Abstract
Tuberculosis patients may harbor both drug-susceptible and -resistant bacteria, i.e., heteroresistance. We used mixtures of rifampin-resistant and -susceptible Mycobacterium tuberculosis strains to simulate heteroresistance in patient samples. Molecular tests can be used for earlier discovery of multidrug resistance (MDR), but the sensitivity to detect heteroresistance is unknown. Conventional phenotypic drug susceptibility testing was the most sensitive, whereas two line probe assays and sequencing were unable to detect the clinically important 1% resistant bacteria.
TEXT
Patients with tuberculosis (TB) that harbor drug-susceptible Mycobacterium tuberculosis strains may also have a small proportion of drug-resistant bacteria that develops spontaneously during replication, normally at a rate of 10−8 to 10−9 mutations/cell division (1). For rifampin (Rif) resistance, mutations are almost exclusively found in a single gene, rpoB (2). Conventional drug susceptibility testing (DST) aims to determine if 1% or more of the bacterial population in clinical specimens is drug resistant (3, 4). In this study, cultures that contain both susceptible and at least 1% resistant bacteria are defined as heteroresistant. Heteroresistance is thought to be an early stage in the development of drug-resistant TB in a patient. In such cases, failing to detect resistance may lead to insufficient treatment and treatment failure. As a consequence, spread of resistant bacteria may occur in the future (5). The prevalence of heteroresistance is unknown and is presumably dependent on the local resistance epidemiology. Findings of heteroresistance are accidental, and simple methods for the detection are needed (6).
In recent years, a number of genotypic methods have become available for rapid detection of mutations that may confer resistance. Molecular tests have been recommended for use worldwide, with the objective of earlier discovery of multidrug resistance (MDR) (http://www.who.int/tb/features_archive/policy_statement.pdf). These assays are important for the global scaling up of detection of MDR-TB. However, little is known of the sensitivity of these methods to detect resistance in heteroresistant specimens. The aim of the present study was to evaluate the ability of different DST methods to detect Rif resistance when heteroresistance is present.
Two freeze-dried strains each of the spoligo families Haarlem and Beijing were obtained from the WHO Tropical Disease Research (TDR) TB Strain Bank. The Haarlem strain TB-TDR-063 was susceptible, and TB-TDR-165 was Rif resistant with the rpoB H526Y mutation. The Beijing strain TB-TDR-077 was susceptible, and TB-TDR-068 was Rif resistant with the rpoB S531L mutation. The susceptible strains from both families had wild-type (WT) DNA in rpoB. The strains were subcultured in Dubos with 0.045% Tween 80 (SSI Diagnostika, Hilleroed, Denmark) with 1 mg/ml Rif (BD, Franklin Lakes, NJ) diluted in water for the resistant strains. After 2 weeks of incubation at 37°C, the bacterial concentrations in liquid media were adjusted to equal densities at 580 nm by adding Dubos-Tween. Subsequently, heteroresistant mixtures of 99% and 1%, 95% and 5%, 90% and 10%, and 50% and 50% were prepared by combining suspensions of susceptible and resistant bacteria belonging to the same family, as shown in Fig. 1.
Fig 1.
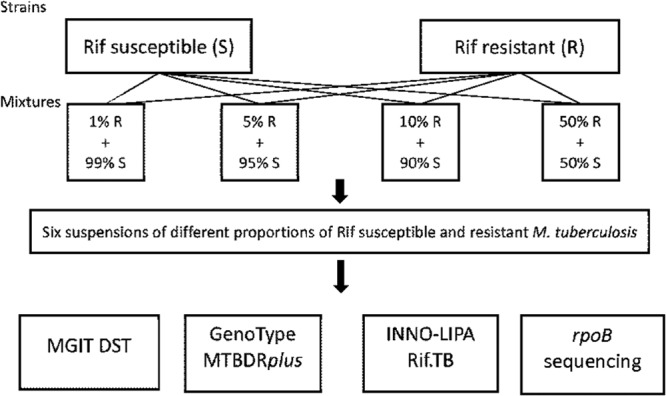
Study design for the Rif-resistant and -susceptible strains of M. tuberculosis. The same designs were applied for the Haarlem and the Beijing strains.
The ability to detect resistance in the suspensions of pure strains and mixtures of resistant and susceptible bacteria was evaluated with genotypic and phenotypic methods. The line probe assays (LPA) GenoType MTBDRplus (Hain Lifescience, Nehren, Germany) and INNO-LiPA Rif.TB (InnoGenetics, Ghent, Belgium) were carried out as previously described (7–9). With these kits, PCR of rpoB is followed by hybridization of mutants to a membrane strip. Resistance is shown as the presence of specific mutation bands and/or by the absence of WT bands (8).
