Abstract
Dengue virus (DENV) is a mosquito-transmitted flavivirus that can cause severe disease in humans and is considered a reemerging pathogen of significant importance to public health. The DENV capsid (C) protein functions as a structural component of the infectious virion; however, it may have additional functions in the virus replicative cycle. Here, we show that the DENV C protein interacts and colocalizes with the multifunctional host protein nucleolin (NCL). Furthermore, we demonstrate that this interaction can be disrupted by the addition of an NCL binding aptamer (AS1411). Knockdown of NCL with small interfering RNA (siRNA) or treatment of cells with AS1411 results in a significant reduction of viral titers after DENV infection. Western blotting and quantitative RT-PCR (qRT-PCR) analysis revealed no differences in viral RNA or protein levels at early time points postinfection, suggesting a role for NCL in viral morphogenesis. We support this hypothesis by showing that treatment with AS1411 alters the migration characteristics of the viral capsid, as visualized by native electrophoresis. Here, we identify a critical interaction between DENV C protein and NCL that represents a potential new target for the development of antiviral therapeutics.
INTRODUCTION
Dengue virus (DENV), a member of the genus Flavivirus, causes a spectrum of disease in humans that can range from a mild febrile illness to potentially fatal hemorrhage or shock syndromes. Four serotypes of DENV (DENV-1, -2, -3, and -4) are transmitted by the bite of a mosquito vector and are responsible for more than 50 million infections each year. Infection with one DENV serotype does not confer lasting immunity to the others (1–3). The most severe clinical manifestations of dengue are associated with secondary infections by a heterologous DENV serotype (2). Increasing urbanization, cocirculation of multiple DENV serotypes within the same geographic area, and expansion of its insect vector contribute to the increasing importance of dengue to global health (2, 3).
DENV contains a positive-sense single-stranded RNA (ssRNA) genome that is translated as a single open reading frame. The resulting polyprotein is cleaved by virus and host proteases into three structural and at least seven nonstructural proteins (4, 5). The capsid (C) protein functions as a structural component of the DENV virion, encapsulating the ssRNA genome of the virus (4, 5). The DENV C protein is composed of four alpha helices with an unstructured amino terminus and forms antiparallel homodimers. In this configuration, one face of the capsid dimer is highly charged and may orchestrate RNA binding, whereas the opposite face contains a hydrophobic cleft that may enable interactions with lipids (6, 7). Packaging of the viral genome within the capsid has been shown to be coupled with RNA replication and is required for the release of infectious flavivirus particles (8, 9). The C protein binds nucleic acids promiscuously; how the DENV genome is targeted to sites of capsid assembly remains an area of active research (10, 11). Although the amino terminus of the C protein is not required for protein integrity (6, 12), deletions in the region can result in defects in particle formation in human cells (13). During virus assembly, the carboxy terminus is cleaved off the immature C protein by the viral NS2B-NS3 protease, resulting in the mature form of the protein. This cleavage of DENV C represents an important step in virus morphogenesis (5, 14).
Flavivirus replication and assembly occur in association with a complex network of membranes derived from the endoplasmic reticulum (15–17). Within the cytoplasm, the DENV C protein not only is localized to sites of virus assembly, but is found in association with lipid droplets, where it may play an important role in the formation of virus particles (18–21). The DENV C protein is also localized in the nuclei of infected cells, where it is primarily associated with the nucleolus (22–25). The role of the C protein in the nucleus has not yet been elucidated. However, it has been shown to interact with a number of (predominantly nuclear) host proteins, including hnRNP-K, DAXX, and Core histones (26–30). The cellular protein nucleolin (NCL) is found primarily within the nucleolus but can also be found in the cytoplasm and on the plasma membrane (31–33). NCL is involved in an array of different cellular processes, including ribosome biogenesis, protein transport, chromatin remodeling, translational control, and RNA processing and stability (31–34). NCL is highly expressed in a number of different malignancies, and several compounds targeting NCL are currently being developed as therapeutics for cancer, as well as for HIV (35–43).
NCL is involved in attachment or entry of several unrelated viruses, including human parainfluenza virus type 3, respiratory syncytial virus, Crimean-Congo hemorrhagic fever virus, Japanese encephalitis virus (JEV), and HIV (41–47). NCL colocalizes with hepatitis D virus delta antigen and interacts with herpes simplex virus 1 protein US11, cytomegalovirus UL44, white-spot syndrome virus VP15, influenza virus (H3N2) NS1, and the NS5B protein of hepatitis C virus. These interactions have been shown to mediate a number of different processes, including protein synthesis, RNA replication, release of virions, and trafficking of viral proteins (48–54). In addition, NCL has been shown to interact with the untranslated regions (UTR) of several other viruses, including tombusvirus, feline calicivirus, Norwalk virus, and poliovirus, where it may play roles in virus replication (55–57).
In this study, we demonstrate for the first time that DENV C protein interacts with NCL. Our data suggest that these interactions are important for the efficient replication of DENV, potentially due to its involvement in virion morphogenesis and production of infectious DENV progeny.
MATERIALS AND METHODS
Viruses, cell culture, and reagents.
The DENV serotype 2 isolate Tonga/74 was generously provided by S. Whitehead (Laboratory of Infectious Diseases [LID], NIAID) and passaged in C6/36 cells. The titer of virus was determined on Vero cells as previously described (58, 59). Encephalomyocarditis virus (EMCV) was obtained from the ATCC (Manassas, VA). HEK293 cells were obtained from Life Technologies (Grand Island, NY), and Vero cells were obtained from the ATCC and cultured in RPMI containing 10% fetal bovine serum and 50 μg/ml of penicillin and streptomycin. DENV C protein polyclonal antibodies were produced by Covance Inc. (Princeton, NJ) in rabbits immunized against the amino terminus of the DENV C protein (amino acids 5 to 26) and affinity purified. Antibody to DENV E (4G2) was provided by S. Whitehead. Antibodies to NCL and RPA1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies specific for green fluorescent protein (GFP) were obtained from Roche Diagnostics (Indianapolis, IN), and nonspecific mouse IgG was obtained from Santa Cruz Biotechnology. Purified nucleolin was obtained from Vaxron Corporation (Rockaway, NJ).
Plasmid constructs and transfections.
A plasmid containing the DENV (Tonga/74) genome was generously provided by S. Whitehead (LID, NIAID) (59). The immature form of the C gene (including the carboxy terminus) was amplified using directional forward and reverse primers (5′-CACCAGCAGATCTCTGATGAATAACCAAC-3′ and 5′-CGCCATCACTGTTGGAGTC-3′) and cloned into a pENTR/D-TOPO vector (Life Technologies, Grand Island, NY). Capsid gene sequences were then transferred to pcDNA 6.2/N-EmGFP-DEST vectors (Life Technologies) via LR recombination according to the manufacturer's protocol. A vector containing the NCL gene was purchased from the ATCC and amplified using the forward and reverse primers 5′-CACCGCCATCATGGTGAAGCTC-3′ and 5′-TTCAAACTTCGTCTTCTTTCCTTGT-3′. The NCL gene was then cloned into a pcDNA 6.2/N-EmGFP-DEST expression vector as described above. All constructs were sequenced to verify the correct sequence and orientation. Negative-control (NC) vectors were obtained from Life Technologies. Transfections were performed in HEK293 cells using Opti-MEM and Fugene HD according to the manufacturer's directions (Roche Diagnostics).
Coimmunoprecipitation and Western blot analysis.
HEK293 cells were transfected with GFP-tagged expression vectors for DENV C or NCL as described above. For NCL coimmunoprecipitation (co-IP), cells were infected with DENV (Tonga/74) at a multiplicity of infection (MOI) of 3 for 48 h. The cells were then washed in phosphate-buffered saline (PBS) and resuspended in lysis buffer (20 mM Tris, pH 7.5, 400 mM NaCL, 1 mM EDTA, 1 mM dithiothreitol [DTT], 1% Triton X-100, and protease and phosphatase inhibitors [Pierce Biotechnology, Rockford, IL]). The lysates were incubated on ice for 30 min and spun down, and the supernatants were removed to a new tube. Co-IP was then performed using protein A Dynabeads (Life Technologies) according to the manufacturer's directions. The immunoprecipitated sample was then resuspended in Novex SDS sample buffer containing NuPAGE sample-reducing agent (Life Technologies) and incubated at 95°C for 10 min. Samples were then run on 10 to 20% Tris-glycine gels and transferred to nitrocellulose membranes. The membranes were blocked in milk for 1 h, followed by overnight incubation with primary antibody. The blots were then washed and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology). Bands were visualized using SuperSignal West Femto maximum-sensitivity substrate (Pierce Biotechnology, Rockford, IL) on a LAS-3000 imaging system (Fujifilm, Edison, NJ). RNA IP was performed using the Magna RIP RNA-Binding Protein Immunoprecipitation kit (EMD Millipore, Billerica, MA) according to the manufacturer's specifications.
Mass spectrometry analysis.
Following co-IP, proteins were separated by SDS-PAGE, with a 4 to 12% NuPAGE gel running in MOPS (morpholinepropanesulfonic acid) buffer (Life Technologies), and stained with Coomassie brilliant blue (Life Technologies). Protein bands were picked with a Uni-Core Multi-Purpose Sampling Tool, and the isolated gel pieces were subjected to in-gel trypsin digestion. The supernatant and two washes (5% formic acid in 50% acetonitrile) of gel digests were pooled and concentrated by SpeedVac (Labconco, Kansas City, MO) in 200-μl polypropylene vials. The recovered peptides were resuspended in 5 μl of 0.1% formic acid, 2% acetonitrile in water and chromatographed directly onto the column without trap cleanup. The bound peptides were separated at 500 nl/min, generating 8 × 106 to 1.2 × 107 Pa pressure, using AQ C18 reverse-phase medium (3-μm particle size and 200-μm pore size) packed in a pulled-tip nanochromatography column (0.100 mm [inside diameter {i.d.}] by 150 mm [length]) from Precision Capillary Columns (San Clemente, CA). The chromatography was performed in line with an LTQ-Velos Orbitrap mass spectrometer (ThermoFisher Scientific, West Palm Beach, FL). Nano-liquid chromatography-tandem mass spectrometry (LC–MS-MS) was performed with a ProXeon Easy-nLC II multidimensional liquid chromatograph and temperature-controlled Ion Max Nanospray source (ThermoFisher Scientific) in line with the LTQ-Velos Orbitrap mass spectrometer. Computer-controlled data-dependent automated switching to MS-MS by Xcalibur 2.1 software was used for data acquisition and provided peptide sequence information. Data processing and data bank searching were performed with PD 1.2 and Mascot software (Matrix Science, Beachwood, OH). The data were searched against protein sequences deposited in the National Center for Biotechnology Information nonredundant protein database (NCBI nr; updated April 2011) and a reverse-sequence decoy database.
