2O2 (Fig. 1C). The relative level of survival of a strain producing both EPS-I and EPS-II (expR101) was higher than that of the wild type. The expR101 exoA double mutant produces only EPS-II. Comparison of the relative survival levels of expR101 exoA and exoA strains showed that EPS-II alone improved S. meliloti survival in H2O2 almost 2-fold. The increase in survival seen in EPS-II-producing strains seems to be similar to the increase seen with EPS-I.
The response of the exoA mutant compared to that of the wild type in liquid culture was surprising, considering that the exoA mutant should also be deficient in EPS-I production. To test the sensitivity of the exoA mutant in other ways, a zone-of-inhibition assay was performed (Table 2). This showed that the exoA mutant is indeed sensitive to H2O2, and the differences in phenotype between the exoA mutant and other EPS-I-deficient mutants may be due to the assay conditions.
Table 2.
Zones of inhibition of EPS-I mutants
| Strain | Zone of inhibition by H2O2 (cm) |
|---|---|
| Wild type | 4.7 ± 0.1 |
| exoY mutant | 4.9 ± 0 |
| exoX mutant | 4.1 ± 0.2 |
| exoA mutant | 4.9 ± 0.1 |
Differences in H2O2 survival between EPS-I-overexpressing and EPS-I-deficient strains do not appear to arise from differences in catalase response. While transcription of the bifunctional catalase/peroxidase katA is induced by H2O2, we found that the degree of katA induction in the exoX and exoY mutants did not differ significantly from that in the wild type (Fig. 2A). Additionally, the total equivalent catalase activities of these three strains were not significantly different (Fig. 2B).
Fig 2.
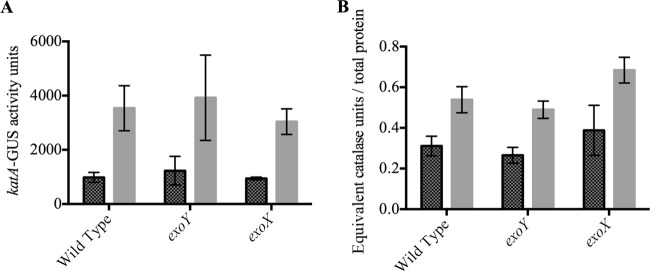
Activity of katA in response to H2O2. (A) Expression of katA as determined by uidA transcriptional fusion with (light shaded bars) or without (dark shaded bars) a 30-min induction with 1 mM H2O2. katA expression is not significantly different in different S. meliloti strain backgrounds. (B) Total equivalent catalase activity from cell lysates left untreated (dark shaded bars) or treated with 1 mM H2O2 (light shaded bars), normalized to total cell protein. Error bars indicate standard deviations. Each strain was tested in triplicate.
EPS-I and EPS-II can protect neighboring cells from H2O2.
To determine if secreted EPS-I protects against H2O2 in trans, we cocultured exoYSp cells (EPS-I− strain carrying the exoY Tn5 insertion, with Nmr replaced with Spr) with either exoY or exoX cells for 3 h prior to challenge with H2O2 (Fig. 3A). Cells were washed in order to remove antibiotics prior to coculture. The relative survival of the exoYSp strain was similar to that of the exoY strain alone, and the exoYSp strain cocultured with the exoY strain showed no significant increase in survival. However, coculture with the exoX strain increased the survival of the exoYSp strain 4-fold. Similarly, coculture of the exoY strain with the EPS-II-producing strain (expR101 exoA) showed a 50% increase in exoY strain survival (Fig. 3B).
Fig 3.

Effects of coculture on the survival of S. meliloti strains expressing different levels of EPS-I and EPS-II. The exoYSp or exoY exopolysaccharide-deficient strain, cocultured with a strain overproducing EPS-I (A) or with a strain expressing EPS-II (B), was challenged with H2O2. Survival was normalized to the average survival of the wild type. Error bars indicate standard deviations. The lowercase letter “a” above a bar indicates a significant difference from the wild type (P, <0.05); the letter “b” indicates a significant difference between coculture and culture of the exoY strain alone (P, <0.05).