The rpoB sequencing was carried out with a 5-μl lysate in PCR amplification at a total volume of 50 μl (PCR buffer, 2 mM MgCl2, 0.2 μM deoxynucleoside triphosphate [dNTP], 0.2 μM primers rpoB-F and rpoB-R [10] [DNA Technology A/S, Risskov, Denmark], 2.5 U GoTaq Flexi [Promega, Madison, WI], and water to 45 μl). Thermocycling was done with initial denaturation at 95°C for 7 min, 40 cycles of 94°C for 30 s, 59°C for 60 s, and 72°C for 60 s, a final extension at 72°C for 10 min, and cooling to 4°C on a GS1 (G-Storm, Somerset, United Kingdom). Sequencing was carried out according to the manufacturer's instructions with a BigDye Terminator version 1.1 cycle sequencing kit (Life Technologies, Naerum, Denmark) using amplification primers, analyzed on an ABI 3730 Genetic Analyzer (Life Technologies), and evaluated with Sequencing Analysis 5.3.1 (Life Technologies) and Sequencer 5.0 (Gene Codes, Ann Arbor, MI). For phenotypic DST, we used Bactec MGIT960 (BD) (11) with a 1/100 diluted control and 1.0 mg/ml Rif. All analyses were interpreted as unknown samples as in routine situations (12, 13).
We observed that the ability to detect heteroresistance was better with conventional phenotypic DST than with the molecular methods. Among the PCR-based methods, the MTBDRplus was most sensitive in detecting heteroresistance (Table 1). This difference in detection limit became more apparent with decreasing concentration of resistant bacteria. The results were somewhat different for the different rpoB mutations in the Haarlem and the Beijing strains. For the Haarlem strains, we found that MGIT DST was able to find the recommended 1% Rif resistance, MTBDRplus was able to detect mutations in rpoB if 5% resistant bacteria were present, and INNO-LiPA and automatic cycle sequencing detected resistance in suspensions with 50% resistant bacteria (Table 1). For the Beijing strains, we found that MGIT DST and MTBDRplus were able to detect Rif resistance when 5% of the bacteria were resistant, and INNO-LiPA and sequencing detected resistance in a suspension with 50% resistant bacteria. In all heteroresistant suspensions, all WT bands were present, as these suspensions also contained a large proportion of susceptible bacteria.
Table 1.
Proportion of rifampin-resistant M. tuberculosis in mixtures of susceptible and resistant bacteria detected by GenoType MTBDRplus, INNO-LiPA Rif.TB, and sequencing in comparison to phenotypic drug susceptibility testing on MGITa
| Suspension | Result |
|||||||
|---|---|---|---|---|---|---|---|---|
| H526Y (Haarlem) strain |
S531L (Beijing) strain |
|||||||
| MGITb,c,d | MTBDRb,c,d | Rif.TBb | Sequencingb,d | MGITb,c | MTBDRb,c | Rif.TBb | Sequencingb | |
| 100% R | R | ΔWT, MUT | ΔWT, MUT | MUT | R | ΔWT, MUT | ΔWT, MUT | MUT |
| 50% S + 50% R | R | WT, MUT | WT, MUT | WT, MUT | R | WT, MUT | WT, MUT | WT, MUT |
| 90% S + 10% R | R | WT, MUT | WT | WT | R | WT, MUT | WT | WT |
| 95% S + 5% R | R | WT, MUT | WT | WT | R | WT, MUT | WT | WT |
| 99% S + 1% R | R | WT | WT | WT | S | WT | WT | WT |
| 100% S | S | WT | WT | WT | S | WT | WT | WT |
The experiments were partly made in duplicate or triplicate at different laboratories. The lowest proportion of resistant bacteria detected in either laboratory is shown. R, resistant; S, susceptible; WT, wild type; ΔWT, loss of wild-type band in LPA; MUT, specific mutation detected.
Experiment carried out at Statens Serum Institut, Copenhagen, Denmark.
Experiment carried out at Swedish Institute for Communicable Diseases Control, Solna, Sweden.
Experiment carried out at Institute of Tropical Medicine, Antwerp, Belgium.
