Abstract
Urinary histoplasma antigen measurement can be useful for diagnosing systemic histoplasmosis and for monitoring treatment response, especially in immunocompromised patients. However, testing has traditionally been limited to specialized reference laboratories, as immunoassay reagents for the antigen were not widely available. Recently, a polyclonal-antibody-based in vitro diagnostic (IVD) kit for histoplasma antigen detection was released, as well as monoclonal-antibody reagents against the target. We evaluated the analytical and clinical performance of the two reagents. Both assays were capable of detecting histoplasma antigen in urine samples over a wide dynamic range, although the monoclonal assay showed improved precision and analytical sensitivity relative to the polyclonal IVD. In a test set of clinically characterized patient samples, the monoclonal laboratory-developed test (LDT) demonstrated 90.5% sensitivity and 96.3% specificity versus 61.9% sensitivity and 79.3% specificity for the polyclonal IVD, with areas under the curve (AUCs) of 0.987 and 0.754, respectively. The major differences between the two assays were higher background reactivity in healthy donors with the polyclonal assay and an increased signal response in positive samples for the monoclonal assay. The impact of these differences on monitoring treatment response was evaluated in a series of patients undergoing treatment for histoplasmosis. While all the assays gave similar qualitative estimates of treatment response, responses were more evident using the monoclonal assay. In summary, we conclude that while multiple assays are available for measuring histoplasma antigen in urine, a monoclonal-antibody-based assay appears to provide improved analytical performance for management of immunocompromised histoplasmosis patients.
INTRODUCTION
Histoplasmosis is the most common endemic mycosis found in immunocompromised patients (1). The disease is caused by Histoplasma capsulatum, a dimorphic soil fungus. The fungus can be found worldwide, but it is most common in North and Central America and is endemic in the Mississippi and Ohio River valleys. While immunocompetent subjects can usually contain the infection via cell-mediated immunity, immunocompromised patients are at higher risk to develop serious disease (2). In addition to direct exposure, immunocompromised patients can also acquire histoplasmosis by reactivation of a latent infection or from a donor organ following transplantation (3). Pneumonia is often the initial manifestation of infection in immunosuppressed patients, but once infected, disseminated infection can occur in more than 50% of patients with conditions such as organ transplantation and AIDS (3–6). Clinical symptoms are usually nonspecific, such as fever, nonproductive cough, and malaise. If the diagnosis can be made early, many patients have a good response to standard antifungal treatment (6). The nonspecific nature of the presenting symptoms coupled with an uncertain exposure history can create diagnostic challenges in immunocompromised patients.
Laboratory methods for the diagnosis of histoplasmosis include fungal culture, serologic testing for antibodies, and antigen detection. The clinical utility of fungal culture in making a diagnosis of histoplasmosis is limited by long incubation times required to see growth (2 to 4 weeks) and a relative lack of sensitivity (7). Serologic testing can be used to document exposure but is less useful for differentiating acute from prior infection and can be unreliable in the immunocompromised-patient populations, who are at the highest risk of disease. For these reasons, detection of histoplasma antigen in the urine has been proposed as a useful method for both diagnosis and monitoring therapeutic response, especially in immunocompromised patients (3).
One of the earliest immunoassays for the detection of urine histoplasma antigen used polyclonal antibodies against histoplasma galactomannan for both capture and detection (8, 9), and early publications showed the utility of this assay for the diagnosis and management of patients with disseminated histoplasmosis. The clinical sensitivity (Sn) and specificity (Sp) of the assay have been well characterized in a variety of populations (10–13), and several generations of laboratory-developed tests (LDTs) based on the approach have been offered by one of the largest histoplasma reference laboratories in the United States (MiraVista Diagnostics, Indianapolis, IN). Because the reagents used in the assay are not commercially available, alternative assays have been evaluated by other investigators with varying success. An LDT assay using a similar format but based on a commercially available polyclonal anti-Histoplasma antibody showed reasonable analytical performance but had limited clinical validation data (14). A similar enzyme immunoassay (EIA) developed by the Centers for Disease Control and Prevention using an in-house-produced polyclonal antibody demonstrated 85% sensitivity in AIDS patients (15). However, the assay is not available commercially. Finally, an inhibition enzyme-linked immunosorbent assay (ELISA) based on a murine monoclonal antibody demonstrated sensitivity similar to that of the polyclonal assay for detecting antigen in serum but was less sensitive for urinary antigen detection (16, 17). However, these monoclonal reagents were also never commercially released. This lack of readily available reagents has limited the widespread implementation of urinary histoplasma antigen testing.