To test if an exported factor is responsible for the increase in survival seen in the coculture of exoYSp and exoX strains, we grew exoYSp cells in heat-killed medium conditioned with either the exoY or the exoX strain. We saw no increase in the relative survival of the exoYSp strain grown in exoX strain-conditioned medium over that of the exoYSp strain alone (data not shown). We grew the exoY strain with increasing concentrations of purified EPS-I in order to determine if EPS-I alone can improve the survival of EPS-I− strains (data not shown). No significant improvement in exoY survival was seen with the concentrations of EPS-I tested.
Iron is required for H2O2-dependent death, independently of EPS-I production.
A mechanism for H2O2-dependent death is the generation of hydroxyl radicals through iron-requiring Fenton chemistry (37). To determine if iron is required for the H2O2-dependent death of S. meliloti, we added the cell-permeant iron chelator 2,2′-dipyridyl (final concentration, 1 mM) to cells 15 min prior to H2O2 addition (Fig. 4A). Dipyridyl increased the survival of the wild-type, exoY, and exoX strains to nearly the levels of non-H2O2-treated cells regardless of EPS-I production, indicating that iron is required for the H2O2-dependent death of S. meliloti. The concentrations (CFU/ml) of non-H2O2-treated wild-type cultures with and without dipyridyl were similar (1.60 × 106 to 1.78 × 106 CFU/ml without dipyridyl compared to 2.00 × 106 to 2.12 × 106 CFU/ml with dipyridyl).
Fig 4.

Chelation of iron with dipyridyl enhances the survival of S. meliloti in H2O2. (A) Survival of S. meliloti strains in 1 mM H2O2 without (dark shaded bars) or with (light shaded bars) the addition of the iron chelator dipyridyl. Survival is normalized to that of the wild-type strain not treated with dipyridyl. Error bars indicate standard deviations. Asterisks indicate significant differences from the wild type without dipyridyl (P, <0.05). (B) Absorbance of ferrozine reagent in response to amounts of Fe2+ (dark shaded bars) or Fe3+ and Fe2+ (light shaded bars) found in EPS-I or the exoY culture supernatant.
To determine if EPS-I is capable of binding to iron, we incubated 50 μg purified EPS-I with either FeSO4 or FeCl3 for 10 to 20 min. We determined the remaining amount of free Fe2+ by using ferrozine (FeCl3-incubated samples were reduced prior to Fe2+ detection). There was no difference between the amounts of Fe2+ detected in the presence and absence of EPS-I (Fig. 4B). This held true even if EPS-I was removed by cetrimide precipitation prior to ferrozine detection (data not shown).
EPS is able to decrease H2O2 levels in vitro.
We tested EPS-I and EPS-II for their abilities to decrease H2O2 levels in vitro (Fig. 5). Exopolysaccharides were purified by cetrimide and acetone precipitation, followed by dialysis against water. We incubated purified EPS-I or EPS-II with various concentrations of H2O2. The final concentrations of H2O2 were assayed using Amplex Red reagent. The addition of 100 μg/ml to 400 μg/ml exopolysaccharide was able to decrease detectable H2O2 levels in a concentration-dependent manner (Fig. 5A; see also Fig. S1 in the supplemental material).
Fig 5.
