Abstract
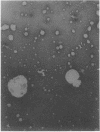
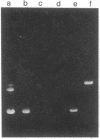
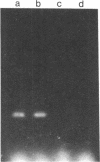
Entrapment of pBR322 DNA within liposomes was demonstrated by (i) its comigration with liposomes on Sepharose 4B columns, (ii) resistance of its biological activity to DNase digestion, and (iii) identification of plasmid DNA on agarose gels after lipid extraction. The biological activity of the liposome-entrapped plasmid was determined by transformation assays. The incubation of intact liposomes, containing entrapped pBR322, with competent Escherichia coli cells in the standard transformation mixture resulted in the appearance of tetracycline-resistant colonies at a frequency of 1% of the control frequency. Importantly, this frequency was unaffected by the addition of DNase to the incubation mixture, whereas transformation by free pBR322 DNA was totally eliminated after treatment with DNase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M. The formation of phosphatidylglycerol and other phospholipids by the transferase activity of phospholipase D. Biochem J. 1967 Jan;102(1):205–210. doi: 10.1042/bj1020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deamer D., Bangham A. D. Large volume liposomes by an ether vaporization method. Biochim Biophys Acta. 1976 Sep 7;443(3):629–634. doi: 10.1016/0005-2736(76)90483-1. [DOI] [PubMed] [Google Scholar]
- Dimitriadis G. J. Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes. Nature. 1978 Aug 31;274(5674):923–924. doi: 10.1038/274923a0. [DOI] [PubMed] [Google Scholar]
- Fraley R. T., Lueking D. R., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Polypeptide insertion into growing membrane. J Biol Chem. 1978 Jan 25;253(2):458–464. [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Interaction of Salmonella typhimurium with phospholipid vesicles. Incorporation of exogenous lipids into intact cells. J Biol Chem. 1977 Oct 25;252(20):7398–7404. [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Translocation of phospholipids between the outer and inner membranes of Salmonella typhimurium. J Biol Chem. 1977 Oct 25;252(20):7405–7412. [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- McIntyre T. M., Bell R. M. Escherichia coli mutants defective in membrane phospholipid synthesis: binding and metabolism of 1-oleoylglycerol 3-phosphate by a plsB deep rough mutant. J Bacteriol. 1978 Jul;135(1):215–226. doi: 10.1128/jb.135.1.215-226.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro M. J., Giacomoni D., Lavelle D., Paxton W., Dray S. Evidence for translation of rabbit globin mRNA after liposome-mediated insertion into a human cell line. Nature. 1978 Aug 31;274(5674):921–923. doi: 10.1038/274921a0. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Weinstein J. N. Interactions of liposomes with mammalian cells. Annu Rev Biophys Bioeng. 1978;7:435–468. doi: 10.1146/annurev.bb.07.060178.002251. [DOI] [PubMed] [Google Scholar]
- Parce J. W., Henry N., McConnell H. M. Specific antibody-dependent binding of complement component C1q to hapten-sensitized lipid vesicles. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1515–1518. doi: 10.1073/pnas.75.3.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorthuis B. J., Yazaki P. J., Hostetler K. Y. An improved two dimensional thin-layer chromatography system for the separation of phosphatidylglycerol and its derivatives. J Lipid Res. 1976 Jul;17(4):433–437. [PubMed] [Google Scholar]
- Studier F. W. Genetic mapping of a mutation that causes ribonucleases III deficiency in Escherichia coli. J Bacteriol. 1975 Oct;124(1):307–316. doi: 10.1128/jb.124.1.307-316.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. N., Yoshikami S., Henkart P., Blumenthal R., Hagins W. A. Liposome-cell interaction: transfer and intracellular release of a trapped fluorescent marker. Science. 1977 Feb 4;195(4277):489–492. doi: 10.1126/science.835007. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Collins T., Evers A., Dunham P. Membrane perturbation: studies employing a calcium-sensitive dye, arsenazo III, in liposomes. Proc Natl Acad Sci U S A. 1976 Feb;73(2):510–514. doi: 10.1073/pnas.73.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T., Papahadjopoulos D., Taber R. Biological properties of poliovirus encapsulated in lipid vesicles: antibody resistance and infectivity in virus-resistant cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3471–3475. doi: 10.1073/pnas.74.8.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]