Abstract
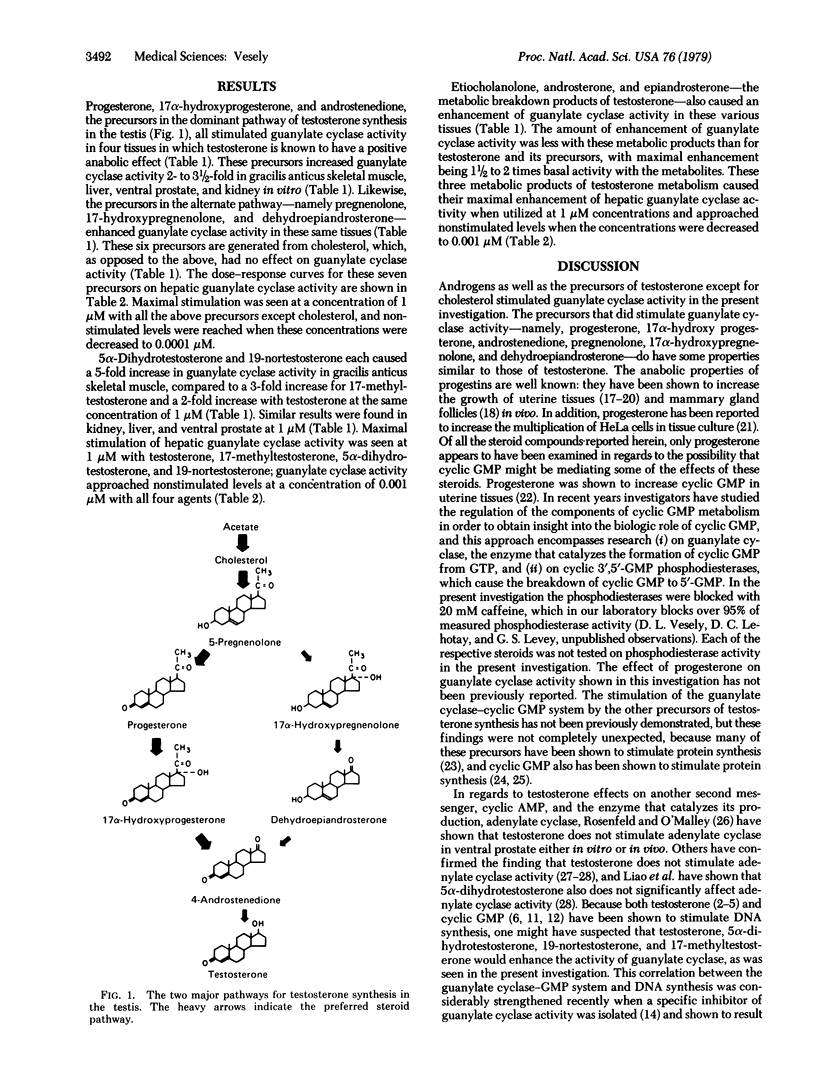
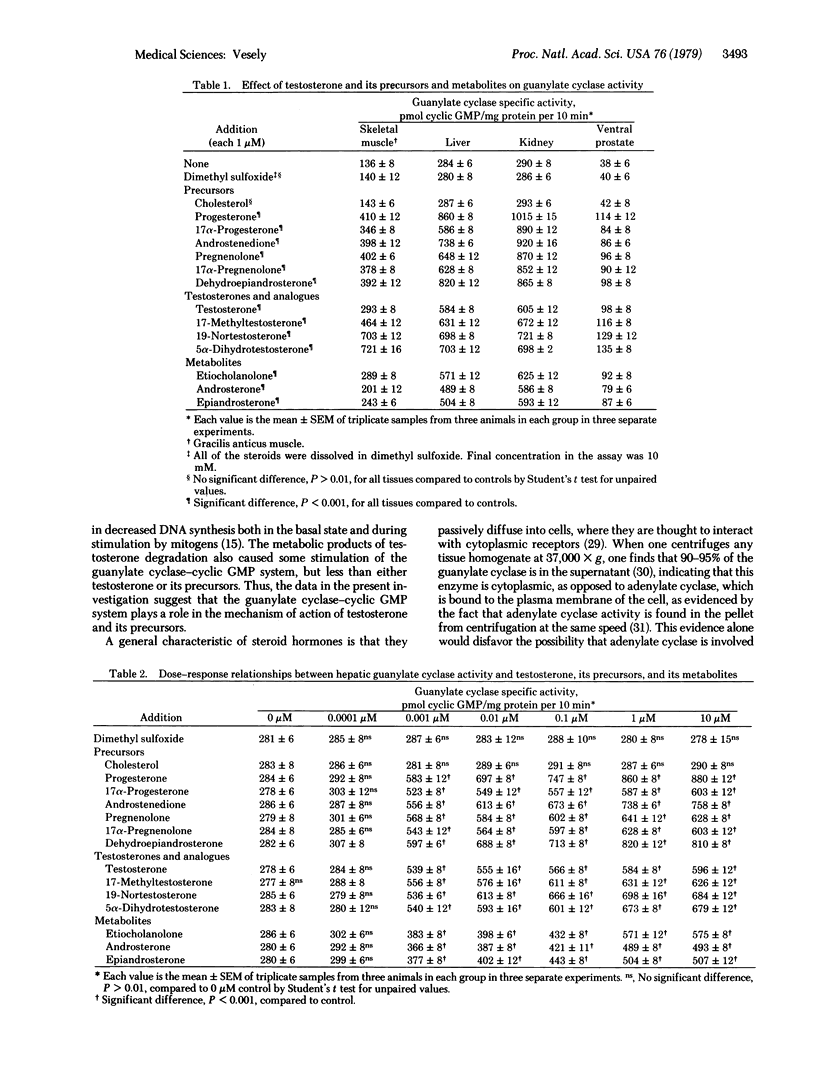
Both testosterone and cyclic GMP stimulate DNA synthesis. Because cyclic GMP and testosterone seem to have similar actions, the objective of this investigation was to determine if testosterone and its precursors might have part of their mechanism of action through stimulation of guanylate cyclase [GTP pyrophosphate-lyase (cyclizing), EC 4.6.1.2], the enzyme that catalyzes the formation of cyclic GMP from GTP. The precursors--namely, progesterone, pregnenolone, 17 alpha-progesterone, 17 alpha-hydroxypregnenolone, androstenedione, and dehydroepiandrosterone--caused a 2- to 3 1/2-fold enhancement of guanylate cyclase activity in rat liver, kidney, skeletal muscle, and ventral prostate at a concentration of 1 microM. These precursors are generated from cholesterol, which had no effect itself on guanylate cyclase activity. Testosterone, 19-nortestosterone, 17-methyltestosterone, and 5 alpha-dihydrotestosterone enhanced guanylate cyclase activity 2- to 5-fold in the same tissues at 1 microM. Etiocholanolone, androsterone, and epiandrosterone, metabolites of testosterone metabolism, enhanced guanylate cyclase activity 1 1/2- to 2-fold at this same concentration. Dose-response relationships revealed that testosterone and its precursors and metabolites had their maximal effect at 1 microM but still had some effect at 0.001 microM. The data in this investigation suggest that the guanylate cyclase-cyclic GMP system plays a role in the mechanism of action of testosterone and its precursors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CULLEN B. M., HARKNESS R. D. The effect of hormones on the physical properties and collagen content of the rat's uterine cervix. J Physiol. 1960 Jul;152:419–436. doi: 10.1113/jphysiol.1960.sp006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claflin A. J., Vesely D. L., Hudson J. L., Bagwell C. B., Lehotay D. C., Lo T. M., Fletcher M. A., Block N. L., Levey G. S. Inhibition of growth and guanylate cyclase activity of an undifferentiated prostate adenocarcinoma by an extract of the balsam pear (Momordica charantia abbreviata). Proc Natl Acad Sci U S A. 1978 Feb;75(2):989–993. doi: 10.1073/pnas.75.2.