Recently, the first in vitro diagnostic (IVD) assay for histoplasma antigen (Alpha Histoplasma Antigen EIA; IMMY, Norman, OK) was approved by the FDA. The assay is based on the same polyclonal reagents previously described (14, 18–20) but has been developed into a standardized analytical kit format. In addition to the IVD assay, analyte-specific reagents (ASRs) for purified Histoplasma galactomannan and monoclonal antibodies against it have recently become available. We used these reagents to develop an LDT assay based on the monoclonal reagents and compared the analytical performance of the monoclonal assay to that of the polyclonal IVD for the detection of histoplasma antigen in urine samples from immunocompromised patients.
MATERIALS AND METHODS
Subjects and samples.
Random urine samples were collected from 70 healthy volunteers between 18 and 65 years old who had normal urinalysis results. Fifty to 100 ml of urine was collected from each donor in a 150-ml polypropylene container. An additional 57 patient controls were obtained using residual material from clinical samples sent for routine testing (for tests other than urine histoplasma antigen). Finally, a clinical test set was developed using residual material from 103 samples submitted for urine histoplasma antigen testing between September 2010 and March 2013. All samples were stored at −80°C until the time of analysis. The use of human specimens and the study protocol for collecting normal urine were approved by the Institutional Review Board at the Cleveland Clinic.
Measurement of urine histoplasma antigen using IVD polyclonal EIA.
The FDA-cleared IVD assay kit (Alpha Histoplasma Antigen EIA) was obtained from Immuno Mycologics, Inc. (Norman, OK). The assay utilizes a rabbit polyclonal antibody for both capture and detection and is labeled for use on urine specimens. The calibration materials for the assay are culture filtrate-derived standards, with results expressed in U/ml. The assay was performed according to the manufacturer's instructions without modifications, and all data were generated from valid runs that met quality control (QC) criteria specified in the product insert.
Development of a monoclonal-antibody-based LDT assay for measurement of urine histoplasma antigen.
The monoclonal reagents were used to develop a two-step sandwich ELISA as follows. Monoclonal anti-Histoplasma galactomannan antibody was immobilized on 96-well plates as the capture antibody, while a second monoclonal anti-Histoplasma galactomannan antibody conjugated to horseradish peroxidase (HRP) was used for detection. The precoated plates and antibody conjugate were both purchased from Immuno Mycologics (Norman, OK). Purified Histoplasma galactomannan (Immuno Mycologics, Norman, OK) was used as a calibrator over seven points ranging from 0.5 to 50 ng/ml. TMB (3,3′,5,5′ tetramethylbenzidine) served as the substrate for color formation.
To perform the assay, 100 μl of calibrator, control, or test specimens was incubated on the coated plate at 37°C for 1 h. After the plate was washed three times with wash buffer and blotted dry, conjugated detection antibody (100 μl) was added for 30 min at room temperature. The plate was washed three more times and blotted, and 100 μl TMB was added. After 20 min of incubation, the stop solution (100 μl) was added to end the reaction. The optical density (absorbance) at 450 nm was determined using a Synergy 2 spectrophotometer with 630 nm as the reference wavelength, and the concentration of histoplasma antigen was calculated relative to a 7-point calibration curve generated using 4-parameter log-logit fitting with Gen5 software (BioTek, Winooski, VT). The total time needed to perform a run was approximately 3.5 h.
Evaluation of the analytical performance of the polyclonal and monoclonal assays.
Assay validation experiments followed guidelines from the Clinical and Laboratory Standards Institute and the Guidance for Industry: Bioanalytical Method Validation from the Food and Drug Administration (21). Assay imprecision was evaluated using four samples that included high and low patient specimens, as well as negative and positive QC materials from the IVD kit. Intra-assay and interassay coefficients of variation (CVs) were generated from a total of 20 replicates (2 replicates per run and 2 runs per day for 5 days). Method comparison was designed and performed in accordance with CLSI (formerly NCCLS) EP9-A2 (22), using the panel of 103 clinical specimens described above. Results from the polyclonal IVD assay and monoclonal LDT were compared to reference laboratory (MiraVista Diagnostics, Indianapolis, IN) results as the “gold standard.” The MiraVista assay utilizes a polyclonal rabbit anti-H. capsulatum IgG as the capture antibody and a modified biotinylated polyclonal antibody as the detection antibody (11, 12). The analytical sensitivity of each assay was evaluated using a panel of normal urine specimens spiked with 0 to 10 U of Histoplasma antigen derived from yeast phase culture filtrate. The analytical specificity was examined against related pathogenic fungi, including Blastomyces dermatitidis, Aspergillus fumigatus, and Aspergillus niger, by spiking normal donor urine with sonicated filtrate from purified colonies of each agent, as well as patient specimens containing high levels of common urinary pathogens, including Candida albicans, Enterococcus faecalis, and Klebsiella oxytoca. Assay linearity was evaluated using a series of 7 specimens spiked with histoplasma culture filtrate. All linearity samples were assayed in duplicate. The lower limit of quantification (LLOQ) was defined as the lowest level at which bias and imprecision were both below 20% and was established using a panel of 5 spiked specimens over 10 days of testing. The reference range for the monoclonal LDT was established using 127 specimens, which included urine samples from 70 healthy donors and 57 patient controls. A subset of 80 of these samples (50 from healthy donors and 30 from patients) was also tested in the polyclonal IVD assay to verify the manufacturer's recommended reference range.