Native agarose gel electrophoresis (AGE).
Supernatants containing DENV were purified through a 20% sucrose cushion and incubated on ice in 0.5% NP-40 buffer for 15 min, followed by incubation at 4, 37, 55, 75, or 95°C for 10 min. Samples were then placed on ice and diluted in native sample running buffer (Life Technologies), followed by electrophoresis on a 1% agarose gel on a horizontal system in Tris-acetate-EDTA (TAE) buffer at 60 V/4°C. Samples were transferred using the TurboBlotter wet-transfer system (Schleicher & Schuell, Keene, NH) onto a nitrocellulose membrane, and Western blot analysis was performed as previously described.
qRT-PCR.
RNA was extracted from DENV-infected HEK293 cells using a Qiagen RNeasy Minikit, including the optional DNase digestion (Qiagen, Valencia, CA). RNA was extracted from cell supernatants using a QIAamp Viral RNA Mini Kit (Qiagen) according to the manufacturer's protocol, with the addition of carrier RNA and norovirus G2 RNA obtained from the ATCC (Manassas, VA), for use as a loading control. Quantitative real-time PCRs (qRT-PCRs) were set up using the Brilliant II SYBR green QRT-PCR Master Mix Kit, 1-step (Agilent Technologies, Santa Clara, CA). Reactions were run on a Stratagene Mx3000P real-time thermocycler (Agilent Technologies, Santa Clara, CA). The forward and reverse DENV primers were as follows: 5′-CAATATGCTGAAACGCGAGA-3′ and 5′-TGCTGTTGGTGGGATTGTTA-3′. The forward and reverse norovirus G2 primers were as follows: 5′-TGTGAATGAAGATGGCGTC-3′ and 5′-GCTTGTACAAAATTATTTCTAATCCA-3′. The qRT-PCR primers for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and IFIT3 were as previously described (60).
Confocal microscopy and FRET-FLIM.
HEK293 cells were plated on poly-d-lysine-coated 35-mm culture dishes (MatTek, Ashland, MA) and infected with DENV as described above. The cells were then fixed with ice-cold methanol and permeabilized with 0.1% Triton X-100, followed by blocking with 5% bovine serum albumin (BSA) for 30 min. Primary antibodies for NCL or DENV C were diluted in blocking solution at 1:200 or 1:1,000, respectively, and samples were incubated overnight at 4°C. The samples were washed three times with PBS, followed by staining with fluorescently labeled secondary antibody (1:500) and nuclear DAPI (4′,6-diamidino-2-phenylindole) stain (Life Technologies). Images were collected on a Leica SP5 inverted confocal microscope with a 63× oil immersion objective (Leica Microsystems, Buffalo Grove, IL). Colocalization analysis was performed using Imaris software (Bitplane Inc., South Windsor, CT). For fluorescence resonance energy transfer by fluorescence lifetime imaging (FRET-FLIM) analysis, fluorescence decays were resolved by time-correlated single-photon counting (TCSPC) using an SPC830 acquisition board (Becker & Hickl, Berlin, Gremany). Two-photon excitation of Alexa 488 fluorophore was performed at 810 nm by a femtosecond mode-locked (80-MHz repetition rate) Mai-Tai HP pulsed multiphoton laser (Spectra Physics). Images were acquired in 256- by 256-pixel format, collecting in excess of 1,000 photons per pixel in 2 to 5 min, and the fluorescence transients were acquired by using SPCIMAGE software (Becker & Hickl, Berlin, Germany). The results were exported and analyzed with an in-house-developed image analysis protocol using Image J NIH imaging software as previously described (61, 62).
siRNA knockdowns and inhibitors.
siRNA knockdowns were performed using Lipofectamine 2000 (Life Technologies) with NCL small interfering RNA (siRNA) (Hs_NCL_10) or AllStars negative-control siRNA obtained from Qiagen (Valencia, CA) at a final concentration of 40 nM. Knockdowns were performed according to the product recommendations, and cells were incubated for 24 h to allow maximal knockdown before infecting cells. Cell viability was tested after siRNA transfection by trypan blue staining and quantified using a Nexcelom Cellometer; no significant differences were observed in cell viability between NCL siRNA and negative-control siRNA treatment groups. AS1411 (5′-GGTGGTGGTGGTTGTGGTGGTGGTGG-3′) and the negative-control CRO (5′-CCTCCTCCTCCTTCTCCTCCTCCTCC-3′) were previously described (63, 64). Oligonucleotides were purchased from Integrated DNA Technologies, Inc., Coralville, IA, and resuspended to a stock concentration of 1 mM in molecular biology grade H2O. Stock AS1411 or CRO was diluted in RPMI medium to a concentration of 1 μM, 3 μM, 4 μM, 5 μM, 7 μM, or 10 μM as indicated in Results below.
Expression and purification of DENV-C protein and direct-interaction studies.
DENV C was amplified using the primers described above, cloned into a pET-101 vector (Life Technologies), and transformed into the Escherichia coli BL21(DE3)-RIL expression cell line. Protein expression was carried out in YT (yeast extract and tryptone) medium by inducing the culture with 1 mM isopropyl-β-d-thiogalactopyranoside at 37°C for 3 h. After induction, cells were harvested by centrifugation and lysed using standard buffer (100 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1% Triton X-100, and 1% [wt/vol] sodium deoxycholate) (65). Protein was solubilized and refolded in an oxidoredox buffer (50 mM MES [morpholineethanesulfonic acid], pH 6.5, 50 mM NaCl, 500 mM arginine-HCl, 2 mM EDTA, 40 mM sucrose, 2 mM DTT, and 5 mM cystamine-HCl), using a rapid-dilution method. The refolded protein was dialyzed against 20 mM MES buffer, pH 6.5, and purified using nickel-nitrilotriacetic acid (Ni-NTA) Sepharose columns and eluted with 0.5 M imidazole-HCl. Fractions containing refolded recombinant capsid protein were pooled and further purified by gel filtration chromatography. The purity of the protein was confirmed by 4 to 20% SDS-PAGE using standard procedures. Native PAGE interaction experiments were performed as previously described (66). In brief, 10 μg of capsid protein in PBS was combined with 0.1 μg of NCL in dilution buffer (Vaxron Corporation) or with 10- or 100-fold dilutions thereof, and AS1411 (10 μM) was added to samples as indicated. Samples were incubated at room temperature for 1 h and run on a 10% Tris-glycine gel at 120 V for 3 h, followed by either staining with Sypro Ruby (Life Technologies) or transfer onto a polyvinylidene difluoride (PVDF) membrane for Western blotting.
Statistical analysis.
Statistical analyses were performed using Graphpad Prism software version 5 (San Diego, CA). Data are represented as means and standard deviations (SD). t tests for figures (see Fig. 6B and C and 9D) were performed using a P value of 0.01. One-way analysis of variance (ANOVA) (see Fig. 3C, 7A and B, 9B, and 10C) was performed using the Tukey-Kramer post hoc test and a P value of 0.01. Analyses of colocalization experiments were performed using Imaris software version 7.6.4 (Bitplane Inc.) and the Pearson product-moment correlation coefficient method.
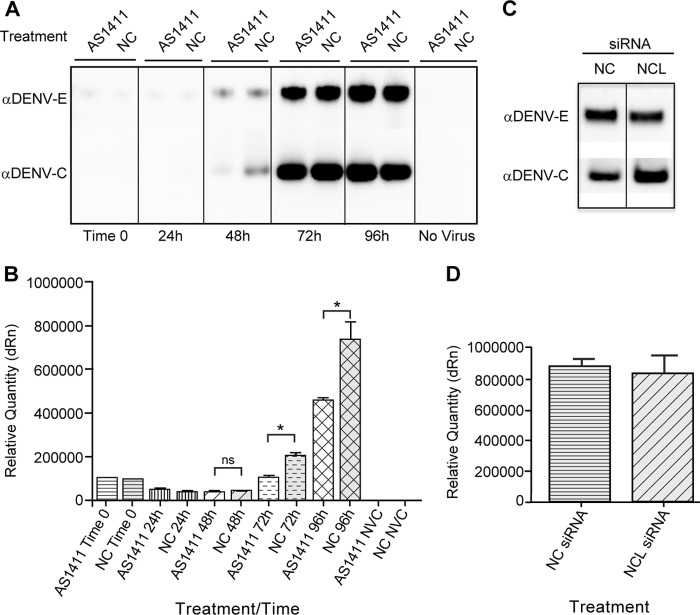
Fig 6.
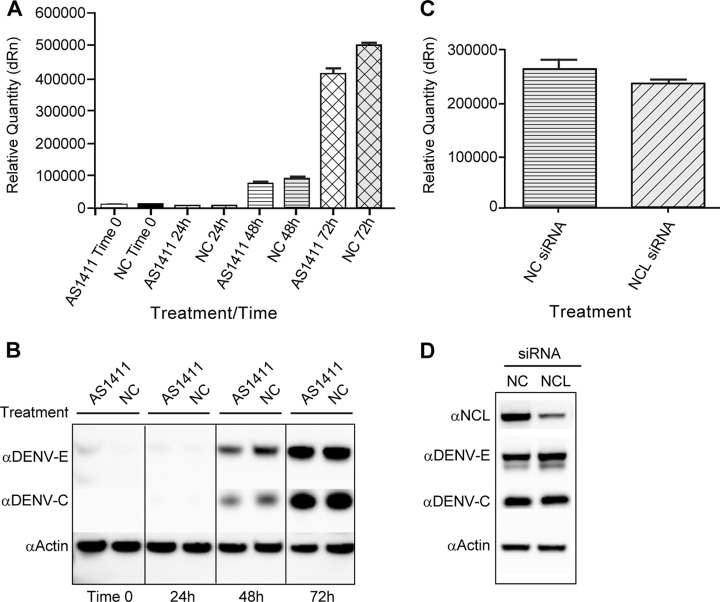
siRNA knockdown of NCL results in decreased titers of DENV. HEK293 cells were treated with NCL siRNA or NC siRNA. Twenty-four hours after siRNA treatment, the cells were infected with DENV at an MOI of 0.1, and samples were collected 72 h after infection. (A) Western blots were performed on cell lysates using antibody to NCL to verify siRNA knockdown and an antibody to actin as a loading control. (B) The titer of DENV collected from NC and NCL siRNA groups was determined in duplicate on Vero cells. Three independent experiments were used to determine significance (P < 0.01). (C) HEK293 cells were treated with NC or NCL siRNA as described for panel A. The cells were then infected with either EMCV (MOI, 0.001) or VSV (MOI, 0.001) for 24 h. The titers of samples were determined in quadruplicate on Vero cells using the limiting-dilution method, and the 50% tissue culture infectious dose (TCID50) was calculated. Two independent experiments were used to determine significance (P < 0.01). The error bars indicate SD.