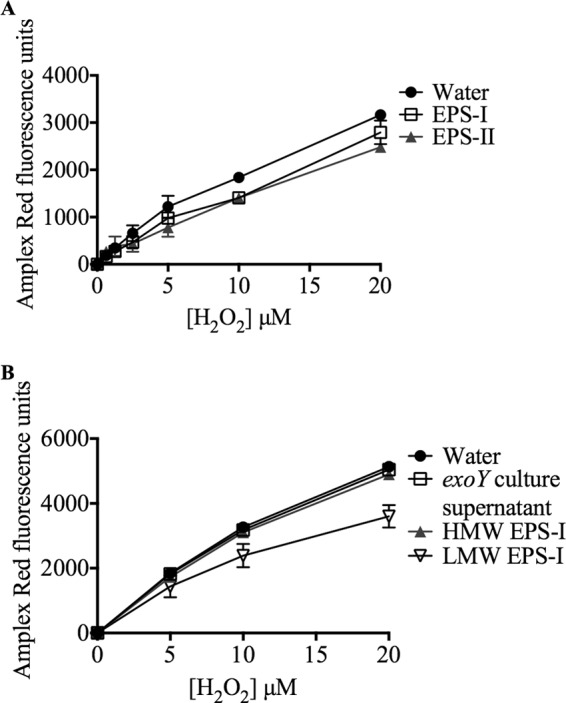
Exopolysaccharides decrease H2O2 concentrations in vitro. (A) Amount of H2O2 detected by Amplex Red when incubated with 7 μg EPS-I (open squares) or 7 μg EPS-II (filled triangles). (b) Amplex Red detection of H2O2 incubated with either the culture supernatant from the exoY strain (open squares), 25 μg HMW EPS-I (filled triangles), or 20 μg LMW EPS-I (open inverted triangles). Error bars indicate standard deviations (not visible in all data points due to small errors).
An exoY cell culture supernatant purified in the same manner as EPS-I or EPS-II had no effect on Amplex Red fluorescence, indicating that the decrease in H2O2 detection is due to exopolysaccharides and is not a by-product of purification (Fig. 5B). In addition, equal amounts of glucose or glucose tetrasaccharide did not decrease H2O2 levels (data not shown). The fluorescence of resorufin (the fluorescent product produced by Amplex Red after H2O2 detection) was not affected by the addition of EPS-I (see Fig. S2 in the supplemental material). From these results, we conclude that both species of S. meliloti exopolysaccharide decrease H2O2 levels in vitro, while glucose monomers and oligomers do not.
Low-molecular-weight (LMW) EPS-I fractionated over a size exclusion column was able to decrease H2O2 levels (Fig. 5B). High-molecular-weight (HMW) EPS-I from size exclusion column fractionation was much less effective at decreasing H2O2 levels, indicating that LMW EPS-I is the active fraction responsible for eliminating H2O2. HMW EPS-I has a high ratio of total sugars to reducing sugars, while LMW EPS has a low ratio (Table 3).
Table 3.
Quantification of total and reducing sugars of HMW and LMW EPS-I
| EPS-I form (source)a | Concn of sugar (μg/ml) |
Total/reducing sugar ratiob | |
|---|---|---|---|
| Totalc | Reducingd | ||
| HMW (exoX strain) | 487 | 1.4 | 348 |
| LMW (Rm1021) | 415 | 6.6 | 62 |
Both HMW EPS-I and LMW EPS-I were fractionated by size exclusion chromatography.
High ratios indicate high-molecular-weight EPS-I (longer sugar chains per reducing end). Conversely, low ratios indicate low-molecular-weight EPS-I.
As determined by an anthrone-sulfuric acid assay.
As determined by a neocuproine assay.
DISCUSSION
Aerobic organisms such as S. meliloti are exposed to both internal and external sources of ROS. H2O2-dependent damage can be avoided if external H2O2 is prevented from entering the cell or if internal H2O2 is detoxified. Here we have shown that the levels of the external polysaccharides produced by S. meliloti, EPS-I and EPS-II, correlate with survival in H2O2.
The protection against H2O2 is independent of the genetic mechanism of altering the levels of exopolysaccharides. Strains that overproduce both Nod factor and EPS-I do not appear to have a survival advantage similar to that of strains that produce only EPS-I. This may be due to differing levels of EPS-I production, or possibly to excess stress on the cells due to the overproduction and export of Nod factor (22). The existing data do not distinguish between these two possibilities.