Diagnostic performance of polyclonal IVD and monoclonal LDT assays in immunosuppressed-patient specimens.
Clinical records for 103 specimens submitted for urine histoplasma testing were reviewed to determine if a final diagnosis of histoplasmosis was made. Patients were classified based on the clinical diagnosis as recorded in the electronic medical record. This diagnosis was based on all information obtained during the original workup, including imaging studies showing characteristic abnormalities on chest computed tomography (CT) (50%); laboratory results, such as positive cultures, serology, or urinary antigen levels (50%, 36%, and 100%, respectively); and clinical findings, such as response to antifungal treatment (93%). For patients tested on multiple occasions, samples were further classified as acute stage versus follow-up, based on the timing of the specimen. Using these criteria, 21 specimens from 14 cases of histoplasmosis (4 pulmonary and 10 disseminated) were identified in the test set. They included a mixture of organ transplant patients and patients with autoimmune disease who were on immunosuppressants. Using this classification, a receiver operating characteristic (ROC) analysis was performed for each assay.
Statistical analyses.
Precision, method comparison, linearity, reference intervals, ROC analysis, and detection limits were calculated using EP Evaluator software (release 9). Comparison between two groups was performed using a t test, and P values of <0.05 were regarded as statistically significant. Box and whisker plots, correlation, and t tests were done using Sigma Plot (version 11.2). Reference intervals were defined as the central 95% of the data for each marker using nonparametric, parametric, or transformed parametric analysis, depending on the data distribution.
RESULTS
Performance characteristics of the monoclonal LDT and polyclonal IVD assays.
The analytical characteristics of the monoclonal LDT and polyclonal IVD assays are summarized in Table 1. The monoclonal assay showed slightly better precision across all levels tested, with CVs generally below 10%, while the polyclonal assay showed a higher degree of variation, particularly in the high patient samples. (CVs could not be calculated for the negative QC material, which gave results below the limit of detection for multiple reads.) Analytical measurement range (AMR) and LLOQ results were similar and proportionate between the two assays. No cross-reactivity was observed against the majority of organisms tested, including C. albicans, E. faecalis, K. oxytoca, and A. fumigatus or A. niger. However, B. dermatitidis showed a positive signal at 1:1,000 dilution in both assays. This organism has previously been shown to cross-react with antibodies against histoplasma antigen (13, 23). The reference interval established for the monoclonal LDT in our population was <1.3 ng/ml, based on the 97.5 percentile of the reference population, and this cutoff value was used in subsequent experiments to classify clinical samples as positive or negative when using the monoclonal LDT.
Table 1.
Analytical performance characteristics of urine histoplasma antigen assays
| Parameter | Value |
|
|---|---|---|
| Polyclonal IVD | Monoclonal LDT | |
| Precisiona | ||
| Assay control, negative | 0.43 (NA/NA) | 0.15 (NA/NA) |
| Assay control, positive | 11.2 (6.0/10.2) | 11.9 (2.3/5.9) |
| Patient control, low | 2.3 (5.4/22.7) | 13.8 (2.5/10.3) |
| Patient control, high | 4.8 (10.6/29.7) | 42.5 (2.1/5.2) |
| AMR | 2–100 U/ml | 0.5–50 ng/ml |
| LLOQ | 1.5 U/ml | 0.5 ng/ml |
| Linear range | Nonlinear | 0.5–50 ng/ml |
| Reference interval | <2 U (product insert) | <1.3 ng/ml (n = 127) |
Mean (CV% [within run/total]) (U/ml); n = 20 repetitions. NA, not applicable.
Notable differences between the two assays were seen in the linearity experiments. The polyclonal IVD assay showed deviations from linearity exceeding 20% in 4 of the 7 levels tested (0.78, 1.56, 3.12, and 25 U/ml). In contrast, the monoclonal assay showed a deviation from linearity of less than 20% at all seven levels tested, indicating that the assay is linear across the range (0.39 to 50 ng/ml galactomannan) (see Fig. S1 in the supplemental material).