Fig 9.
NCL does not significantly affect release of DENV particles. (A) Western blot of HEK293 cells infected with DENV (MOI, 3), followed by treatment with AS1411 or the NC. Cell supernatants were collected at time zero and at 24-h intervals until 96 h postinfection. Virus was purified through a sucrose cushion, and samples were analyzed by SDS-PAGE, followed by Western blotting of DENV C and DENV E proteins. The data are representative of three independent experiments. (B) qRT-PCR of viral RNA extracted from cell supernatants of DENV-infected HEK293 cells (as described for panel A) and no-virus control (NVC). Samples were analyzed using primers to DENV and normalized to norovirus G2 RNA added to the sample prior to extraction. The data are representative of two independent experiments performed in triplicate. (C) Western blot of HEK293 cells treated with siRNA to NCL or the NC, followed by infection with DENV (MOI, 3). Cell supernatants were collected at 72 h postinfection. Virus was purified through a sucrose cushion, and samples were analyzed by SDS-PAGE, followed by Western blotting of DENV C and DENV E proteins. (D) qRT-PCR of viral RNA extracted from cell supernatants of DENV-infected HEK293 cells after treatment with siRNA for NCL or negative-control siRNA. Samples were analyzed using primers to DENV and normalized to norovirus G2 RNA added to the sample prior to extraction. The data are representative of two independent experiments performed in triplicate. The error bars indicate SD.
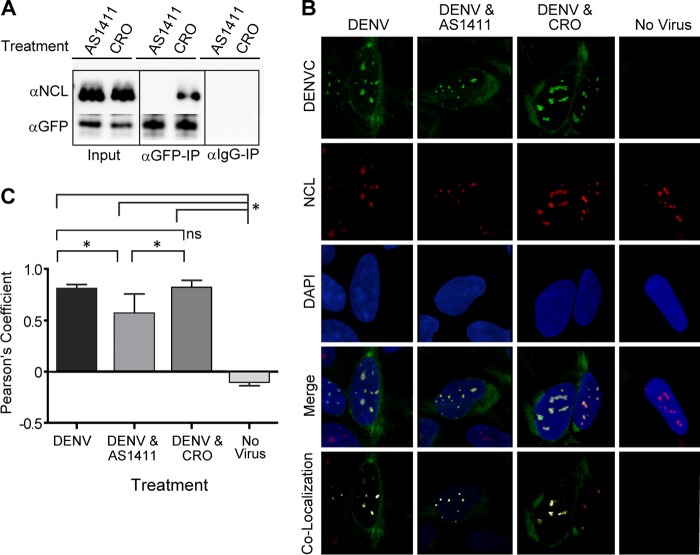
Fig 3.
Treatment with AS1411 blocks interaction between NCL and DENV C and affects colocalization. (A) Co-IP of HEK293 cells transfected with expression vector GFP-DVC. The cells were treated with AS1411 or negative-control CRO (10 μM). Co-IP was performed as previously described, and the Western blots were stained with antibodies to NCL or GFP. (B) Confocal microscopy of HEK293 cells left uninfected or infected with DENV, followed by no treatment or treatment with AS1411 or CRO (10 μM). Samples were examined for localization of DENV C (green) and NCL (red). The nucleus was stained with DAPI (blue). Colocalization of DENV C and NCL is shown in white. (C) Colocalization coefficients of DENV C and NCL in uninfected cells or cells infected with DENV and left untreated or treated with AS1411 or CRO (10 μM), as determined by Pearson's linear correlation coefficient. Significance was determined using a P value of 0.01 (indicated by an asterisk [*]) from 10 images collected from two independent experiments. ns, not significant. The error bars indicate SD.
Fig 7.
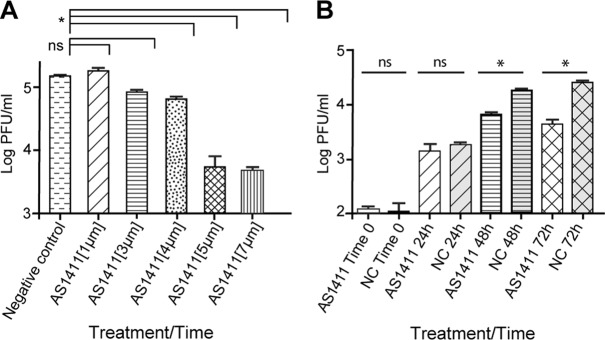
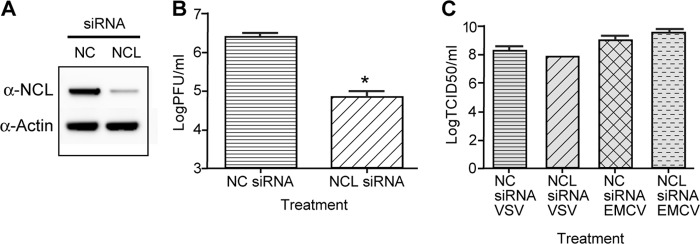
Treatment with AS1411 results in decreased titers of DENV. (A) Virus titers from HEK293 cells infected with DENV (MOI, 0.1) and treated with AS1411 at 1 μM, 3 μM, 4 μM, 5 μM, or 7 μM or with negative-control CRO at 7 μM per ml. Samples were collected at 72 h postinfection, and the titers were determined in duplicate on Vero cells (P < 0.01). (B) Virus titers from HEK293 cells infected with DENV (MOI, 3), followed by treatment with AS1411 or the NC. Samples were collected at time zero and at 24-h intervals for 72 h postinfection. The titers of samples were determined in duplicate on Vero cells. The data are representative of three independent experiments (P < 0.01). The error bars indicate SD.
Fig 10.
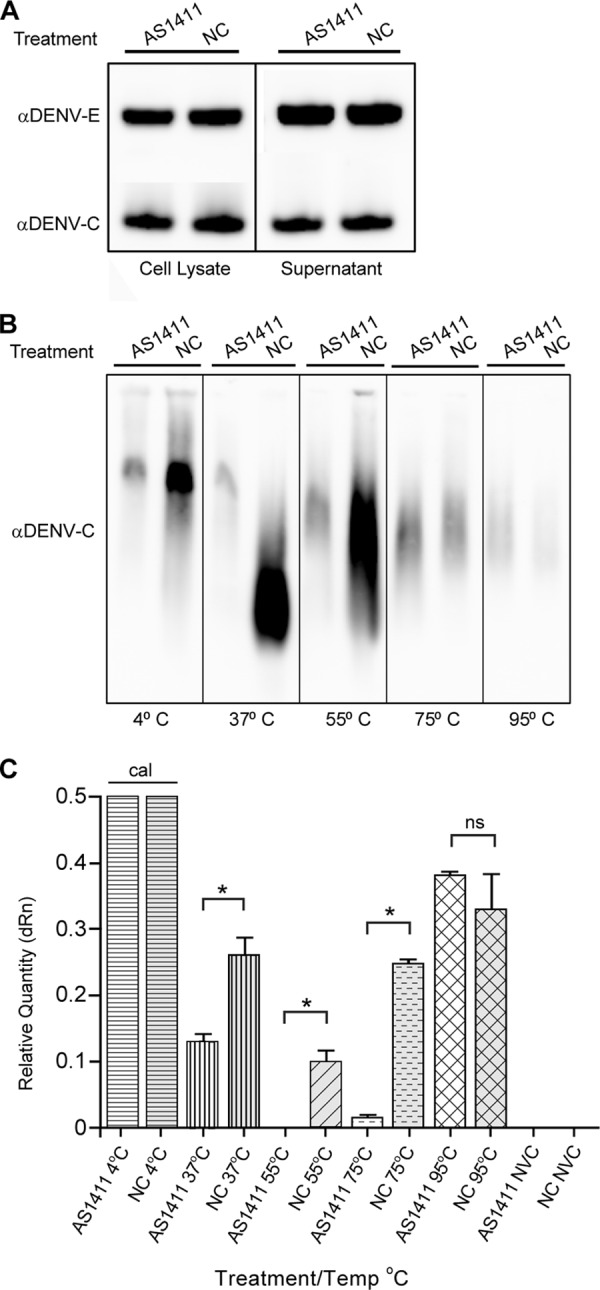
NCL affects DENV capsid migration characteristics. HEK293 cells were infected with DENV (MOI, 3), followed by treatment with AS1411 or the negative control. Cell supernatants were collected at 72 h postinfection, and virus was purified through a sucrose cushion. (A) Sucrose-purified virus was examined by SDS-PAGE, followed by Western blotting for DENV C and E proteins. (B) The sucrose-purified samples were also incubated for 10 min at the indicated temperatures and run on a native AGE gel. Samples were analyzed by Western blotting for DENV C protein. (C) qRT-PCR of sucrose-purified virus after incubation at the indicated temperatures and NVC. Viral RNA was detected by qRT-PCR using primers to DENV and normalized to the 4°C input samples. The data are representative of two independent experiments performed in triplicate. The error bars indicate SD.
RESULTS
Identification of NCL as a DENV C protein binding partner by coimmunoprecipitation and mass spectrometry analysis.
To identify potential host interaction partners of the DENV C protein in mammalian cells, DENV C was cloned into an expression vector encoding an amino-terminal (N-terminal) GFP fusion (GFP-DVC) and used as bait in co-IP studies. HEK293 cells were transfected with GFP-DVC or a negative-control expression vector carrying the chloramphenicol acetyltransferase (CAT) gene with an N-terminal GFP tag. Co-IPs were performed using a monoclonal antibody to the GFP tag. Samples were analyzed by SDS-PAGE and visualized by Coomassie staining. Individual bands were then cut for MS analysis. MS data were screened against the NCBI nr database and a reverse-sequence decoy database. Proteins were selected for further study based on a 1% false-discovery rate cutoff and a 2-peptide-per-protein minimum. Using these criteria, nine candidates were identified as potential DENV C interaction partners, including the protein NCL.