We have also shown that S. meliloti strains producing EPS-II have improved survival in H2O2 compared to wild-type strains that produce only EPS-I. To our knowledge, this is the first report of EPS-II protection against ROS-related damage. While EPS-II is not required for symbiosis with Medicago spp., production of EPS-II in the absence of EPS-I can allow productive symbiosis with Medicago sativa, though not with M. truncatula (26).
In coculture, S. meliloti mutants expressing increased levels of either EPS-I or EPS-II can protect exopolysaccharide-deficient cells against H2O2. This is interesting, given that coinoculation of S. meliloti Nod factor and EPS-I mutants allows for the formation of functional nodules that are not formed by either mutant alone (38, 39). This could result partly from the coprotection of cells against H2O2, but that explanation does not rule out the possibility of exopolysaccharides acting as specific signals.
While there was variability in survival from day to day, which makes comparisons across assays difficult, the correlation between the amount of exopolysaccharide produced and survival was reproducible and consistent. There can be limitations to this assay if the strain tested has growth problems independent of H2O2 stress. An example of this type of problem was seen with the exoA mutant, which had longer doubling times and an apparently wild type level of survival after exposure to H2O2. However, an alternative assay (zone of inhibition) showed that the exoA mutant is indeed sensitive to H2O2. The technical differences between these two assays (different growth stages, levels of H2O2 exposure, and culture media) show that sensitivity to H2O2 can depend on other growth conditions. However, both of these assays showed that EPS-I-deficient mutants are sensitive to H2O2 while EPS-I-overproducing mutants are resistant.
We wondered whether the decrease in the survival of the exoY strain and other EPS-I-deficient strains was due to a general inability to handle environmental stresses. However, this would not explain why EPS-I-overproducing strains are resistant to H2O2 but not discernibly different in total catalase (anti-H2O2) activity. Additionally, the ability of a 3-h coculture to increase the survival of EPS-I-deficient strains in H2O2 indicates that the protective moiety in EPS-I-overproducing strains can act in trans, though this does not rule out the possibility that something coexpressed with EPS-I or EPS-II is responsible for protection against H2O2.
Heat-killed conditioned medium did not improve the survival of the exoY strain; likewise, addition of exogenous EPS-I to the culture medium did not appear to improve the survival of EPS-I-deficient strains under the conditions tested. These observations are at odds with our inference that exopolysaccharides are able to act in trans, which we based on the observation that exoY cells are protected in coculture. One possible reason for this lack of protection by medium or exogenous EPS-I is that living cells are required for coprotection. For example, perhaps the quantity of purified EPS-I, or that of EPS-I in the conditioned medium, is not sufficient, while living cells produce EPS-I continuously, such that levels are kept high. Another possibility is that the purified EPS-I may not be chemically identical to native EPS-I produced in culture. It is possible that heat treatment or purification changes the chemistry. On the other hand, we observe that purified S. meliloti exopolysaccharides, specifically the LMW EPS-I fraction, are capable of decreasing the detectable levels of H2O2 in vitro. For this reason, we favor the idea that the concentrations of EPS-I added to the culture medium do not accurately replicate the local concentrations needed to protect cells from H2O2 damage. Nonetheless, we keep open the possibility that another factor, coproduced with EPS-I in culture and disturbed by mutations of the exo genes, plays a larger role in vivo than EPS-I and is lost or changed in heat-treated culture and in EPS-I purification.
Both EPS-I and EPS-II appear to be competent to decrease H2O2 levels as detected by Amplex Red. The concentrations of EPS-I and EPS-II used in these assays are within the range needed to complement the symbiotic defect of EPS-I-defective cells (24). The detection of the fluorescent product of this assay, resorufin, is unchanged with the addition of EPS-I. An increase in the concentration of EPS-I leads to a greater decrease in H2O2 levels. We believe that these data, taken together, indicate that EPS-I and EPS-II can act as antioxidants, rather than interfering with the Amplex Red assay itself. These data do not rule out the possibility that something that copurifies with EPS-I and EPS-II, and is not present in the culture supernatants from EPS-I-deficient strains, is responsible for decreasing H2O2 levels. Also, these experiments do not show whether EPS-I acts as an antioxidant during symbiosis.