Direct comparison of analytical sensitivity across assays.
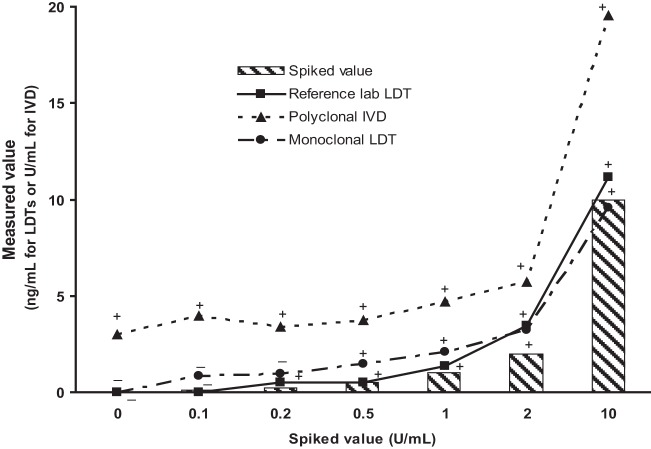
Because the polyclonal and monoclonal assays utilize different calibrators and reportable units (U/ml of culture filtrate versus ng/ml of purified galactomannan), we performed a head-to-head comparison of the two assays using a panel of spiked specimens to directly compare the analytical sensitivities of the various assays. The monoclonal and reference methods showed similar response curves, with low signals in the normal urine and detectable signal above the unspiked sample down to approximately 0.2 to 0.5 U/ml of spiked antigen (Fig. 1). In contrast, the polyclonal assay showed a high level of signal in the normal-urine pool. As a result, samples with levels below 1 U/ml of spiked antigen were indistinguishable from background signal with this assay.
Fig 1.
Direct comparison of analytical sensitivities across assays. Specimens were prepared by spiking pooled healthy-donor urine with histoplasma culture filtrate. Each point represents the mean of duplicate measurements, and whether the sample would have been called positive (+) or negative (−) relative to the reference range for that specific assay is indicated.
Correlation with the reference method.
We next compared urine histoplasma antigen levels in a total of 103 clinical specimens sent for urine histoplasma testing and correlated the IVD and monoclonal LDT results with results from the reference assay. Quantitative correlation between histoplasma antigen levels was calculated using Deming regression for all specimens with detectable results from all laboratories. In addition, qualitative classification of samples as “positive” or “negative” was assessed for each assay using assay-specific cutoffs: <2 U/ml for polyclonal IVD (package insert); “not detectable” for the reference laboratory (MiraVista reports); <1.3 ng/ml for monoclonal LDT (experimentally derived). In the quantitative analysis, the antigen levels generated by the three assays varied significantly and showed little agreement, with correlation coefficients near 0.6 (Fig. 2A). However, despite the variation in the absolute levels measured, the qualitative classification of samples as positive or “negative” relative to assay-specific cutoff points agreed in the majority of cases, with the polyclonal and monoclonal assays matching the reference method 76% and 95% of the time, respectively (Fig. 2B).
Fig 2.
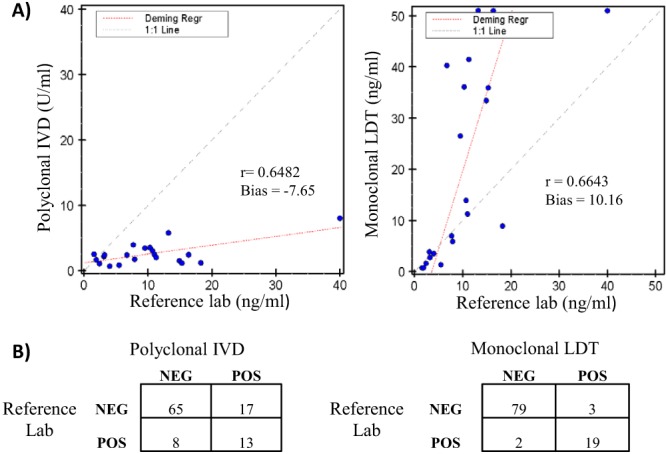
Method comparison of urine histoplasma results for IVD and LDT versus a reference laboratory. (A) Quantitative correlation of antigen levels for the 21 specimens with detectable results in all three assays. The regression (Regr) line was calculated by the Deming method. (B) Qualitative comparison of sample classification as positive (POS) or negative (NEG) using assay-specific cutoff points.
Clinical discrimination of the polyclonal and monoclonal assays.