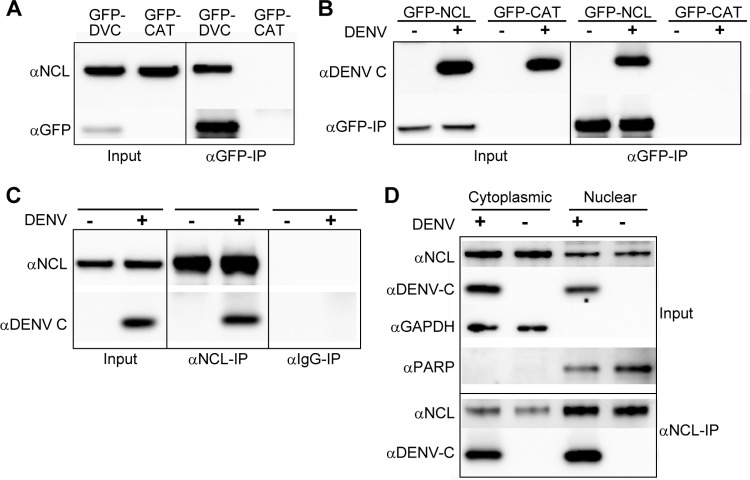
In order to validate our MS findings, interactions between DENV C and NCL were further characterized by co-IP followed by Western blotting. HEK293 cells were transfected with the GFP-DVC expression vector or a negative-control vector expressing GFP-CAT, and co-IP was performed. Western blot analysis using an antibody to the NCL protein showed a band corresponding to NCL for the GFP-DVC co-IP, indicating an interaction between DENV C and NCL (Fig. 1A). Reciprocal co-IP experiments were performed in which NCL instead of DENV C was used as bait. Here, HEK293 cells were transfected with a GFP-tagged NCL expression vector and infected with DENV for 48 h or left uninfected. Co-IPs were performed, and Western blots were stained with polyclonal antibody to the DENV C protein. Western blot analysis showed bands corresponding to the C protein for cells transfected with NCL and infected with DENV, but not for uninfected samples or cells transfected with the empty-vector control (Fig. 1B). To further validate our findings without the use of overexpressed tagged fusion proteins, endogenous co-IP was performed on nontransfected cells infected with DENV, using a monoclonal antibody to NCL or nonspecific mouse IgG as a negative control. Analysis revealed bands corresponding to the C protein in cells infected with DENV and immunoprecipitated with an anti-NCL antibody, but not from uninfected samples or samples in which co-IP was performed with nonspecific IgG (Fig. 1C). Because both NCL and DENV C have been shown to localize to the cytoplasm and nuclei of infected cells, additional endogenous co-IP experiments were performed examining interactions between NCL and DENV C in nuclear and cytoplasmic fractions from uninfected or DENV-infected cells. Nuclear/cytoplasmic fractionation was performed as previously described, and endogenous co-IPs were performed as described above (67). Western blot analysis revealed interactions between NCL and DENV C in both cytoplasmic and nuclear fractions of DENV-infected cells (Fig. 1D).
Fig 1.
NCL interacts with DENV C. (A) Co-IP of HEK293 cells transfected with an expression vector containing the DENV C gene (GFP-DVC) or a negative-control vector (GFP-CAT). Co-IP was performed using anti-GFP (αGFP) antibody, and Western blots were stained using antibodies to NCL or GFP. (B) Reciprocal co-IP of HEK293 cells transfected with expression vectors containing NCL (GFP-NCL) or GFP-CAT. Cells were infected with DENV for 48 h or left uninfected. Co-IPs were performed as described for panel A, and Western blots were stained with antibodies to DENV C or GFP. (C) Endogenous co-IP of HEK293 cells infected with DENV for 48 h or left uninfected. Co-IP was performed using αNCL antibody or a nonspecific mouse IgG antibody, and Western blots were stained with antibodies to DENV C or NCL. (D) Endogenous co-IP of HEK293 cells infected with DENV for 48 h or left uninfected. After nuclear/cytoplasmic fractionation, co-IP was performed using αNCL antibody or a nonspecific mouse IgG antibody, and Western blots were stained with antibodies to DENV C or NCL. Antibodies to GAPDH and poly(ADP-ribose) polymerase (PARP) were used as markers for the cytoplasmic and nuclear fractions, respectively. All Western blots are representative of three or more independent experiments.
DENV C interacts with NCL in an RNA-independent manner.
Both DENV C and NCL can bind RNA (25, 34). In order to determine if interactions between DENV C and NCL were occurring through RNA intermediates, co-IP experiments were performed using RNase A-treated lysates from cells transfected with the GFP-DVC expression vector. Samples treated with RNase A did not show any differences in interaction between DENV C and NCL compared to untreated samples (Fig. 2A). In contrast, treatment with RNase A abolished interactions between DENV C and PABPC1, another DENV C-interacting protein identified in our MS studies. Furthermore, RNA IP experiments performed using GFP-tagged NCL as bait failed to pull down a significant quantity of DENV RNA compared to the previously identified DENV RNA binding protein PABP (68), indicating that NCL is not strongly associated with the DENV genome (Fig. 2B). These data indicate that NCL interacts with the DENV C protein and that RNA is not required as a binding intermediate.
Fig 2.
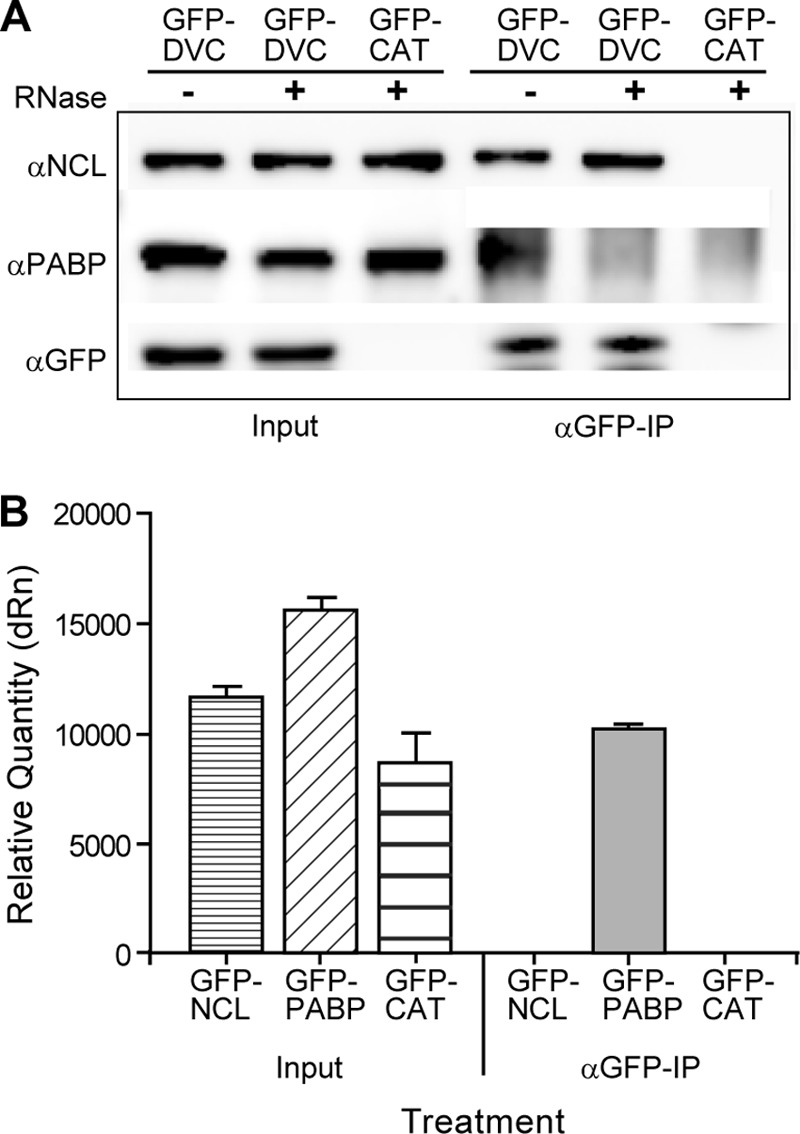
NCL interaction with DENV C is RNA independent. (A) Co-IP of HEK293 cells transfected with either GFP-DVC or GFP-CAT. Cell lysates were treated with RNase A or left untreated, and co-IP was performed using αGFP antibody as previously described. Western blots were stained using antibodies to NCL, PABP, or GFP. The Western blots are representative of three independent experiments. (B) RNA-IP of HEK293 cells transfected with GFP-NCL, GFP-PABP, or GFP-CAT expression vectors. Co-IP was performed using αGFP antibody, and RNA was extracted from the input or co-IP sample. Samples were analyzed by qRT-PCR using DENV forward and reverse primers. The data are representative of two independent experiments performed in triplicate. dRn, change in the normalized reporter signal.
Treatment of cells with the NCL binding aptamer AS1411 blocks interaction between DENV C and NCL and results in decreased colocalization.
To further characterize NCL-DENV C interactions, cells were treated with a previously characterized NCL binding aptamer (AS1411) (69, 70). HEK293 cells were transfected with the GFP-DVC expression vector, followed by treatment with AS1411 or the non-NCL binding control aptamer CRO (10 μM), and samples were analyzed by co-IP. Western blot analysis of immunoprecipitated samples revealed that treatment with AS1411 resulted in a loss of DENV C-NCL interactions, while treatment with the negative-control CRO had no effect (Fig. 3A). Cells were also examined by confocal microscopy after treatment with AS1411 and infection with DENV (Fig. 3B). Although our co-IP studies show that AS1411 was effective at blocking DENV C-NCL interactions, confocal images indicated both NCL and DENV C remained colocalized after AS1411 treatment of DENV-infected cells. However, analysis of colocalization coefficients as determined by Pearson's linear correlation coefficient revealed a moderate but statistically significant decrease in colocalization after AS1411 treatment (n = 10; P < 0.01) (Fig. 3C).
Treatment with AS1411 affects in vivo interactions between DENV C and NCL as determined by FRET-FLIM analysis.
