Abstract
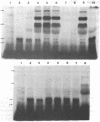
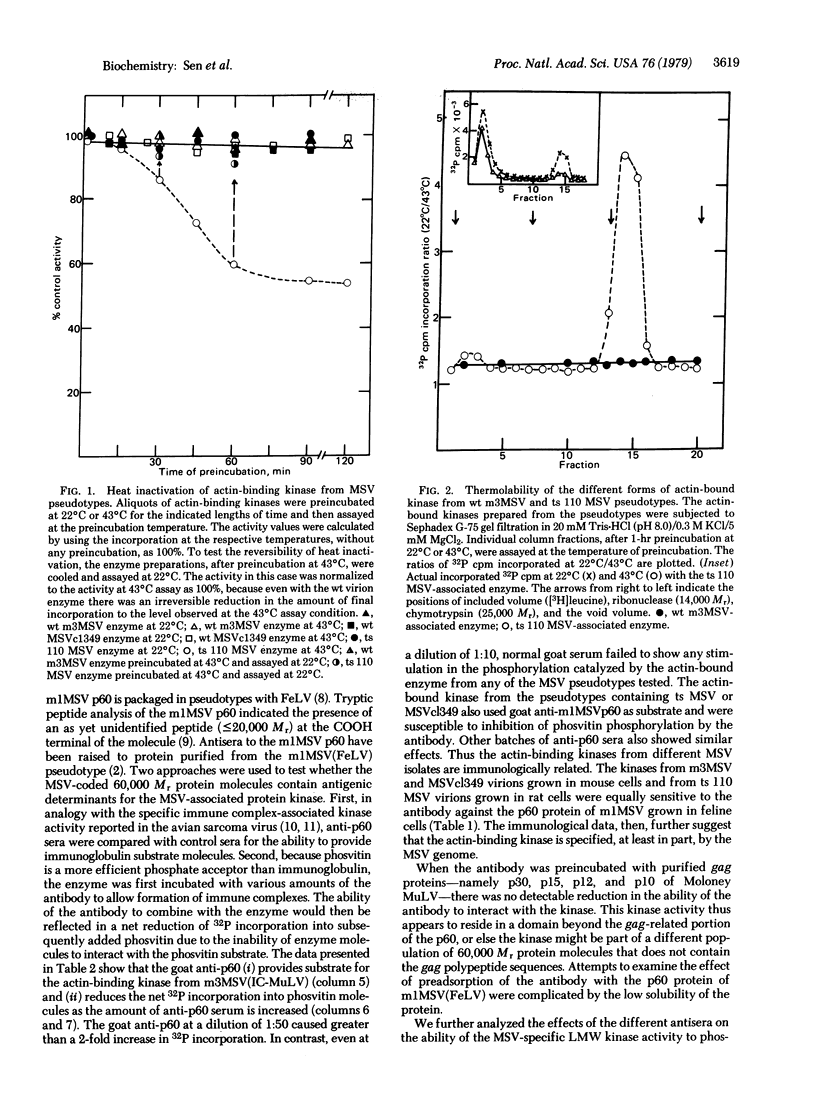
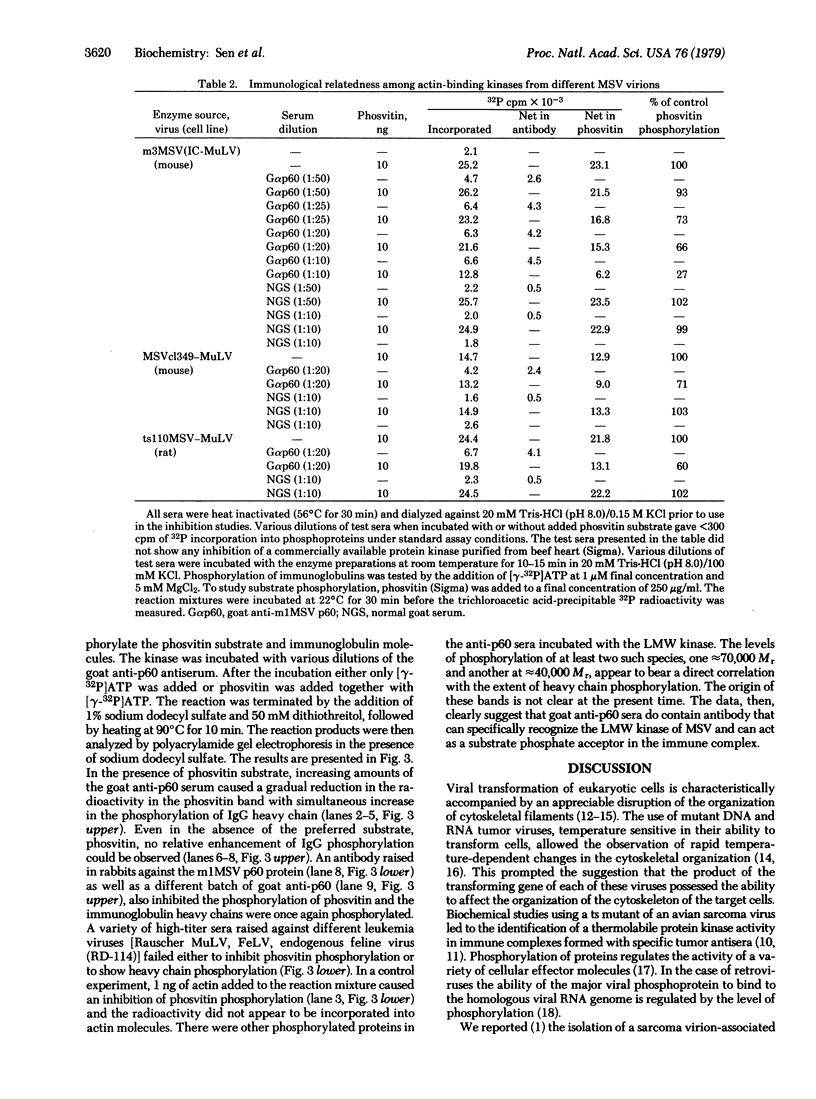
Murine sarcoma virus-associated protein kinases that bind to actin have been purified by affinity chromatography on actin coupled to Sepharose. Heat inactivation studies showed the presence of thermolabile enzyme activity in pseudotypes containing a temperature-sensitivity mutant of murine sarcoma virus (MSV) but not in two independent wild-type MSV pseudotypes. Studies with Sephadex G-75 column fractions showed that a low molecular weight form, approximately 15,000, is the major thermolabile kinase in the temperature-sensitive MSV virions. Antibodies raised against the MSV-coded p60 protein, when added to the in vitro reaction mixtures, showed specific phosphorylation of the IgG heavy chain and a simultaneous reduction in the extent of phosvitin phosphorylation catalyzed by the various MSV pseudotype kinases. Thus a transforming retrovirus-coded enzyme activity that interacts directly with a major cytoskeletal protein and whose activity parallels the transforming ability of a conditional MSV mutant has now been identified.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash J. F., Vogt P. K., Singer S. J. Reversion from transformed to normal phenotype by inhibition of protein synthesis in rat kidney cells infected with a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3603–3607. doi: 10.1073/pnas.73.10.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball J., McCarter J. A., Sunderland S. M. Evidence for helper independent murine sarcoma virus. I. Segregation of replication-defective and transformation-defective viruses. Virology. 1973 Nov;56(1):268–284. doi: 10.1016/0042-6822(73)90305-x. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Hull M. A., Finch E. A. The isolation and preliminary characterization of temperature-sensitive transformation mutants of Moloney sarcoma virus. Virology. 1979 Jun;95(2):303–316. doi: 10.1016/0042-6822(79)90486-0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I. Temperature-sensitive changes in surface modulating assemblies of fibroblasts transformed by mutants of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2047–2051. doi: 10.1073/pnas.73.