LMW EPS-I is reported to be the active fraction required for symbiosis (23, 24). The ability of LMW EPS-I to suppress the symbiotic defects of EPS-I-deficient strains seems to be inhibited by the presence of HMW EPS-I (24). The EPS-I added to cultures to test survival in H2O2 contained a mixture of both HMW and LMW forms. Perhaps the reason we do not see improved survival of EPS-I-deficient strains when purified EPS-I is added to the growth medium is that HMW EPS-I has an inhibitory effect. Alternatively, the addition of mixed-molecular-weight EPS-I may provide insufficient amounts of LMW EPS-I to be effective against H2O2.
We have not investigated the chemical mechanisms by which exopolysaccharides could decrease H2O2 levels. Both exopolysaccharides are produced in HMW and LMW forms, and in both cases, the LMW form is reported to rescue symbiotic defects (24, 40). The improved activity of LMW EPS-I may be due to an increased number of reducing ends compared to total sugars (Table 3), or the improved mobility or diffusibility of the smaller EPS-I may lead to better antioxidant activity. LMW EPS-I also has a higher degree of succinylation than HMW EPS-I (14). These succinyl groups may also be possible targets of H2O2.
We cannot rule out the possibility that the antioxidant activity of these exopolysaccharides is due to something, such as an ion or small molecule, that coprecipitates with EPS-I and EPS-II rather than to the exopolysaccharides themselves. While we did not see iron binding to EPS-I, likely due to a lower sensitivity of our assay, specific LMW EPS-I forms have recently been reported to have iron-chelating properties (41). It is not clear if the ability of LMW EPS-I to bind iron results in protection of that iron and, therefore, decreased production of hydroxyl radicals. If this is the case, it might explain the ability of exopolysaccharides to protect against H2O2 in vivo but would not necessarily explain the ability to decrease H2O2 levels in vitro. Calcium also binds EPS-I (42), raising the question of whether other ions might do so as well. One candidate is manganese, since Mn(II) is capable of scavenging superoxide (2, 43). The levels of manganese present during symbiosis have not been analyzed.
Earlier studies found that exogenous addition of exopolysaccharides from other rhizobial species or EPS-I lacking nonsugar modifications is not competent to rescue the symbiotic defects of S. meliloti EPS-I mutants (24). However, recent work in the Rhizobium leguminosarum– Vicia sativa system indicates that specific exopolysaccharides may not be required for early stages of symbiosis (44, 45). Perhaps heterologous exopolysaccharides capable of reducing H2O2 can reconstitute some of the functions of native exopolysaccharides.
The requirement for EPS-I during symbiosis may be due in part to its ability to protect the bacteria from ROS. This could explain how two structurally different exopolysaccharides can function to allow productive symbiosis (26), since they both have the ability to decrease H2O2 levels in vitro. Whether exopolysaccharides actually function during symbiosis to modulate H2O2 levels, and whether this is the only role for these polysaccharides, remains an open question. The fact that EPS-II cannot overcome the requirement for EPS-I in symbiosis with M. truncatula and several other host plants, despite its ability to decrease H2O2 levels, implies that exopolysaccharides have additional functions during symbiosis.
We have shown that both EPS-I and EPS-II are protective against H2O2-dependent death in S. meliloti. Our work suggests that the exopolysaccharides may provide this protection by decreasing the amount of H2O2 in the surrounding environment. The LMW fraction of EPS-I is the effective form for decreasing H2O2 levels. Future studies are needed to address the symbiotic importance of decreased levels of H2O2 and the mechanism of such a decrease by exopolysaccharides.
Supplementary Material
ACKNOWLEDGMENTS
We thank Virginia Walbot for the use of her fluorescence plate reader and the members of the Long laboratory for comments on the manuscript. We also thank the anonymous reviewers of the manuscript for critical comments and suggestions, including alternative explanations.