In order to further quantify the influence of AS1411 on in vivo interactions between DENV C and NCL, HEK293 cells were infected with DENV for 72 h, followed by treatment with AS1411 (10 μM) for 24 h, and examined by FRET-FLIM analysis (Fig. 4A). DENV-infected cells displayed decreased lifetime values (1.8 to 2.2 ns) compared to uninfected controls (2.6 to 2.9 ns), representing 25 to 35% FRET efficiency and direct interactions between DENV C and NCL. Regions of high FRET occurred at discrete areas within the cytoplasm of infected cells, as well as within the nucleolus. Furthermore, treatment of DENV-infected cells with AS1411 showed a significant reduction in interaction, represented by lifetime values between 2.5 and 2.7 ns, resulting in 5 to 10% FRET efficiency and a 70 to 80% reversal in comparison to DENV-infected cells without AS1411 treatment (Fig. 4B). These data indicate that NCL interacts directly with DENV C in the nucleus and in the cytoplasm of infected cells and that addition of AS1411 can significantly impede these interactions, further validating our co-IP results. It is notable that even though interactions are affected by AS1411 treatment, both NCL and DENV C remain localized to the same regions of the cell, indicating that interactions are blocked on site.
Fig 4.
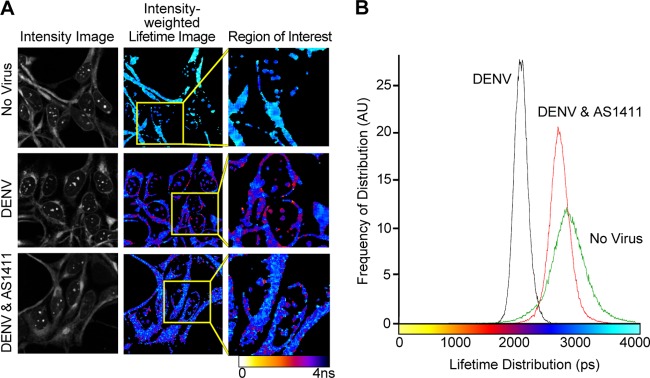
Treatment with AS1411 affects in vivo interactions between DENV C and NCL as determined by FRET-FLIM analysis. (A) Representative image of FRET-FLIM showing the image intensity, intensity-weighted lifetime, and selected region of interest in uninfected HEK293 cells and cells infected with DENV and left untreated or treated with AS1411 (10 μM). The image is representative of 7 images from each treatment group collected from two independent experiments. The color table corresponds to fluorescence lifetime values. (B) Lifetime histogram of FRET-FLIM of uninfected HEK293 cells, DENV-infected cells, and DENV-infected cells treated with AS1411. The histogram was calculated from 7 images collected from two independent experiments showing lifetime distribution in picoseconds versus frequency of distribution in arbitrary units (AU).
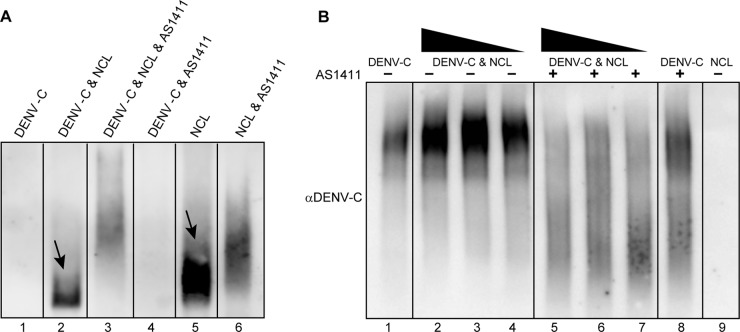
Treatment with AS1411 affects interactions between purified recombinant DENV C and purified NCL.
In order to determine if DENV C and NCL interact directly in the absence of other viral or cellular factors and how AS1411 affects this interaction, purified recombinant DENV C and purified NCL were incubated in the presence or absence of AS1411 (10 μM) and examined by native PAGE. Western blot analysis of the purified NCL protein demonstrated a clear shift in migration and a change in the staining intensity of NCL after incubation with purified recombinant DENV C protein (Fig. 5A, lanes 2 and 5), signifying a direct interaction and validating our previous FRET-FLIM results. Addition of AS1411 resulted in a marked decrease in the staining intensity of NCL (Fig. 5A, lanes 3 and 6), due to interactions between AS1411 and NCL. The NCL antibody showed no cross-reactivity to DENV C (Fig. 5A, lanes 1 and 4).
Fig 5.
Treatment with AS1411 affects interactions between purified recombinant DENV C and purified NCL. (A) Purified recombinant DENV C protein was incubated for 1 h either alone or in the presence of purified NCL, with or without AS1411 (10 μM). Samples were analyzed by native PAGE, followed by Western blotting of purified NCL protein. The arrows indicate bands corresponding to NCL. (B) Purified recombinant DENV C protein was incubated with a 1,000-fold lower concentration (by molar ratio) of purified NCL or with 10- or 100-fold dilutions thereof, with or without AS1411. Samples were analyzed by native PAGE, followed by Western blotting of recombinant DENV C protein.
Western blots from native PAGE were also analyzed for recombinant DENV C protein. Here, recombinant DENV C was incubated with a 1,000-fold lower concentration (by molar ratio) of purified NCL or with 10- or 100-fold dilutions thereof. Western blot analysis of the recombinant DENV C protein also revealed differences in band intensity between DENV C and DENV C-NCL groups (Fig. 5B, lanes 1 to 4). Treatment of DENV C-NCL complexes with AS1411 had a marked influence on band intensity (Fig. 5B, lanes 5 to 7), suggesting that interactions are affected. Treatment of DENV C with AS1411 appears to also have some influence on DENV C band intensity compared to DENV C alone, albeit with different characteristics than those observed with DENV C-NCL or DENV C-NCL-AS1411 (Fig. 5B, lane 8). This is likely due to the DENV C protein's established role in binding nucleic acids. The specificity of DENV C-AS1411 interactions and how they might contribute to AS1411's influence on NCL-DENV C interactions remains a point for future inquiry. The DENV C antibody showed no cross-reactivity to NCL (Fig. 5B, lane 9).
Knockdown of NCL results in a reduction of viral titers after infection with DENV.
To examine the effect of NCL on DENV replication, HEK293 cells were treated with either NCL siRNA or a negative-control siRNA. After 24 h of treatment with siRNA, cells were infected with DENV (MOI, 0.1), and samples were collected at 3 days postinfection. Western blot analysis verified knockdown of NCL (Fig. 6A). Knockdown of NCL resulted in a 1.6-log-unit reduction in virus titers compared to the negative-control treatment group (n = 3; P < 0.01) (Fig. 6B). To determine if the reduction in viral titers was a result of induction of a type I interferon-mediated antiviral response to treatment with NCL siRNA, infected cells were analyzed for induction of interferon-stimulated genes. qRT-PCR and Western blot analysis found no differences in the expression of the interferon-stimulated gene IFIT3 (data not shown). In addition, siRNA knockdown of NCL did not significantly reduce titers of EMCV or vesicular stomatitis virus (VSV) (P < 0.01), indicating the observed decrease in DENV titers was due to a virus-specific mechanism (Fig. 6C). Trypan blue staining showed no differences in cell viability at 72 h post-siRNA treatment.
Treatment of cells with the NCL binding aptamer AS1411 results in a reduction of DENV titers.
To determine if AS1411-mediated interference in NCL-DENV C interactions reduced DENV replication, HEK293 cells were infected with DENV, followed by treatment with various concentrations of AS1411 (1 μM, 3 μM, 4 μM, 5 μM, or 7 μM). Treatment with AS1411 resulted in a dose-dependent decrease in DENV titers, with maximum reduction occurring at a concentration of 5 μM (Fig. 7A). In addition, cells infected with DENV were treated with AS1411 (5 μM) or the negative control, and samples were collected at time zero and at 24-hour intervals thereafter until 72 h postinfection. Titration of supernatants from infected cells revealed differences in viral titers starting at 48 h (3-fold reduction; P < 0.01) and increasing in magnitude at 72 h (5.7-fold reduction; P < 0.01) (Fig. 7B). Cells treated with AS1411 or CRO were also infected with EMCV or VSV. Differences in viral titers were not observed for either virus, indicating that the effect of AS1411 is virus specific (data not shown). Cells treated with AS1411 or the negative control were also examined for inhibition of proliferation by counting trypan blue-stained cells at 72 h posttreatment on a Cellometer (Nexcelom Bioscience, Lawrence, MA). Although antiproliferative activity of AS1411 has previously been described for certain cell lines (71, 72), an effect on cell proliferation was not observed in HEK293 cells treated with inhibitory concentrations of AS1411 (data not shown).
NCL does not significantly affect DENV RNA replication or translation of viral proteins.
To examine the effects of NCL on virus replication, HEK293 cells were infected with DENV at an MOI of 3 and treated with AS1411 or CRO 2 h postadsorption. Supernatants and cell lysates were collected at time zero and at 24-hour intervals thereafter until 72 h postinfection. qRT-PCR analysis of cell-associated viral RNA and Western blot analysis of viral proteins showed little difference between the AS1411 and CRO treatment groups (Fig. 8A and B). In addition, HEK293 cells were treated with siRNA for NCL or negative-control siRNA, followed by infection with DENV for 72 h. Samples were then collected for qRT-PCR or Western blotting as described above. The results for siRNA knockdown of NCL were consistent with those for AS1411 treatment, with little difference in viral RNA (Fig. 8C) or proteins (Fig. 8D) between treatment groups.
Fig 8.
NCL does not significantly affect DENV RNA replication or protein translation. (A) qRT-PCR of viral RNA extracted from DENV-infected (MOI, 3) HEK293 cells after treatment with AS1411. Samples were analyzed using primers to DENV and normalized to GAPDH. (B) Western blot of cell lysates collected from DENV-infected cells, followed by treatment with AS1411 or the NC and collection at the indicated time points. The Western blots were stained with antibodies to the DENV C and DENV E proteins, as well as with actin. The Western blots are representative of three independent experiments. (C) qRT-PCR of viral RNA extracted from HEK293 cells treated with siRNA to NCL or nonspecific siRNA, followed by infection with DENV (MOI, 3) for 72 h. Samples were analyzed using primers to DENV and normalized to GAPDH. (D) Western blot of HEK293 cells treated with siRNA to NCL or the NC, followed by infection with DENV (MOI, 3). Samples were collected at 72 h postinfection and examined by Western blotting for NCL, DENV C, DENV E, and actin. The error bars indicate SD.