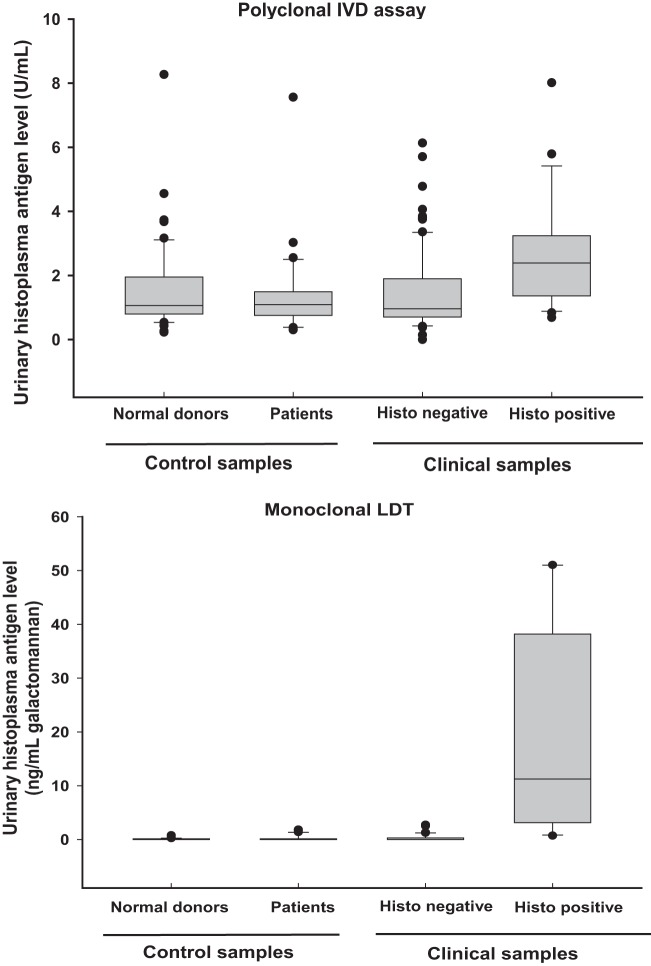
The primary difference between the polyclonal IVD and monoclonal LDT appeared to be related to the magnitude of the signal seen in infected patients. Although urinary antigen levels in histoplasmosis patients were significantly elevated compared to control groups for both the polyclonal and monoclonal assays (P < 0.05), the polyclonal assay showed substantially more overlap with controls (Fig. 3). This was reflected in the ROC analysis for the two assays in the clinical test set (Fig. 4), where the monoclonal LDT exhibited better discrimination than the IVD assay (areas under the curve [AUC], 0.98 versus 0.76). Using the reference range cutoffs described above, the calculated sensitivities and specificities were as follows: Sn = 61.9% and Sp 79.3% for the polyclonal assay; Sn = 90.5% and Sp 96.3% for the monoclonal assay.
Fig 3.
Population distribution of histoplasma antigen levels in patients and controls. The boxes indicate the median and the central 50th percentile, while the whiskers indicate the central 95%.
Fig 4.
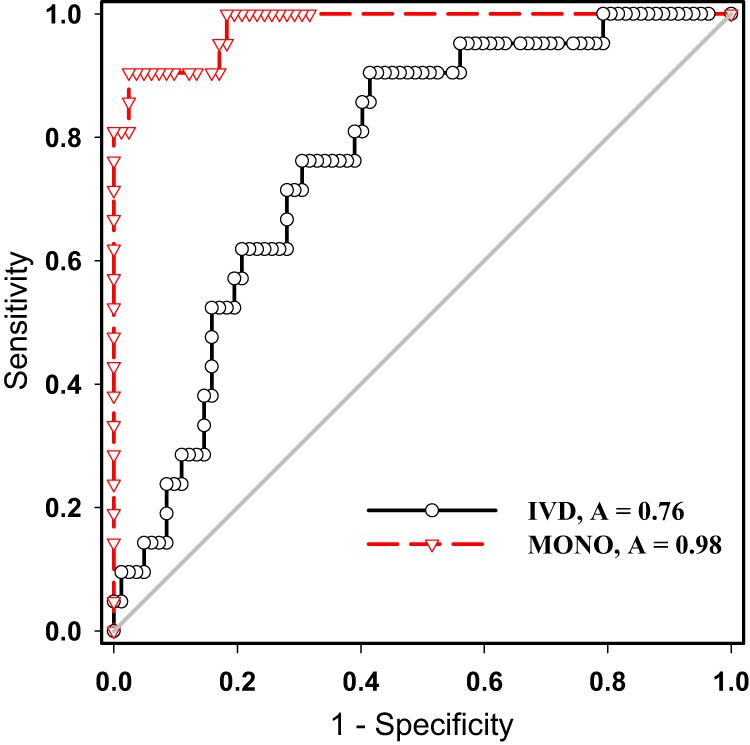
ROC curves of the polyclonal IVD assay and monoclonal LDT in the clinical-test set. The results are based on 103 specimens sent for urine histoplasma analysis, with clinical classification defined by chart review. A, AUC.