This work was supported by National Institutes of Health grant GM093628 (to S.R.L.). A.P.L. was supported by the ASM Robert D. Watkins Graduate Research Fellowship.
Footnotes
Published ahead of print 27 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00681-13.
REFERENCES
- 1.Imlay JA. 2002. How oxygen damages microbes: oxygen tolerance and obligate anaerobiosis. Adv. Microb. Physiol. 46:111–153 [DOI] [PubMed] [Google Scholar]
- 2.Imlay JA. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 77:755–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halliwell B. 2006. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 141:312–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capela D, Barloy-Hubler F, Gouzy J, Bothe G, Ampe F, Batut J, Boistard P, Becker A, Boutry M, Cadieu E, Dréano S, Gloux S, Godrie T, Goffeau A, Kahn D, Kiss E, Lelaure V, Masuy D, Pohl T, Portetelle D, Pühler A, Purnelle B, Ramsperger U, Renard C, Thébault P, Vandenbol M, Weidner S, Galibert F. 2001. Analysis of the chromosome sequence of the legume symbiont Sinorhizobium meliloti strain 1021. Proc. Natl. Acad. Sci. U. S. A. 98:9877–9882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haag AF, Arnold MFF, Myka KK, Kerscher B, Dall'angelo S, Zanda M, Mergaert P, Ferguson GP. 2013. Molecular insights into bacteroid development during Rhizobium-legume symbiosis. FEMS Microbiol. Rev. 37:364–383 [DOI] [PubMed] [Google Scholar]
- 6.Santos R, Hérouart D, Sigaud S, Touati D, Puppo A. 2001. Oxidative burst in alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol. Plant Microbe Interact. 14:86–89 [DOI] [PubMed] [Google Scholar]
- 7.Rubio MC, James EK, Clemente MR, Bucciarelli B, Fedorova M, Vance CP, Becana M. 2004. Localization of superoxide dismutases and hydrogen peroxide in legume root nodules. Mol. Plant Microbe Interact. 17:1294–1305 [DOI] [PubMed] [Google Scholar]
- 8.Jamet A, Mandon K, Puppo A, Hérouart D. 2007. H2O2 is required for optimal establishment of the Medicago sativa/Sinorhizobium meliloti symbiosis. J. Bacteriol. 189:8741–8745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cárdenas L, Martínez A, Sánchez F, Quinto C. 2008. Fast, transient and specific intracellular ROS changes in living root hair cells responding to Nod factors (NFs). Plant J. 56:802–813 [DOI] [PubMed] [Google Scholar]
- 10.Shaw S, Long S. 2003. Nod factor inhibition of reactive oxygen efflux in a host legume. Plant Physiol. 132:2196–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lohar DP, Haridas S, Gantt JS, VandenBosch KA. 2007. A transient decrease in reactive oxygen species in roots leads to root hair deformation in the legume-rhizobia symbiosis. New Phytol. 173:39–49 [DOI] [PubMed] [Google Scholar]
- 12.Király Z, El-Zahaby HM, Klement Z. 1997. Role of extracellular polysaccharide (EPS) slime of plant pathogenic bacteria in protecting cells to reactive oxygen species. J. Phytopathol. 145:59–68 [Google Scholar]
- 13.Davies B, Walker G. 2007. Identification of novel Sinorhizobium meliloti mutants compromised for oxidative stress protection and symbiosis. J. Bacteriol. 189:2110–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang LX, Wang Y, Pellock B, Walker GC. 1999. Structural characterization of the symbiotically important low-molecular-weight succinoglycan of Sinorhizobium meliloti. J. Bacteriol. 181:6788–6796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.González JE, Semino CE, Wang LX, Castellano-Torres LE, Walker GC. 1998. Biosynthetic control of molecular weight in the polymerization of the octasaccharide subunits of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Proc. Natl. Acad. Sci. U. S. A. 95:13477–13482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glucksmann MA, Reuber TL, Walker GC. 1993. Genes needed for the modification, polymerization, export, and processing of succinoglycan by Rhizobium meliloti: a model for succinoglycan biosynthesis. J. Bacteriol. 