NCL does not significantly affect release of virus particles from infected cells.
To study the effect of NCL on DENV release, supernatants from cells infected with DENV at an MOI of 3 and treated with AS1411 or the negative control were collected at time zero and subsequently at 24-h intervals until 96 h postinfection. DENV particles were partially purified and analyzed by Western blotting for DENV C or DENV E protein (Fig. 9A). No differences were observed in the presence of extracellular DENV E protein, and only minor differences were observed in extracellular DENV C protein.
The RNA contents of virus preparations produced in the presence of AS1411 were evaluated. Viral RNA was isolated from cell supernatants collected from DENV-infected cells treated with AS1411 or the negative control at time zero and at 24- h intervals until 96 h postinfection and analyzed by qRT-PCR (Fig. 9B). At 48 h postinfection, there was no significant difference in the relative quantities of DENV RNA between the AS1411 and negative-control groups, despite a 3-fold reduction in virus titers at the time. At 72 h postinfection, qRT-PCR showed approximately 2-fold differences in the relative quantities of DENV RNA between AS1411 and the negative control. Supernatants from cells infected with DENV were also analyzed after treatment with siRNA for NCL or with negative-control siRNA. Here, no significant differences were observed in viral proteins (Fig. 9C) or RNA (Fig. 9D) between siRNA treatment groups.
NCL affects DENV capsid migration characteristics under native conditions.
Because the infectious titer of DENV is reduced in response to disruption of DENV C-NCL interactions but levels of extracellular DENV E protein remain relatively consistent, we next investigated whether the DENV capsid within assembled virions was altered by treatment with AS1411. One possibility is that NCL interactions are critical for the packaging of capsid into the virus particle, an aspect of DENV biology about which little is known. To investigate whether inhibiting NCL-DENV C interactions alters or perturbs capsid structure within the virion, purified virions grown in the absence or presence of AS1411 were collected at 72 h postinfection and examined by gel electrophoresis. As previously described, Western blot analysis of purified virus after denaturing SDS-PAGE revealed equivalent levels of DENV C or E protein between treatments (Fig. 10A). However, when the same samples were treated with a nondenaturing detergent followed by exposure to the indicated temperatures and analyzed by native AGE, differences between AS1411 and NC groups were evident (Fig. 10B). Treatment with AS1411 resulted in reduced staining by the DENV C antibody compared to the no-AS1411 treatment group. This difference between denaturing and native gels could reflect the availability of the DENV C epitope for binding by the DENV C antibody. In addition, different migration patterns were evident between the two groups after incubation at 37°C and 55°C, but less so after incubation at higher temperatures. This result suggests a difference in the structure or arrangement of DENV capsid in AS1411-treated samples in comparison to the negative-control group. Alterations in capsid organization or structure within the particle could render it more sensitive to treatments expected to disrupt tertiary or quaternary protein-protein interactions, such as elevated temperature.
We next examined the accessibility of encapsidated viral RNA using RT-PCR. Virus-containing supernatants were collected at 72 h and purified as previously described. Samples were then incubated at 4°C or the indicated temperatures for 10 min, followed by qRT-PCR analysis (Fig. 10C). Samples held at 4°C did not differ significantly (mean threshold cycle [CT] values, 25.6 and 25.5 for AS11411 and NC treatment, respectively) and were used as calibrators. Significant differences between AS1411 and NC treatment groups were observed after incubation at 37°C, 55°C, and 75°C, but not after incubation at 95°C (P < 0.01). This may be due to temperature-dependent changes in capsid structure affecting the accessibility of viral RNA to PCR amplification or influencing RNA stability. Altogether, both approaches suggest undefined differences in the capsid structure of AS1411-treated DENV and may explain the reduction in infectivity of DENV observed when C-NCL interactions are disrupted.
DISCUSSION
DENV relies on many host factors to support its replication and pathogenesis (5, 73–75). Here, we demonstrate that the C protein of DENV interacts with the cellular protein NCL and that this interaction plays a role in the formation of infectious virus particles. Our data suggest that the antiviral effects associated with inhibiting the interaction between NCL and DENV C are related to virion morphogenesis.
Disruption of the interactions between DENV C and NCL, whether by siRNA knockdown of NCL or treatment with an NCL binding aptamer, results in a modest but significant reduction in viral titers. We did not observe a significant difference in intracellular DENV RNA or in DENV C and E proteins in response to these treatments, suggesting that viral genome replication and protein translation are not influenced.
Closer examination of the DENV capsid under native conditions showed differential staining by DENV C antibodies and different migration characteristics after incubation at elevated temperature, both suggestive of differences in capsid conformation between the two groups. Additional RNA accessibility studies strengthened this hypothesis. We show that RNA from AS1411-treated samples is less accessible as a substrate to RT-PCR after incubation at elevated temperatures. As these differences were not observed after purification of the viral RNA, this may be due to conformational differences in the viral capsid. When viral RNA was measured from cell supernatants after DENV infection, significant differences between AS1411 and the negative-control groups were not observed at early time points. However, differences were observed at 72 h, increasing in magnitude at 96 h. In the absence of observable differences in DENV C and E proteins, this observation could be explained by variation in particle stability between the two groups. While structurally inconclusive, these data hint that treatment with AS1411 may disrupt the assembly of a stable and functional nucleocapsid complex. Virus-infected cells release both infectious virions and subviral particles that do not incorporate capsid or RNA (76, 77). How the capsid is incorporated into the infectious virus is unknown. It is also possible that NCL could play a role in modulating incorporation of the capsid into the virus particle, thus influencing the ratio of infectious virus to subviral particles.
Little is known about how the C protein is structured within the nucleocapsid. High-resolution reconstructions of DENV suggest that the C protein is disordered within the virus particle (6, 7). Flavivirus C proteins demonstrate remarkable plasticity and are able to tolerate large deletions while maintaining protein integrity and the ability to produce viable virus (12, 13, 19, 78–82). Notably, deletions in the N termini of DENV C proteins have been shown to affect virus particle formation (13). In addition, it has recently been shown that amino acid substitutions in the capsid protein of West Nile virus (WNV) can influence the virus' sensitivity to acidotropic compounds, potentially influencing virus infectivity (83). Our data suggest that interactions of NCL with DENV C may also affect the virus particle.
It is interesting that NCL plays a role in ribosome assembly and in the maturation of rRNA. It is tempting to speculate that NCL could play a similar role in the assembly of the viral nucleocapsid complex. Indeed, NCL has been shown to enhance the release and infectivity of HIV-1 and to be involved in trafficking and nuclear egress of herpesvirus nucleocapsids (49–51). NCL could act as a chaperone for DENV C, assisting in the assembly of stable nucleoprotein complexes and perhaps aiding in the transport of the nucleocapsid to sites of maturation and egress.
DENV C has been shown to associate with lipid droplets in the cytoplasm of infected cells (18, 19). The localization of DENV C to lipid droplets has been linked to the formation of virus particles (13, 18, 19). The presence of DENV C in the nucleoli of infected cells has also been observed in several prior studies (22–24, 27, 84). In addition, the DENV C protein has been shown to interact with a number of other nuclear proteins, including DAXX, hnRNP-K, and Core histones (27–29). There is evidence suggesting a role for nuclear DENV C in induction of FAS-mediated apoptosis in hepatoma cells (27). However, it has also been reported that DENV C may act to inhibit apoptosis through interactions with cyclophilin-binding ligand (30). Additionally, it has been suggested that DENV C may inhibit the formation of nucleosomes by acting as a histone mimic (29). Mutational analysis of DENV C nuclear localization signals did not demonstrate a clear correlation between nuclear localization of DENV C and viral replication (23). In JEV, however, it was shown that disrupting nuclear localization by mutagenesis affected virus replication and pathogenesis in mice (85). It is notable that interaction with NCL does not appear to be a requirement for nuclear localization of DENV C. Although treatment with AS1411 results in decreased interaction between DENV C and NCL, nuclear localization does not appear to change significantly.
In summary, we demonstrate that DENV C interacts with NCL. Both knockdown of NCL with siRNA and blocking the interaction between NCL and DENV C with the NCL binding aptamer AS1411 resulted in decreased production of infectious virus. In both cases, viral RNA replication and synthesis of viral proteins were unaffected, and virus particles could be isolated from cell supernatants. This, combined with the late appearance of differences in titers between the two groups, indicates that DENV genome replication and protein translation are not affected. Rather, it appears that NCL interaction with DENV C plays a role in virion morphogenesis, possibly resulting in differences in the stability of the virus structure. Viral proteins are capable of multiple and diverse functions within the cell and enlist an array of host factors to support viral replication. Likewise, DENV C is an integral factor in DENV replication and pathogenesis and will likely be assigned additional functions as our knowledge of flaviviruses expands.
ACKNOWLEDGMENTS
We thank the NIAID Research Technologies Branch (Ming Zhao, David Garboczi, and Steven Becker) for providing excellent technical assistance; Joseph Bekisz, Swati Mukherjee, and Falko Schmeisser for providing reagents and support; S. Whitehead and S. Best for valuable insights and discussion; and Christopher Obara and Kimberly Dowd for critical reading of the manuscript.
This work was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID).