We have also tested the ability of the different methods to detect Rif resistance after different additional culture conditions altering culture media and time of culturing and culturing in the presence of CO2 and found no consistency in the limits of detecting heteroresistance, except for the finding that MGIT DST detects a smaller proportion of resistant bacteria than MTBDRplus (data not shown). All the different analyses were done once at Statens Serum Institut, Copenhagen, Denmark, and were partly repeated at the Swedish Institute for Communicable Diseases Control, Solna, Sweden, and Mycobacteriology Unit, Institute of Tropical Medicine, Antwerp, Belgium. Due to the complexity and large scale of the experiment, it was not feasible to repeat the whole study. However, the different additional culture conditions may serve as repetitions as we compare different methods for detecting resistance. When we pairwise compare the two methods in the 131 analyzed suspensions (one failed), MGIT DST found more resistance than MTBDRplus (P ≤ 0.001, signed-rank test) irrespective of subcultivation conditions.
When testing the Haarlem strains (WT and the H526Y mutant), we were able to find the targeted 1% Rif resistance with MGIT DST but not with the molecular methods. We could not find the same sensitivity for the Beijing strains (WT and the S531L mutant), in contrast to our study on isoniazid heteroresistance that did not show a difference among the test strains (13). One possible explanation is a loss of fitness in the mutated Beijing strain. This was evaluated by comparing curves of the resistant and susceptible strains in the MGIT960 system using Epicenter software from BD. There was an increased lag phase of the mutated strains, compared to that of their susceptible counterpart (Beijing or Haarlem), suggesting loss of fitness. Previous studies have, however, shown relatively low fitness costs in Rif-resistant strains (14–16). As our strains are not isogenic mutants, interstrain variability within the lineage cannot be excluded as causing the observed decrease. Another possible explanation is increased clumping of the resistant Beijing bacteria during preparation of the suspensions.
MTBDRplus could generally detect smaller proportions of resistance in a heterogeneous mixture than INNO-LiPA. This difference probably lies in the PCR primers or in the hybridization. To evaluate the LPAs' PCR cycling programs, we used the protocol for INNO-LiPA on MTBDRplus and found no differences in detection. As the INNO-LiPA Rif.TB kit became unavailable on the market, further comparisons were not possible.
In this, and in our previous study on isoniazid heteroresistance (13), the conventional phenotypic DST was more sensitive than genotypic DST in detecting the resistance. The reason for the inability of the molecular methods to detect heteroresistance in suspensions with a low proportion of resistant bacteria is probably that the concentration of the amplified fragments containing mutations is too low to be detected (see Fig. 3 in reference 13). We believe that this is true for most cases of heteroresistance. However, the very low number of studied strains is a limitation of this study. The difficulty in studying heteroresistance makes it complicated and expensive to evaluate the effectiveness of different detection methods in experimental studies and to perform epidemiological studies to assess the frequency of which heteroresistance occurs. Other important aspects of difficulties in detection of resistance are less arduous to study, such as the presence of resistant strains with undetected mutations and the presence of low-level resistant strains. For example, 314 selected strains were retrospectively sequenced for resistance mutations in various genes, including rpoB, and compared with previous DST results (17). In the other study (18), discordant rifampin DST results in a proficiency panel were analyzed. In future heteroresistance prevalence studies, the analyses need to be done on the primary samples or primary culture. In such studies, the presence of resistant strains with undetected mutations (17) or other resistance mechanisms and the presence of low-level resistant strains (18) may complicate the interpretation of the study results. In our experimental study, we did not meet those complications. Presence or absence of resistance was never difficult to detect by any method in suspensions and cultures containing 100% resistant or 100% susceptible bacteria.
In conclusion, it may be difficult to detect Rif-heteroresistant M. tuberculosis with any method, but MGIT DST was more sensitive than the tested molecular methods in this study. To our knowledge, this is the first study to test and quantify the performance of the molecular methods MTBDRplus, INNO-LiPA, and automatic cycle sequencing for Rif drug susceptibility testing and to compare them with MGIT DST when mixtures of resistant and susceptible bacteria are present in an M. tuberculosis culture.
ACKNOWLEDGMENTS
For this publication, the research leading to these results received funding from the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement FP7-223681 (TB PAN-NET).
We thank the technicians Karin Øhrberg Lund and Pia Kristiansen for their skillful work, work package colleagues for good discussions, and the WHO TDR-TB Strain Bank in Antwerp for providing strains for the study.