Because urine antigen levels are routinely used in monitoring patient response to antifungal therapy, we examined the impact of analytical differences on determining treatment response. Fourteen patients who had follow-up specimens drawn during the study period, ranging from 2 to 20 months after diagnosis, were identified. For most patients, longitudinal data fell into one of two distinct patterns of urinary antigen change following antifungal treatment: a rapid reduction in antigen levels or a slower, small decline (Fig. 5). The three assays showed general agreement on the response pattern of each patient despite the notable variation of antigen levels. However, the polyclonal IVD assay gave significantly lower measurements than the other two assays in follow-up specimens (P < 0.01) and, as a result, was more likely to convert to a negative result following treatment than either the monoclonal or reference laboratory assay (Table 2).
Fig 5.
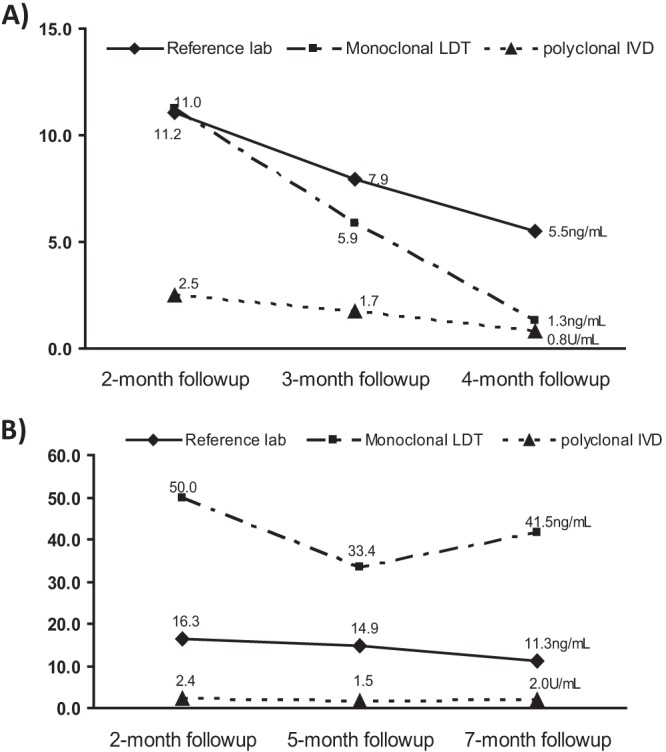
Serial changes in urine histoplasma antigen levels with various assays. The units on the y axis are ng/ml for monoclonal LDT and reference laboratory values and U/ml for polyclonal IVD. (A) Representative graph for a patient showing rapid reduction of antigen levels following treatment. (B) Representative graph for a patient showing stable levels of urinary antigen in response to treatment.
Table 2.
Positive rates for urine histoplasma antigen assays in different patient groups
| Clinical-diagnosis group | N | No. (%) positive |
|
|---|---|---|---|
| Polyclonal IVD | Monoclonal LDT | ||
| Histoplasmosis patients | 21 | 13 (61.9) | 19 (90.5) |
| Acute stage | 9 | 8 (88.9) | 9 (100) |
| Follow-up | 12 | 5 (41.6) | 10 (83.3) |
| Noninfected patients | 82 | 17 (20.7) | 3 (3.7) |
DISCUSSION
Disseminated histoplasmosis is a potentially life-threatening disease in immunosuppressed patients. The nonspecific nature of the symptoms, coupled with the extremely slow growth of histoplasma in culture, has led to the use of urinary antigen testing for early diagnosis in these patients. Historically, analytical options have been limited for laboratories wishing to perform this testing on site, due to a lack of commercially available reagents. However, the release of an FDA-approved IVD kit for urine histoplasma antigen, coupled with the development of monoclonal antibodies and purified standards for histoplasma antigen, presents new opportunities for diagnostic laboratories.