Footnotes
Published ahead of print 11 September 2013
REFERENCES
- 1.WHO 2006. Scientific working group report on dengue. WHO reference number TDR/SWG/08. WHO, Geneva, Switzerland [Google Scholar]
- 2.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martínez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. 2010. Dengue: a continuing global threat. Nat. Rev. Microbiol. 8:S7–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyle JL, Harris E. 2008. Global spread and persistence of dengue. Annu. Rev. Microbiol. 62:71–92 [DOI] [PubMed] [Google Scholar]
- 4.Perera R, Kuhn RJ. 2008. Structural proteomics of dengue virus. Curr. Opin. Microbiol. 11:369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodenhuis-Zybert IA, Wilschut J, Smit JM. 2010. Dengue virus life cycle: viral and host factors modulating infectivity. Cell. Mol. Life Sci. 67:2773–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones CT, Ma L, Burgner JW, Groesch TD, Post CB, Kuhn RJ. 2003. Flavivirus capsid is a dimeric alpha-helical protein. J. Virol. 77:7143–7149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma L, Jones CT, Groesch TD, Kuhn RJ, Post CB. 2004. Solution structure of dengue virus capsid protein reveals another fold. Proc. Natl. Acad. Sci. U. S. A. 101:3414–3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khromykh AA, Varnavski AN, Sedlak PL, Westaway EG. 2001. Coupling between replication and packaging of flavivirus RNA: evidence derived from the use of DNA-based full-length cDNA clones of Kunjin virus. J. Virol. 75:4633–4640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanyi-Nagy R, Darlix J-L. 2012. Core protein-mediated 5′-3′ annealing of the West Nile virus genomic RNA in vitro. Virus Res. 167:226–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tseng H-N, Lee C-C, Wong M-L, Chen S-O, Liu J-J. 2007. DNA-binding property of recombinant capsid protein of Japanese encephalitis virus. Virus Genes 35:483–488 [DOI] [PubMed] [Google Scholar]
- 11.López C, Gil L, Lazo L, Menéndez I, Marcos E, Sánchez J, Valdés I, Falcón V, De la Rosa MC, Márquez G, Guillén G, Hermida L. 2009. In vitro assembly of nucleocapsid-like particles from purified recombinant capsid protein of dengue-2 virus. Arch. Virol. 154:695–698 [DOI] [PubMed] [Google Scholar]
- 12.Patkar CG, Jones CT, Chang Y, Warrier R, Kuhn RJ. 2007. Functional requirements of the yellow fever virus capsid protein. J. Virol. 81:6471–6481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samsa MM, Mondotte JA, Caramelo JJ, Gamarnik AV. 2012. Uncoupling cis-acting RNA elements from coding sequences revealed a requirement of the N-terminal region of dengue virus capsid protein in virus particle formation. J. Virol. 86:1046–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrauf S, Mandl CW, Bell-Sakyi L, Skern T. 2009. Extension of flavivirus protein C differentially affects early RNA synthesis and growth in mammalian and arthropod host cells. J. Virol. 83:11201–11210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peña J, Harris E. 2012. Early dengue virus protein synthesis induces extensive rearrangement of the endoplasmic reticulum independent of the UPR and SREBP-2 pathway. PLoS One 7:e38202. 10.1371/journal.pone.0038202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillespie LK, Hoenen A, Morgan G, Mackenzie JM. 2010. The endoplasmic reticulum provides the membrane platform for biogenesis of the flavivirus replication complex. J. Virol. 84:10438–10447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackenzie JM, Westaway EG. 2001. Assembly and maturation of the flavivirus Kunjin virus appear to occur in the rough endoplasmic reticulum and along the secretory pathway, respectively. J. Virol. 75:10787–10799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carvalho FA, Carneiro FA, Martins IC, Assunção-Miranda I, Faustino AF, Pereira RM, Bozza PT, Castanho MARB, Mohana-Borges R, Da Poian AT, Santos NC. 2012. Dengue virus capsid protein binding to hepatic lipid droplets (LD) is potassium ion dependent and is mediated by LD surface proteins. J. Virol. 86:2096–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samsa MM, Mondotte JA, Iglesias NG, Assunção-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV. 2009. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 5:e1000632. 10.1371/journal.ppat.1000632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martins IC, Gomes-Neto F, Faustino AF, Carvalho FA, Carneiro FA, Bozza PT, Mohana-Borges R, Castanho MARB, Almeida FCL, Santos NC, Da Poian AT. 2012. The disordered N-terminal region of dengue virus capsid protein contains a lipid-droplet-binding motif. Biochem. J. 444:405–415 [DOI] [PubMed] [Google Scholar]
- 21.Byrd CM, Dai D, Grosenbach DW, Berhanu A, Jones KF, Cardwell KB, Schneider C, Wineinger KA, Page JM, Harver C, Stavale E, Tyavanagimatt S, Stone MA, Bartenschlager R, Scaturro P, Hruby DE, Jordan R. 2013. A novel inhibitor of dengue virus replication that targets the capsid protein. Antimicrob. Agents Chemother. 57:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulich R, Aaskov JG. 1992. Nuclear localization of dengue 2 virus core protein detected with monoclonal antibodies. J. Gen. Virol. 73:2999–3003 [DOI] [PubMed] [Google Scholar]
- 23.Sangiambut S, Keelapang P, Aaskov J, Puttikhunt C, Kasinrerk W, Malasit P, Sittisombut N. 2008. Multiple regions in dengue virus capsid protein contribute to nuclear localization during virus infection. J. Gen. Virol. 89:1254–1264 [DOI] [PubMed] [Google Scholar]
- 24.Wang S-H, Syu W-J, Huang K-J, Lei H-Y, Yao C-W, King C-C, Hu S-T. 2002. Intracellular localization and determination of a nuclear localization signal of the core protein of dengue virus. J. Gen. Virol. 83:3093–3102 [DOI] [PubMed] [Google Scholar]
- 25.Pong W-L, Huang Z-S, Teoh P-G, Wang C-C, Wu H-N. 2011. RNA binding property and RNA chaperone activity of dengue virus core protein and other viral RNA-interacting proteins. FEBS Lett. 585:2575–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Limjindaporn T, Netsawang J, Noisakran S, Thiemmeca S, Wongwiwat W, Sudsaward S, Avirutnan P, Puttikhunt C, Kasinrerk W, Sriburi R, Sittisombut N, Yenchitsomanus P-T, Malasit P. 2007. Sensitization to Fas-mediated apoptosis by dengue virus capsid protein. Biochem. Biophys. Res. Commun. 362:334–339 [DOI] [PubMed] [Google Scholar]
- 27.Netsawang J, Noisakran S, Puttikhunt C, Kasinrerk W, Wongwiwat W, Malasit P, Yenchitsomanus P, Limjindaporn T. 2010. Nuclear localization of dengue virus capsid protein is required for DAXX interaction and apoptosis. Virus Res. 147:275–283 [DOI] [PubMed] [Google Scholar]
- 28.Chang CJ, Luh HW, Wang SH, Lin HJ, Lee SC, Hu ST. 2001. The heterogeneous nuclear ribonucleoprotein K (hnRNP K) interacts with dengue virus core protein. DNA Cell Biol. 20:569–577 [DOI] [PubMed] [Google Scholar]
- 29.Colpitts TM, Barthel S, Wang P, Fikrig E. 2011. Dengue virus capsid protein binds core histones and inhibits nucleosome formation in human liver cells. PLoS One 6:e24365. 10.1371/journal.pone.0024365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Huang R, Liao W, Chen Z, Zhang S, Huang R. 2012. Dengue virus utilizes calcium modulating cyclophilin-binding ligand to subvert apoptosis. Biochem. Biophys. Res. Commun. 418:622–627 [DOI] [PubMed] [Google Scholar]
- 31.Mongelard F, Bouvet P. 2007. Nucleolin: a multiFACeTed protein. Trends Cell Biol. 17:80–86 [DOI] [PubMed] [Google Scholar]
- 32.Tajrishi MM, Tuteja R, Tuteja N. 2011. Nucleolin: the most abundant multifunctional phosphoprotein of nucleolus. Commun. Integr. Biol. 4:267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo SJ, Lee C-C, Lai H-J. 2006. The nucleolus: reviewing oldies to have new understandings. Cell Res. 16:530–538 [DOI] [PubMed] [Google Scholar]
- 34.Abdelmohsen K, Gorospe M. 2012. RNA-binding protein nucleolin in disease. RNA Biol. 9:799–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mongelard F, Bouvet P. 2010. AS-1411, a guanosine-rich oligonucleotide aptamer targeting nucleolin for the potential treatment of cancer, including acute myeloid leukemia. Curr. Opin. Mol. Ther. 12:107–114 [PubMed] [Google Scholar]
- 36.Soundararajan S, Wang L, Sridharan V, Chen W, Courtenay-Luck N, Jones D, Spicer EK, Fernandes DJ. 2009. Plasma membrane nucleolin is a receptor for the anticancer aptamer AS1411 in MV4-11 leukemia cells. Mol. Pharmacol. 76:984–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krust B, El Khoury D, Soundaramourty C, Nondier I, Hovanessian AG. 2011. Suppression of tumorigenicity of rhabdoid tumor derived G401 cells by the multivalent HB-19 pseudopeptide that targets surface nucleolin. Biochimie 93:426–433 [DOI] [PubMed] [Google Scholar]
- 38.Destouches D, Page N, Hamma-Kourbali Y, Machi V, Chaloin O, Frechault S, Birmpas C, Katsoris P, Beyrath J, Albanese P, Maurer M, Carpentier G, Strub J-M, Van Dorsselaer A, Muller S, Bagnard D, Briand JP, Courty J. 2011. A simple approach to cancer therapy afforded by multivalent pseudopeptides that target cell-surface nucleoproteins. Cancer Res. 71:3296–3305 [DOI] [PubMed] [Google Scholar]
- 39.Zhuo W, Luo C, Wang X, Song X, Fu Y, Luo Y. 2010. Endostatin inhibits tumour lymphangiogenesis and lymphatic metastasis via cell surface nucleolin on lymphangiogenic endothelial cells. J. Pathol. 222:249–260 [DOI] [PubMed] [Google Scholar]
- 40.Shi H, Huang Y, Zhou H, Song X, Yuan S, Fu Y, Luo Y. 2007. Nucleolin is a receptor that mediates antiangiogenic and antitumor activity of endostatin. Blood 110:2899–2906 [DOI] [PubMed] [Google Scholar]
- 41.Hovanessian AG. 2006. Midkine, a cytokine that inhibits HIV infection by binding to the cell surface expressed nucleolin. Cell Res. 16:174–181 [DOI] [PubMed] [Google Scholar]
- 42.Said EA, Courty J, Svab J, Delbé J, Krust B, Hovanessian AG. 2005. Pleiotrophin inhibits HIV infection by binding the cell surface-expressed nucleolin. FEBS J. 272:4646–4659 [DOI] [PubMed] [Google Scholar]
- 43.Nisole S, Krust B, Callebaut C, Guichard G, Muller S, Briand JP, Hovanessian AG. 1999. The anti-HIV pseudopeptide HB-19 forms a complex with the cell-surface-expressed nucleolin independent of heparan sulfate proteoglycans. J. Biol. Chem. 274:27875–27884 [DOI] [PubMed] [Google Scholar]