Footnotes
Published ahead of print 25 September 2013
REFERENCES
- 1.David HL. 1970. Probability distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl. Microbiol. 20:810–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams DL, Waguespack C, Eisenach K, Crawford JT, Portaels F, Salfinger M, Nolan CM, Abe C, Sticht-Groh V, Gillis TP. 1994. Characterization of rifampin-resistance in pathogenic mycobacteria. Antimicrob. Agents Chemother. 38:2380–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canetti G, Froman S, Grosset J, Hauduroy P, Langerová M, Mahler HT, Meissner G, Mitchison DA, Šula L. 1963. Mycobacteria: laboratory methods for testing drug sensitivity and resistance. Bull. World Health Organ. 29:565–578 [PMC free article] [PubMed] [Google Scholar]
- 4.Canetti G, Fox W, Khomenko A, Mahler HT, Menon NK, Mitchison DA, Rist N, Smelev NA. 1969. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull. World Health Organ. 41:21–43 [PMC free article] [PubMed] [Google Scholar]
- 5.Espinal M. 2004. What is the “fall and rise” phenomenon and the “sequential regimen” mechanism?, p 200–202 In Frieden T. (ed), Toman's tuberculosis: case detection, treatment, and monitoring: questions and answers. World Health Organization, Geneva, Switzerland [Google Scholar]
- 6.Hingley-Wilson SM, Casey R, Connell D, Bremang S, Evans JT, Hawkey PM, Smith GE, Jepson A, Philip S, Kon OM, Lalvani A. 2013. Undetected multidrug-resistant tuberculosis amplified by first-line therapy in mixed infection. Emerg. Infect. Dis. 19:1138–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vijdea R, Stegger M, Sosnovskaja A, Andersen AB, Thomsen VØ, Bang D. 2008. Multidrug-resistant tuberculosis: rapid detection of resistance to rifampin and high or low levels of isoniazid in clinical specimens and isolates. Eur. J. Clin. Microbiol. Infect. Dis. 27:1079–1086 [DOI] [PubMed] [Google Scholar]
- 8.Hillemann D, Rüsch-Gerdes S, Richter E. 2007. Evaluation of the GenoType MTBDRplus assay for rifampin and isoniazid susceptibility testing of Mycobacterium tuberculosis strains and clinical specimens. J. Clin. Microbiol. 45:2635–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansen IS, Lundgren B, Sosnovskaja A, Thomsen VØ. 2003. Direct detection of multidrug-resistant Mycobacterium tuberculosis in clinical specimens in low- and high-incidence countries by line probe assay. J. Clin. Microbiol. 41:4454–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekiguchi J, Miyoshi-Akiyama T, Augustynowicz-Kopeć E, Zwolska Z, Kirikae F, Toyota E, Kobayashi I, Morita K, Kudo K, Kato S, Kuratsuji T, Mori T, Kirikae T. 2007. Detection of multidrug resistance in Mycobacterium tuberculosis. J. Clin. Microbiol. 45:179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palaci M, Ueki SY, Sato DN, Da Silva Telles MA, Curcio M, Silva EA. 1996. Evaluation of mycobacteria growth indicator tube for recovery and drug susceptibility testing of Mycobacterium tuberculosis isolates from respiratory specimens. J. Clin. Microbiol. 34:762–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rinder H, Mieskes KT, Löscher T. 2001. Heteroresistance in Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 5:339–345 [PubMed] [Google Scholar]
- 13.Folkvardsen DB, Svensson E, Thomsen VØ, Rasmussen EM, Bang D, Werngren J, Hoffner S, Hillemann D, Rigouts L. 2013. Can molecular methods detect 1% isoniazid resistance in Mycobacterium tuberculosis? J. Clin. Microbiol. 51:1596–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spies FS, von Groll A, Ribeiro AW, Ramos DF, Ribeiro MO, Dalla Costa ER, Martin A, Palomino JC, Rossetti ML, Zaha A, da Silva PEA. 2012. Biological cost in Mycobacterium tuberculosis with mutations in the rpsL, rrs, rpoB, and katG genes. Tuberculosis 93:150–154 [DOI] [PubMed] [Google Scholar]
- 15.Billington OJ, McHugh TD, Gillespie SH. 1999. Physiological cost of rifampin resistance induced in vitro in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 43:1866–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariam DH, Mengistu Y, Hoffner SE, Andersson DI. 2004. Effect of rpoB mutations conferring rifampin resistance on fitness of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 48:1289–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell PJ, Morlock GP, Sikes RD, Dalton TL, Metchock B, Starks AM, Hooks DP, Cowan LS, Plikaytis BB, Posey JE. 2011. Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 55:2032–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Deun A, Barrera L, Bastian I, Fattorini L, Hoffmann H, Kam KM, Rigouts L, Rüsch-Gerdes S, Wright A. 2009. Mycobacterium tuberculosis strains with highly discordant rifampin susceptibility test results. J. Clin. Microbiol. 47:3501–3506 [DOI] [PMC free article] [PubMed] [Google Scholar]


