Abstract
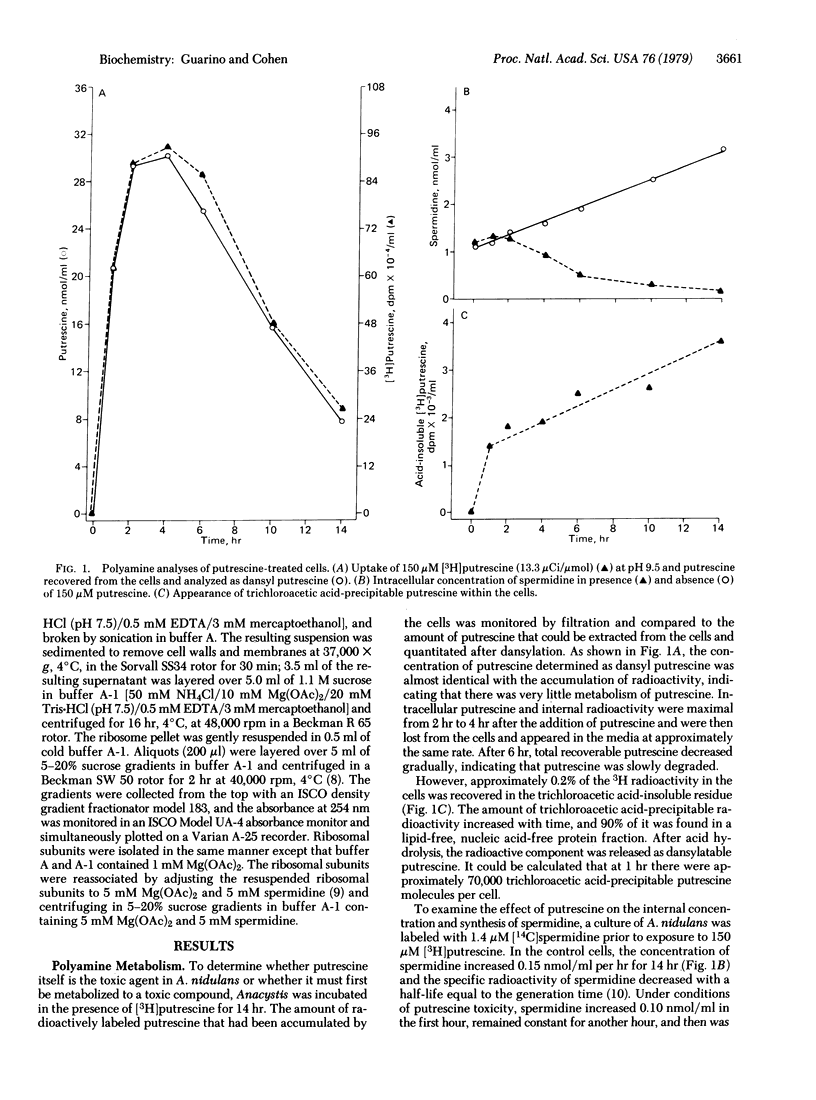
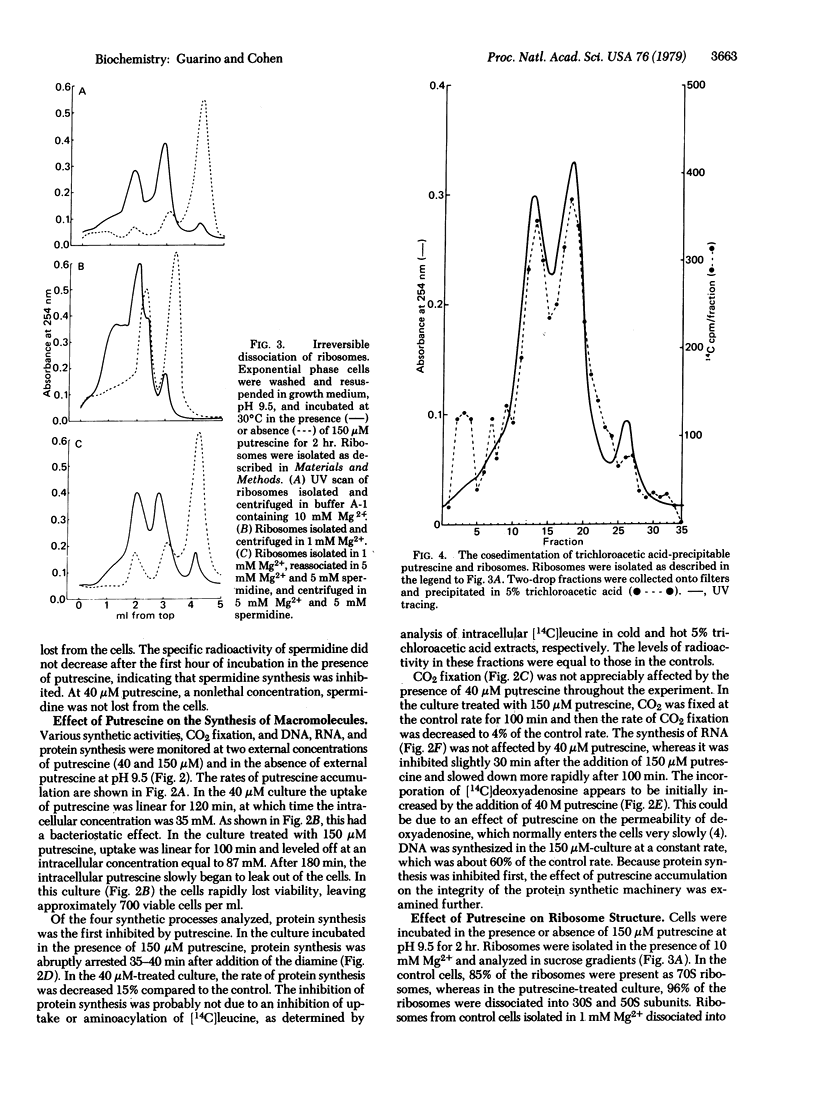
Putrescine is lethal to the cyanobacterium Anacystis nidulans at extracellular pH values at which significant concentrations of the nonprotonated diamine rapidly diffuse into the cell and accumulate as the charged form. Although over 98% of the accumulated putrescine is not metabolized, a small fraction is rendered trichloroacetic acid-insoluble, and about 90% of this is bound as putrescinie to proteins and cell structures. Various synthetic functions were studied in the presence of a bacteriostatic (40 microM) and a bacteriocidal (150 microM) concentration of putrescine at pH 9.5. Under lethal conditions, protein synthesis was completely inhibited after 45 min and CO2 fixation after 100 min, whereas nucleic acid synthesis was less affected. Spermidine was lost from the cell and its synthesis was arrested. These functions were much less inhibited at 40 microM putrescine. Ribosomes from putrescine-killed cells were found to be irreversibly dissociated into 30S and 50S subunits. Some putrescine (1-4 molecules) cosedimented with each subunit.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN S. S., LICHTENSTEIN J. Polyamines and ribosome structure. J Biol Chem. 1960 Jul;235:2112–2116. [PubMed] [Google Scholar]
- Goldberg M. L., Steitz J. A. Cistron specificity of 30S ribosomes heterologously reconstituted with components from Escherichia coli and Bacillus stearothermophilus. Biochemistry. 1974 May 7;13(10):2123–2129. doi: 10.1021/bi00707a020. [DOI] [PubMed] [Google Scholar]
- Guarino L. A., Cohen S. S. The estimation of turnover of spermidine in Anacystis nidulans. Anal Biochem. 1979 May;95(1):73–76. doi: 10.1016/0003-2697(79)90186-6. [DOI] [PubMed] [Google Scholar]
- Guarino L. A., Cohen S. S. Uptake and accumulation of putrescine and its lethality in Anacystis nidulans. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3184–3188. doi: 10.1073/pnas.76.7.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. E., Rossetti G. P., Van Holde K. E. Physical studies of ribosomes from Escherichia coli. J Mol Biol. 1969 Sep 14;44(2):263–277. doi: 10.1016/0022-2836(69)90174-0. [DOI] [PubMed] [Google Scholar]