- 44.Bose S, Basu M, Banerjee AK. 2004. Role of nucleolin in human parainfluenza virus type 3 infection of human lung epithelial cells. J. Virol. 78:8146–8158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tayyari F, Marchant D, Moraes TJ, Duan W, Mastrangelo P, Hegele RG. 2011. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat. Med. 17:1132–1135 [DOI] [PubMed] [Google Scholar]
- 46.Xiao X, Feng Y, Zhu Z, Dimitrov DS. 2011. Identification of a putative Crimean-Congo hemorrhagic fever virus entry factor. Biochem. Biophys. Res. Commun. 411:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thongtan T, Wikan N, Wintachai P, Rattanarungsan C, Srisomsap C, Cheepsunthorn P, Smith DR. 2012. Characterization of putative Japanese encephalitis virus receptor molecules on microglial cells. J. Med. Virol. 84:615–623 [DOI] [PubMed] [Google Scholar]
- 48.Izumi RE, Valdez B, Banerjee R, Srivastava M, Dasgupta A. 2001. Nucleolin stimulates viral internal ribosome entry site-mediated translation. Virus Res. 76:17–29 [DOI] [PubMed] [Google Scholar]
- 49.Greco A, Arata L, Soler E, Gaume X, Couté Y, Hacot S, Callé A, Monier K, Epstein AL, Sanchez J-C, Bouvet P, Diaz J-J. 2012. Nucleolin interacts with US11 protein of herpes simplex virus 1 and is involved in its trafficking. J. Virol. 86:1449–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sagou K, Uema M, Kawaguchi Y. 2010. Nucleolin is required for efficient nuclear egress of herpes simplex virus type 1 nucleocapsids. J. Virol. 84:2110–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueno T, Tokunaga K, Sawa H, Maeda M, Chiba J, Kojima A, Hasegawa H, Shoya Y, Sata T, Kurata T, Takahashi H. 2004. Nucleolin and the packaging signal, psi, promote the budding of human immunodeficiency virus type-1 (HIV-1). Microbiol. Immunol. 48:111–118 [DOI] [PubMed] [Google Scholar]
- 52.Shimakami T, Honda M, Kusakawa T, Murata T, Shimotohno K, Kaneko S, Murakami S. 2006. Effect of hepatitis C virus (HCV) NS5B-nucleolin interaction on HCV replication with HCV subgenomic replicon. J. Virol. 80:3332–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hiscox JA. 2002. The nucleolus—a gateway to viral infection? Arch. Virol. 147:1077–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melén K, Tynell J, Fagerlund R, Roussel P, Hermandez-Verdun D, Julkunen I. 2012. Influenza A H3N2 subtype virus NS1 protein targets into the nucleus and binds primarily via its C-terminal NLS2/NoLS to nucleolin and fibrillarin. Virol. J. 9:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang Y, Li Z, Nagy PD. 2010. Nucleolin/Nsr1p binds to the 3′ noncoding region of the tombusvirus RNA and inhibits replication. Virology 396:10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cancio-Lonches C, Yocupicio-Monroy M, Sandoval-Jaime C, Galvan-Mendoza I, Ureña L, Vashist S, Goodfellow I, Salas-Benito J, Gutiérrez-Escolano AL. 2011. Nucleolin interacts with the feline calicivirus 3′ untranslated region and the protease-polymerase NS6 and NS7 proteins, playing a role in virus replication. J. Virol. 85:8056–8068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waggoner S, Sarnow P. 1998. Viral ribonucleoprotein complex formation and nucleolar-cytoplasmic relocalization of nucleolin in poliovirus-infected cells. J. Virol. 72:6699–6709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gubler DJ, Reed D, Rosen L, Hitchcock JR., Jr 1978. Epidemiologic, clinical, and virologic observations on dengue in the Kingdom of Tonga. Am. J. Trop. Med. Hyg. 27:581–589 [DOI] [PubMed] [Google Scholar]
- 59.Blaney JE, Jr, Hanson CT, Hanley KA, Murphy BR, Whitehead SS. 2004. Vaccine candidates derived from a novel infectious cDNA clone of an American genotype dengue virus type 2. BMC Infect. Dis. 4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmeisser H, Mejido J, Balinsky CA, Morrow AN, Clark CR, Zhao T, Zoon KC. 2010. Identification of alpha interferon-induced genes associated with antiviral activity in Daudi cells and characterization of IFIT3 as a novel antiviral gene. J. Virol. 84:10671–10680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ganesan S, Rohde G, Eckermann K, Sroka K, Schaefer MKE, Dohm CP, Kermer P, Haase G, Wouters F, Bähr M, Weishaupt JH. 2008. Mutant SOD1 detoxification mechanisms in intact single cells. Cell Death Differ. 15:312–321 [DOI] [PubMed] [Google Scholar]
- 63.Ireson CR, Kelland LR. 2006. Discovery and development of anticancer aptamers. Mol. Cancer Ther. 5:2957–2962 [DOI] [PubMed] [Google Scholar]
- 64.Reyes-Reyes EM, Teng Y, Bates PJ. 2010. A new paradigm for aptamer therapeutic AS1411 action: uptake by macropinocytosis and its stimulation by a nucleolin-dependent mechanism. Cancer Res. 70:8617–8629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh K, Gitti RK, Diouf A, Zhou H, Gowda DC, Miura K, Ostazeski SA, Fairhurst RM, Garboczi DN, Long CA. 2010. Subdomain 3 of Plasmodium falciparum VAR2CSA DBL3x is identified as a minimal chondroitin sulfate A-binding region. J. Biol. Chem. 285:24855–24862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmeisser H, Gorshkova I, Brown PH, Kontsek P, Schuck P, Zoon KC. 2007. Two interferons alpha influence each other during their interaction with the extracellular domain of human type interferon receptor subunit 2. Biochemistry 46:14638–14649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schreiber E, Matthias P, Müller MM, Schaffner W. 1989. Rapid detection of octamer binding proteins with “mini-extracts”, prepared from a small number of cells. Nucleic Acids Res. 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Polacek C, Friebe P, Harris E. 2009. Poly(A)-binding protein binds to the non-polyadenylated 3′ untranslated region of dengue virus and modulates translation efficiency. J. Gen. Virol. 90:687–692 [DOI] [PubMed] [Google Scholar]
- 69.Dapić V, Bates PJ, Trent JO, Rodger A, Thomas SD, Miller DM. 2002. Antiproliferative activity of G-quartet-forming oligonucleotides with backbone and sugar modifications. Biochemistry 41:3676–3685 [DOI] [PubMed] [Google Scholar]
- 70.Dapić V, Abdomerović V, Marrington R, Peberdy J, Rodger A, Trent JO, Bates PJ. 2003. Biophysical and biological properties of quadruplex oligodeoxyribonucleotides. Nucleic Acids Res. 31:2097–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bates PJ, Kahlon JB, Thomas SD, Trent JO, Miller DM. 1999. Antiproliferative activity of G-rich oligonucleotides correlates with protein binding. J. Biol. Chem. 274:26369–26377 [DOI] [PubMed] [Google Scholar]
- 72.Bates PJ, Laber DA, Miller DM, Thomas SD, Trent JO. 2009. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 86:151–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagy PD, Pogany J. 2012. The dependence of viral RNA replication on co-opted host factors. Nat. Rev. Microbiol. 10:137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pastorino B, Nougairède A, Wurtz N, Gould E, De Lamballerie X. 2010. Role of host cell factors in flavivirus infection: implications for pathogenesis and development of antiviral drugs. Antiviral Res. 87:281–294 [DOI] [PubMed] [Google Scholar]
- 75.Paranjape SM, Harris E. 2010. Control of dengue virus translation and replication. Curr. Top. Microbiol. Immunol. 338:15–34 [DOI] [PubMed] [Google Scholar]
- 76.Konishi E, Fujii A. 2002. Dengue type 2 virus subviral extracellular particles produced by a stably transfected mammalian cell line and their evaluation for a subunit vaccine. Vaccine 20:1058–1067 [DOI] [PubMed] [Google Scholar]
- 77.Wang P-G, Kudelko M, Lo J, Siu LYL, Kwok KTH, Sachse M, Nicholls JM, Bruzzone R, Altmeyer RM, Nal B. 2009. Efficient assembly and secretion of recombinant subviral particles of the four dengue serotypes using native prM and E proteins. PLoS One 4:e8325. 10.1371/journal.pone.0008325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu W, Qin C, Chen S, Jiang T, Yu M, Yu X, Qin E. 2007. Attenuated dengue 2 viruses with deletions in capsid protein derived from an infectious full-length cDNA clone. Virus Res. 126:226–232 [DOI] [PubMed] [Google Scholar]
- 79.Kofler RM, Heinz FX, Mandl CW. 2002. Capsid protein C of tick-borne encephalitis virus tolerates large internal deletions and is a favorable target for attenuation of virulence. J. Virol. 76:3534–3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schlick P, Kofler RM, Schittl B, Taucher C, Nagy E, Meinke A, Mandl CW. 2010. Characterization of West Nile virus live vaccine candidates attenuated by capsid deletion mutations. Vaccine 28:5903–5909 [DOI] [PubMed] [Google Scholar]
- 81.Schlick P, Taucher C, Schittl B, Tran JL, Kofler RM, Schueler W, Von Gabain A, Meinke A, Mandl CW. 2009. Helices alpha2 and alpha3 of West Nile virus capsid protein are dispensable for assembly of infectious virions. J. Virol. 83:5581–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Groat-Carmona AM, Orozco S, Friebe P, Payne A, Kramer L, Harris E. 2012. A novel coding-region RNA element modulates infectious dengue virus particle production in both mammalian and mosquito cells and regulates viral replication in Aedes aegypti mosquitoes. Virology 432:511–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martín-Acebes MA, Blázquez A-B, De Oya NJ, Escribano-Romero E, Shi P-Y, Saiz J-C. 2013. A single amino acid substitution in the core protein of West Nile virus increases resistance to acidotropic compounds. PLoS One 8:e69479. 10.1371/journal.pone.0069479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Makino Y, Tadano M, Anzai T, Ma SP, Yasuda S, Fukunaga T. 1989. Detection of dengue 4 virus core protein in the nucleus. II. Antibody against dengue 4 core protein produced by a recombinant baculovirus reacts with the antigen in the nucleus. J. Gen. Virol. 70:1417–1425 [DOI] [PubMed] [Google Scholar]
- 85.Mori Y, Okabayashi T, Yamashita T, Zhao Z, Wakita T, Yasui K, Hasebe F, Tadano M, Konishi E, Moriishi K, Matsuura Y. 2005. Nuclear localization of Japanese encephalitis virus core protein enhances viral replication. J. Virol. 79:3448–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]