175:7045–7055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leigh JA, Signer ER, Walker GC. 1985. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc. Natl. Acad. Sci. U. S. A. 82:6231–6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellweg C, Pühler A, Weidner S. 2009. The time course of the transcriptomic response of Sinorhizobium meliloti 1021 following a shift to acidic pH. BMC Microbiol. 9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossbach S, Mai DJ, Carter EL, Sauviac L, Capela D, Bruand C, de Bruijn FJ. 2008. Response of Sinorhizobium meliloti to elevated concentrations of cadmium and zinc. Appl. Environ. Microbiol. 74:4218–4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breedveld MW, Zevenhuizen L, Zehnder AJB. 1990. Osmotically induced oligo- and polysaccharide synthesis by Rhizobium meliloti SU-47. J. Gen. Microbiol. 136:2511–2519 [Google Scholar]
- 21.Cheng HP, Walker GC. 1998. Succinoglycan production by Rhizobium meliloti is regulated through the ExoS-ChvI two-component regulatory system. J. Bacteriol. 180:20–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulligan JT, Long SR. 1989. A family of activator genes regulates expression of Rhizobium meliloti nodulation genes. Genetics 122:7–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urzainqui A, Walker GC. 1992. Exogenous suppression of the symbiotic deficiencies of Rhizobium meliloti exo mutants. J. Bacteriol. 174:3403–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Battisti L, Lara JC, Leigh JA. 1992. Specific oligosaccharide form of the Rhizobium meliloti exopolysaccharide promotes nodule invasion in alfalfa. Proc. Natl. Acad. Sci. U. S. A. 89:5625–5629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González JE, York GM, Walker GC. 1996. Rhizobium meliloti exopolysaccharides: synthesis and symbiotic function. Gene 179:141–146 [DOI] [PubMed] [Google Scholar]
- 26.Glazebrook J, Walker GC. 1989. A novel exopolysaccharide can function in place of the calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell 56:661–672 [DOI] [PubMed] [Google Scholar]
- 27.Rinaudi LV, González JE. 2009. The low-molecular-weight fraction of exopolysaccharide II from Sinorhizobium meliloti is a crucial determinant of biofilm formation. J. Bacteriol. 191:7216–7224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glazebrook J, Walker GC. 1991. Genetic techniques in Rhizobium meliloti. Methods Enzymol. 204:398–418 [DOI] [PubMed] [Google Scholar]
- 29.De Vos GF, Walker GC, Signer ER. 1986. Genetic manipulations in Rhizobium meliloti utilizing two new transposon Tn5 derivatives. Mol. Gen. Genet. 204:485–491 [DOI] [PubMed] [Google Scholar]
- 30.Elgrably-Weiss M, Park S, Schlosser-Silverman E, Rosenshine I, Imlay J, Altuvia S. 2002. A Salmonella enterica serovar Typhimurium hemA mutant is highly susceptible to oxidative DNA damage. J. Bacteriol. 184:3774–3784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo L, Qi M, Yao S, Cheng H, Zhu J, Yu G. 2005. Role of oxyR from Sinorhizobium meliloti in regulating the expression of catalases. Acta Biochim. Biophys. Sin. 37:421–428 [DOI] [PubMed] [Google Scholar]
- 32.Swanson JA, Mulligan JT, Long SR. 1993. Regulation of syrM and nodD3 in Rhizobium meliloti. Genetics 134:435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells D, Chen E, Fisher R, Long S. 2007. ExoR is genetically coupled to the ExoS-ChvI two-component system and located in the periplasm of Sinorhizobium meliloti. Mol. Microbiol. 64:647–664 [DOI] [PubMed] [Google Scholar]
- 34.Chaplin MF, Kennedy JF. 1986. Carbohydrate analysis: a practical approach. IRL, Oxford, United Kingdom [Google Scholar]