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey D. S., Shimazaki J., Williams-Ashman H. G. Polymerization of deoxyribonucleotides in relation to androgen-induced prostatic growth. Arch Biochem Biophys. 1968 Mar 20;124(1):184–198. doi: 10.1016/0003-9861(68)90319-6. [DOI] [PubMed] [Google Scholar]
- DeRubertis F. R., Chayoth R., Field J. B. The content and metabolism of cyclic adenosine 3', 5'-monophosphate and cyclic guanosine 3', 5'-monophosphate in adenocarcinoma of the human colon. J Clin Invest. 1976 Mar;57(3):641–649. doi: 10.1172/JCI108320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandroy L., Galand P. Changes in cGMP and cAMP content in the estrogen-stimulated rat uterus: temporal relationship with other parameters of hormonal stimulation. J Cyclic Nucleotide Res. 1978 Apr;4(2):145–158. [PubMed] [Google Scholar]
- Golder M. P., Boyns A. R., Harper M. E., Griffiths K. An effect of prolactin on prostatic adenylate cyclase activity. Biochem J. 1972 Jul;128(3):725–727. doi: 10.1042/bj1280725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON T. H. SEQUENCES OF RNA AND PROTEIN SYNTHESIS DURING EARLY ESTROGEN ACTION. Proc Natl Acad Sci U S A. 1964 Jan;51:83–89. doi: 10.1073/pnas.51.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWADA I. Effects of progesterone and estrogens on the multiplication of chick embryo fibroblasts, ratascites hepatoma cells, and strainHeLa cells (cervical carcinoma of human uterus) in tissue culture. Jpn J Exp Med. 1959 Dec;29:615–626. [PubMed] [Google Scholar]
- Kimura H., Murad F. Increased particulate and decreased soluble guanylate cyclase activity in regenerating liver, fetal liver, and hepatoma. Proc Natl Acad Sci U S A. 1975 May;72(5):1965–1969. doi: 10.1073/pnas.72.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kram R., Tomkins G. M. Pleiotypic control by cyclic AMP: interaction with cyclic GMP and possible role of microtubules. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1659–1663. doi: 10.1073/pnas.70.