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- McClain D. A., D'Eustachio P., Edelman G. M. Role of surface modulating assemblies in growth control of normal and transformed fibroblasts. Proc Natl Acad Sci U S A. 1977 Feb;74(2):666–670. doi: 10.1073/pnas.74.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson M. K., Elder J. H., Gautsch J. W., Lerner R. A., Vande Woude G. F. Chemical determination of the m1 Moloney sarcoma virus pP60gag gene order: evidence for unique peptides in the carboxy terminus of the polyprotein. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4694–4698. doi: 10.1073/pnas.75.10.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson M. K., Long C. W., Robey W. G., Scherer M. A., Vande Woude G. F. Phosphorylation and nucleic acid binding properties of m1 Moloney murine sarcoma virus-specific pP60gag. J Virol. 1977 Jul;23(1):196–204. doi: 10.1128/jvi.23.1.196-204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson M. K., Robey W. G., Harris C. L., Fischinger P. J., Haapala D. K., Vande Woude G. F. A p60 polypeptide in the feline leukemia virus pseudotype of Moloney sarcoma virus with murine leukemia virus p30 antigenic determinants. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2380–2384. doi: 10.1073/pnas.72.6.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey W. G., Oskarsson M. K., Vande Woude G. F., Naso R. B., Arlinghaus R. B., Haapala D. K., Fischinger P. J. Cells transformed by certain strains of Moloney sarcoma virus contain murine p60. Cell. 1977 Jan;10(1):79–89. doi: 10.1016/0092-8674(77)90142-8. [DOI] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Phosphorylation of murine type C viral p12 proteins regulates their extent of binding to the homologous viral RNA. Cell. 1977 Mar;10(3):489–496. doi: 10.1016/0092-8674(77)90036-8. [DOI] [PubMed] [Google Scholar]
- Sen A., Todaro G. J. A murine sarcoma virus-associated protein kinase: interaction with actin and microtubular protein. Cell. 1979 Jun;17(2):347–356. doi: 10.1016/0092-8674(79)90161-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Lazarides E., Goldman R. D., Vogel A., Pollack R. Localization and distribution of actin fibers in normal transformed and revertant cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):363–369. doi: 10.1101/sqb.1974.039.01.047. [DOI] [PubMed] [Google Scholar]