To our knowledge, this is the first study providing both analytical and clinical evaluation of the recently approved IVD assay and monoclonal anti-Histoplasma galactomannan antibodies for the detection of urinary histoplasma antigen. Our comparison of the IVD kit with a monoclonal-antibody-based LDT assay demonstrated some key performance differences between the two assays. The analytical parameters of the IVD assay were fairly typical for a plate-based ELISA in terms of precision, AMR, and ease of use, but the assay was found to be nonlinear in the manufacturer-indicated reportable range of 2 to 100 U/ml. This finding was also reported in an earlier study using an LDT assay based on these reagents (14) and may be an inherent characteristic of the polyclonal antibody used for the assay. The monoclonal-antibody-based LDT showed improved performance relative to the IVD assay in terms of precision, dynamic range, and linearity but requires more effort to prepare and characterize components, such as calibrators and controls.
In terms of clinical utility, the overall sensitivity of the monoclonal assay was notably better than that of the IVD in our patient population. The monoclonal LDT showed 90.5% sensitivity and 96.3% specificity in our clinical test set and good agreement (95.1%) with reference laboratory results in qualitative detection of antigenuria. The sensitivity is similar to that of the commonly used reference method, which showed 93% sensitivity in patients with immunocompromising conditions other than AIDS (13). However, all three parameters were lower for the polyclonal IVD assay (61.9% Sn, 79.3% Sp, 75.7% concordance). Much of this difference appeared to be due to an increased rate of weakly positive results detected with the IVD relative to the other assays.
Another interesting finding was the relationship of assay performance to the stage of disease. Both assays did a good job of identifying histoplasma antigen in the acute-stage specimens (89 and 100% for IVD and LDT, respectively). However, the IVD assay was much less likely to detect antigen in posttreatment follow-up specimens than the monoclonal (and reference laboratory) assays. While this may be an analytical sensitivity issue related to the lower concentrations of antigen seen in follow-up specimens, it may also indicate that antigens released in urine may be changed by antifungal treatment and that antibodies used in different assays respond differently to various antigens. So far, no study has been done to investigate the chemical natures and variations in different stages of histoplasma infection.
The analytical characteristics of the two assays have implications for how one could apply them in the clinical laboratory. Histoplasmosis is a relatively rare disease but is often included as part of a broader workup for fever of unknown origin in immunosuppressed patients. As a result, for most laboratories, the majority of samples submitted for urine histoplasma antigen analysis will be negative. Given the assay characteristics of the IVD, one could imagine a scenario where the IVD assay could be used on site to rapidly screen out the majority of negative samples, while positive samples (in the absence of other diagnostic findings) could be reflexively sent to a reference laboratory for confirmatory testing.
In summary, we evaluated two commercially available systems for measuring urine histoplasma antigen levels and found that the analytical characteristics of the assays present different options for clinical laboratories wishing to offer this testing. The IVD provides a relatively straightforward assay in standardized kit form that can be readily implemented in routine workflow for institutions with a desire to perform the testing in house. However, users should be aware of the potential for false-positive results, particularly in weakly positive specimens, and may want to incorporate reflex algorithms for confirmation of screen-positive samples. For high-complexity laboratories with experience in LDT development and validation, the monoclonal reagents may offer improved analytical characteristics that could be beneficial. More widespread adoption of this testing will hopefully allow larger multisite clinical trials to provide more data on the utilization and clinical performance of assays for this relatively rare disease.
Supplementary Material
Footnotes
Published ahead of print 2 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02298-13.
REFERENCES
- 1.Kauffman CA. 2008. Diagnosis of histoplasmosis in immunosuppressed patients. Curr. Opin. Infect. Dis. 21:421–425 [DOI] [PubMed] [Google Scholar]
- 2.Kurowski R, Ostapchuk M. 2002. Overview of histoplasmosis. Am. Fam. Physician 66:2247–2252 [PubMed] [Google Scholar]
- 3.Grim SA, Proia L, Miller R, Alhyraba M, Costas-Chavarri A, Oberholzer J, Clark NM. 2012. A multicenter study of histoplasmosis and blastomycosis after solid organ transplantation. Transpl. Infect. Dis. 14:17–23 [DOI] [PubMed] [Google Scholar]