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Ham E. A., Zanetti M. E., Sanford C. H., Nicol S. E., Goldberg N. D. Estrogen-related increases in uterine guanosine 3':5'-cyclic momophosphate levels. Proc Natl Acad Sci U S A. 1974 May;71(5):1866–1870. doi: 10.1073/pnas.71.5.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanzani G. A., Giannattasio M., Manzocchi L. A., Bollini R., Soffientini A. N., Macchia V. The influence of cyclic GMP on polypeptide synthesis in a cell-free system derived from wheat embryos. Biochem Biophys Res Commun. 1974 May 7;58(1):172–177. doi: 10.1016/0006-291x(74)90907-3. [DOI] [PubMed] [Google Scholar]
- Lesser B., Bruchovsky N. The effects of testosterone, 5 -dihydrotestosterone and adenosine 3',5'-monophosphate on cell proliferation and differentiation in rat prostate. Biochim Biophys Acta. 1973 May 18;308(3):426–437. doi: 10.1016/0005-2787(73)90336-5. [DOI] [PubMed] [Google Scholar]
- Liao S., Lin A. H., Tymoczko J. L. Adenyl cyclase of cell nuclei isolated from rat ventral prostate. Biochim Biophys Acta. 1971;230(3):535–538. doi: 10.1016/0304-4165(71)90185-1. [DOI] [PubMed] [Google Scholar]
- MUELLER G. C. Incorporation of glycine-2-C14 into protein by surviving uteri from alpha-estradiol-treated rats. J Biol Chem. 1953 Sep;204(1):77–90. [PubMed] [Google Scholar]
- NOTEBOOM W. D., GORSKI J. AN EARLY EFFECT OF ESTROGEN ON PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1963 Aug;50:250–255. doi: 10.1073/pnas.50.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris J. S., Gorski J., Kohler P. O. Androgen receptors in a Syrian hamster ductus deferens tumour cell line. Nature. 1974 Mar 29;248(447):422–424. doi: 10.1038/248422a0. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L., Kohler P. O., Korenman S. G. Studies on the mechanism of steroid hormone regulation of synthesis of specific proteins. Recent Prog Horm Res. 1969;25:105–160. doi: 10.1016/b978-0-12-571125-8.50006-5. [DOI] [PubMed] [Google Scholar]
- Oka T., Schimke R. T. Interaction of estrogen and progesterone in chick oviduct development. II. Effects of estrogen and progesterone on tubular gland cell function. J Cell Biol. 1969 Oct;43(1):123–137. doi: 10.1083/jcb.43.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. G., O'Malley B. W. Steroid hormones: effects on adenyl cyclase activity and adenosine 3',5'-momophosphate in target tissues. Science. 1970 Apr 10;168(3928):253–255. doi: 10.1126/science.168.3928.253. [DOI] [PubMed] [Google Scholar]