- Kimes B. W., Morris D. R. Cations and ribosome structure. II. Effects on the 50S subunit of substituting polyamines for magnesium ion. Biochemistry. 1973 Jan 30;12(3):442–449. doi: 10.1021/bi00727a013. [DOI] [PubMed] [Google Scholar]
- Kondo M., Eggerston G., Eisenstadt J., Lengyel P. Ribosome formation from subunits: dependence on formylmethionyl-transfer RNA in extracts from E. coli. Nature. 1968 Oct 26;220(5165):368–371. doi: 10.1038/220368a0. [DOI] [PubMed] [Google Scholar]
- Pigott G. H., Carr N. G. The assimilation of nucleic acid precursors by intact cells and protoplasts of the blue-green alga anacystis nidulans. Arch Mikrobiol. 1971;79(1):1–6. doi: 10.1007/BF00412035. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S., Guarino L., Cohen S. S. Polyamines of Anacystis nidulans and metabolism of exogenous spermidine and spermine. J Bacteriol. 1978 Jun;134(3):744–750. doi: 10.1128/jb.134.3.744-750.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restaino L., Frampton E. W. Labeling the deoxyribonucleic acid of Anacystis nidulans. J Bacteriol. 1975 Oct;124(1):155–160. doi: 10.1128/jb.124.1.155-160.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN J. L., SEKIGUCHI M., BARNER H. D., COHEN S. S. THE SYNTHESIS OF MESSENGER RNA WITHOUT PROTEIN SYNTHESIS. I. STUDIES WITH THYMINELESS STRAINS OF ESCHERICHIA COLI. J Mol Biol. 1964 May;8:629–637. doi: 10.1016/s0022-2836(64)80113-3. [DOI] [PubMed] [Google Scholar]
- Scafati A. R., Stornaiuolo M. R., Novaro P. Physicochemical and light scattering studies on ribosome particles. Biophys J. 1971 Apr;11(4):370–384. doi: 10.1016/S0006-3495(71)86221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L., Morris D. R. Cations and ribosome structure. I. Effects on the 30S subunit of substituting polyamines for magnesium ion. Biochemistry. 1973 Jan 30;12(3):435–441. doi: 10.1021/bi00727a012. [DOI] [PubMed] [Google Scholar]
- Weiss R. L., Morris D. R. The inality of polyamines to maintain ribosome structure and function. Biochim Biophys Acta. 1970 Apr 15;204(2):502–511. doi: 10.1016/0005-2787(70)90170-x. [DOI] [PubMed] [Google Scholar]