- 4.Wheat LJ, Connolly-Stringfield PA, Baker RL, Curfman MF, Eads ME, Israel KS, Norris SA, Webb DH, Zeckel ML. 1990. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine 69:361–374 [DOI] [PubMed] [Google Scholar]
- 5.Cuellar-Rodriguez J, Avery RK, Lard M, Budev M, Gordon SM, Shrestha NK, van Duin D, Oethinger M, Mawhorter SD. 2009. Histoplasmosis in solid organ transplant recipients: 10 years of experience at a large transplant center in an endemic area. Clin. Infect. Dis. 49:710–716 [DOI] [PubMed] [Google Scholar]
- 6.Freifeld AG, Wheat LJ, Kaul DR. 2009. Histoplasmosis in solid organ transplant recipients: early diagnosis and treatment. Curr. Opin. Organ Transplant. 14:601–605 [DOI] [PubMed] [Google Scholar]
- 7.Kauffman CA. 2007. Histoplasmosis: a clinical and laboratory update. Clin. Microbiol. Rev. 20:115–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wheat LJ, Connolly-Stringfield P, Kohler RB, Frame PT, Gupta MR. 1989. Histoplasma capsulatum polysaccharide antigen detection in diagnosis and management of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome. Am. J. Med. 87:396–400 [DOI] [PubMed] [Google Scholar]
- 9.Wheat LJ, Garringer T, Brizendine E, Connolly P. 2002. Diagnosis of histoplasmosis by antigen detection based upon experience at the histoplasmosis reference laboratory. Diagn. Microbiol. Infect. Dis. 43:29–37 [DOI] [PubMed] [Google Scholar]
- 10.Durkin MM, Connolly PA, Wheat LJ. 1997. Comparison of radioimmunoassay and enzyme-linked immunoassay methods for detection of Histoplasma capsulatum var. capsulatum antigen. J. Clin. Microbiol. 35:2252–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheat LJ, Connolly P, Durkin M, Book BK, Pescovitz MD. 2006. Elimination of false-positive Histoplasma antigenemia caused by human anti-rabbit antibodies in the second-generation Histoplasma antigen assay. Transpl. Infect. Dis. 8:219–221 [DOI] [PubMed] [Google Scholar]
- 12.Connolly PA, Durkin MM, Lemonte AM, Hackett EJ, Wheat LJ. 2007. Detection of histoplasma antigen by a quantitative enzyme immunoassay. Clin. Vaccine Immunol. 14:1587–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hage CA, Ribes JA, Wengenack NL, Baddour LM, Assi M, McKinsey DS, Hammoud K, Alapat D, Babady NE, Parker M, Fuller D, Noor A, Davis TE, Rodgers M, Connolly PA, El Haddad B, Wheat LJ. 2011. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin. Infect. Dis. 53:448–454 [DOI] [PubMed] [Google Scholar]
- 14.Cloud JL, Bauman SK, Neary BP, Ludwig KG, Ashwood ER. 2007. Performance characteristics of a polyclonal enzyme immunoassay for the quantitation of Histoplasma antigen in human urine samples. Am. J. Clin. Pathol. 128:18–22 [DOI] [PubMed] [Google Scholar]
- 15.Scheel CM, Samayoa B, Herrera A, Lindsley MD, Benjamin L, Reed Y, Hart J, Lima S, Rivera BE, Raxcaco G, Chiller T, Arathoon E, Gomez BL. 2009. Development and evaluation of an enzyme-linked immunosorbent assay to detect Histoplasma capsulatum antigenuria in immunocompromised patients. Clin. Vaccine Immunol. 16:852–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton AJ, Bartholomew MA, Fenelon LE, Figueroa J, Hay RJ. 1990. A murine monoclonal antibody exhibiting high species specificity for Histoplasma capsulatum var. capsulatum. J. Gen. Microbiol. 136:331–335 [DOI] [PubMed] [Google Scholar]
- 17.Guimaraes AJ, Nosanchuk JD, Zancope-Oliveira RM. 2006. Diagnosis of histoplasmosis. Braz. J. Microbiol. 37:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cloud JL, Bauman SK, Pelfrey JM, Ashwood ER. 2007. Biased report on the IMMY ALPHA Histoplasma antigen enzyme immunoassay for diagnosis of histoplasmosis. Clin. Vaccine Immunol. 14:1389–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeMonte A, Egan L, Connolly P, Durkin M, Wheat LJ. 2007. Evaluation of the IMMY ALPHA histoplasma antigen enzyme immunoassay for diagnosis of histoplasmosis marked by antigenuria. Clin. Vaccine Immunol. 14:802–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheat LJ. 2008. Invalid comparison of the IMMY Histoplasma antigen assay with the “gold standard”. Am. J. Clin. Pathol. 129:661–665 [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services 2001. Guidance for industry; bioanalytical method validation. U.S. Department of Health and Human Services, Rockville, MD [Google Scholar]
- 22.Krouwer JS, Tholen DW, Garber CC, Goldschmidt HMJ, Kroll MH, Linnet K, Meier K, Robinowitz M, Kennedy JW. 2002. Method comparison and bias estimation using patient samples. Approved guideline, 2nd ed. NCCLS document EP9-A2 NCCLS, Wayne, PA [Google Scholar]
- 23.Wheat J, Wheat H, Connolly P, Kleiman M, Supparatpinyo K, Nelson K, Bradsher R, Restrepo A. 1997. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin. Infect. Dis. 24:1169–1171 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.