- SHEPPARD H., TSIEN W. H., MAYER P., HOWIE N. METABOLISM OF THE ACCESSORY SEX ORGANS OF THE IMMATURE MALE RAT: CHANGES IN NUCLEIC ACID COMPOSITION AND UPTAKE OF THYMIDINE-3-H INDUCED BY CASTRATION AND METHANDROSTENOLONE. Biochem Pharmacol. 1965 Jan 1;14:41–51. doi: 10.1016/0006-2952(65)90056-0. [DOI] [PubMed] [Google Scholar]
- Seifert W. E., Rudland P. S. Possible involvement of cyclic GMP in growth control of cultured mouse cells. Nature. 1974 Mar 8;248(5444):138–140. doi: 10.1038/248138a0. [DOI] [PubMed] [Google Scholar]
- VELARDO J. T. Steroid hormones and uterine growth. Ann N Y Acad Sci. 1959 Jan 9;75:441–462. doi: 10.1111/j.1749-6632.1959.tb44567.x. [DOI] [PubMed] [Google Scholar]
- Vesely D. L., Castro A., Levey G. S. Decreased rat hepatic guanylate cyclase activity in streptozotocin-induced diabetes mellitus. Diabetes. 1977 Apr;26(4):308–313. doi: 10.2337/diab.26.4.308. [DOI] [PubMed] [Google Scholar]
- Vesely D. L., Chown J., Levey G. S. Guanylate cyclase activity in fetal, neonatal and adult rat heart. J Mol Cell Cardiol. 1976 Nov;8(11):909–913. doi: 10.1016/0022-2828(76)90073-0. [DOI] [PubMed] [Google Scholar]
- Vesely D. L., Graves W. R., Lo T. M., Fletcher M. A., Levey G. S. Isolation of a guanylate cyclase inhibitor from the balsam pear (Momordica charantia abreviata). Biochem Biophys Res Commun. 1977 Aug 22;77(4):1294–1299. doi: 10.1016/s0006-291x(77)80120-4. [DOI] [PubMed] [Google Scholar]
- Vesely D. L., Rovere L. E., Levey G. S. Activation of guanylate cyclase by streptozotocin and 1-methyl-1-nitrosourea. Cancer Res. 1977 Jan;37(1):28–31. [PubMed] [Google Scholar]
- Watson J., Epstein R., Cohn M. Cyclic nucleotides as intracellular mediators of the expression of antigen-sensitive cells. Nature. 1973 Dec 14;246(5433):405–409. doi: 10.1038/246405a0. [DOI] [PubMed] [Google Scholar]
- Weinstein Y., Chambers D. A., Bourne H. R., Melmon K. L. Cyclic GMP stimulates lymphocyte nucleic acid synthesis. Nature. 1974 Sep 27;251(5473):352–353. doi: 10.1038/251352a0. [DOI] [PubMed] [Google Scholar]