- 35.Viollier E, Inglett PW, Hunter K, Roychoudhury AN, Van Cappellen P. 2000. The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl. Geochem. 15:785–790 [Google Scholar]
- 36.Stookey LI. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. 42:779–781 [Google Scholar]
- 37.Touati D. 2000. Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 373:1–6 [DOI] [PubMed] [Google Scholar]
- 38.Klein S, Hirsch AM, Smith CA, Signer ER. 1988. Interaction of nod and exo Rhizobium meliloti in alfalfa nodulation. Mol. Plant Microbe Interact. 1:94–100 [DOI] [PubMed] [Google Scholar]
- 39.Kapp D, Niehaus K, Quandt J, Muller P, Puhler A. 1990. Cooperative action of Rhizobium meliloti nodulation and infection mutants during the process of forming mixed infected alfalfa nodules. Plant Cell 2:139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.González JE, Reuhs BL, Walker GC. 1996. Low molecular weight EPS II of Rhizobium meliloti allows nodule invasion in Medicago sativa. Proc. Natl. Acad. Sci. U. S. A. 93:8636–8641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho E, Choi JM, Kim H, Tahir MN, Choi Y, Jung S. 2013. Ferrous iron chelating property of low-molecular weight succinoglycans isolated from Sinorhizobium meliloti. Biometals 26:321–328 [DOI] [PubMed] [Google Scholar]
- 42.Aslam SN, Newman M-A, Erbs G, Morrissey KL, Chinchilla D, Boller T, Jensen TT, De Castro C, Ierano T, Molinaro A, Jackson RW, Knight MR, Cooper RM. 2008. Bacterial polysaccharides suppress induced innate immunity by calcium chelation. Curr. Biol. 18:1078–1083 [DOI] [PubMed] [Google Scholar]
- 43.Gray B, Carmichael AJ. 1992. Kinetics of superoxide scavenging by dismutase enzymes and manganese mimics determined by electron spin resonance. Biochem. J. 281:795–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laus MC, van Brussel AAN, Kijne JW. 2005. Exopolysaccharide structure is not a determinant of host-plant specificity in nodulation of Vicia sativa roots. Mol. Plant Microbe Interact. 18:1123–1129 [DOI] [PubMed] [Google Scholar]
- 45.van Rhijn P, Fujishige NA, Lim PO, Hirsch AM. 2001. Sugar-binding activity of pea lectin enhances heterologous infection of transgenic alfalfa plants by Rhizobium leguminosarum biovar viciae. Plant Physiol. 126:133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meade HM, Long SR, Ruvkun GB, Brown SE, Ausubel FM. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 149:114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long S, Reed JW, Himawan J, Walker GC. 1988. Genetic analysis of a cluster of genes required for synthesis of the calcofluor-binding exopolysaccharide of Rhizobium meliloti. J. Bacteriol. 170:4239–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reed JW, Walker GC. 1991. The exoD gene of Rhizobium meliloti encodes a novel function needed for alfalfa nodule invasion. J. Bacteriol. 173:664–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wells DH, Long SR. 2002. The Sinorhizobium meliloti stringent response affects multiple aspects of symbiosis. Mol. Microbiol. 43:1115–1127 [DOI] [PubMed] [Google Scholar]
- 50.Fisher RF, Egelhoff TT, Mulligan JT, Long SR. 1988. Specific binding of proteins from Rhizobium meliloti cell-free extracts containing NodD to DNA sequences upstream of inducible nodulation genes. Genes Dev. 2:282–293 [DOI] [PubMed] [Google Scholar]
- 51.Barnett MJ, Swanson JA, Long SR. 1998. Multiple genetic controls on Rhizobium meliloti syrA, a regulator of exopolysaccharide abundance. Genetics 148:19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cronan GE, Keating DH. 2004. Sinorhizobium meliloti sulfotransferase that modifies lipopolysaccharide. J. Bacteriol. 186:4168–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


