Abstract
Introduction
The efficiency of islet graft survival following intra-portal implantation is compromised by host innate immune responses and the production of pro-inflammatory cytokines that cause acute cellular injury. This reaction activates intra-islet NF-κB causing production of gene products that have detrimental effects on β cell survival and function. We hypothesized that siRNA targeting of IKKβ, a crucial kinase in the NF-κB activation pathway, in islets prior to transplantation would ameliorate the detrimental effects of cytokines and improve islet survival post transplantation.
Methods
To test this hypothesis, we prepared siRNA-based spherical nucleic acid nanoparticle conjugates targeting IKKβ (IKKβ SNA-NCs). We treated isolated islets with IKKβ SNA-NCs and assessed the functional consequences of IKKβ knockdown in vitro and after intra-portal transplantation in mice.
Results
Treatment of freshly isolated mouse islets with IKKβ SNA-NCs reduced constitutive IKKβ expression and protected against pro-inflammatory cytokine-induced NF-κB activation, resulting in improved cell viability and decreased expression of gene products associated with β cell dysfunction. Intra-portal transplantation of a marginal mass (50 islets) of syngeneic islets treated with nanoparticle conjugates targeting IKKβ resulted in reversion to normoglycemia in 50% of streptozotocin-induced diabetic recipients (n=12) compared with 0% of controls (n=12). Histologic analyses showed reduced CD11b+ cellular infiltration and decreased islet apoptosis.
Conclusions
These results are consistent with the hypothesis that inhibition of intra-islet NF-κB activation ameliorates the detrimental effects of host cytokines and demonstrates that preconditioning freshly isolated islets in culture with IKKβ SNA-NCs may be a promising therapy to enhance islet graft function and survival post-transplant.
Keywords: Beta cell, Islet transplantation, Cytokines, Nanotechnology
Introduction
Islet transplantation provides a highly effective and safe means to restore endogenous, regulated insulin secretion to stabilize labile glycemic control and correct hemoglobin A1c to levels predicted to prevent secondary complications of diabetes (1). However, it is estimated that 50-70% of the intra-portally transplanted islet mass is lost early post-infusion (2-4), making the treatment less efficient.
Following intra-portal delivery, islet graft injury is multi-factorial. It is due, in part, to the early host innate immune responses involving infiltration of the islet graft by macrophages and monocytes and the production of pro-inflammatory cytokines, such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α and interferon (IFN)-γ (5-8). Strategies of TNF-α and/or IL-1β blockade improve islet engraftment in experimental islet transplant models (9, 10) as well as human clinical pilot studies of islet transplantation (11, 12).
In β cells, and other cells within the islet, TNF-α and IL-1β activate NF-κB primarily via the IκB kinase (IKK) subunit, IKKβ. NF-κB activation induces expression of noxious gene products, including inducible nitric oxide synthase (iNOS) and Fas; as well as the chemokines monocyte chemoattractant protein (MCP)-1 and IFN-γ inducible protein (IP)-10; and the T-cell growth factor and activator, IL-15. Collectively, these mediators contribute to β cell dysfunction and apoptosis (13-17). Additionally, the accumulation of islet-infiltrating macrophages associated with pro-inflammatory cytokine expression contributes to β cell dysfunction and death (6, 18). Using a transgenic mouse model that conditionally and specifically expresses a non-degradable mutant IκBα protein in β cells, we have demonstrated inhibition of cytokine-induced NF-κB activity, which prevented islet dysfunction and enhanced intra-portal survival and β cell function in a syngeneic marginal mass islet transplant model (19).
The current methods for inhibiting NF-κB activity in human islets have significant drawbacks that limit their effectiveness. Small molecule inhibitors must be applied systemically, either to the donor prior to harvesting the pancreas, or to the islet graft recipient, with the latter leading to adverse effects in non-islet tissues (20). Genetic manipulating technologies, including lipid-based transfection reagents and viral vectors, fail to deliver genetic material to the cells located in the core of the islets, and are toxic at concentrations that would make them efficient delivery vehicles (21-23).
We have previously published on the ability of DNA-functionalized gold nanoparticle conjugates to act as a transfection and antisense agent in isolated islets without altering islet function or survival (24). These conjugates penetrated to the cells at the core of the islet and deliver functional oligonucleotides to alter islet gene expression. The current term, spherical nucleic acid-nanoparticle conjugates (SNA-NC), is more appropriate, as the origins of SNA-NCs effects are derived from the size of the gold nanoparticle core and the spherical shell of oligonucleotides attached to their surface (25-29).
In addition to antisense-DNA oligonucleotides, siRNAs can be densely loaded onto the surface of SNA-NCs (25-30). These siRNA based SNA-NCs are also able to efficiently enter cells, protect loaded siRNAs from nuclease degradation, and enable target gene regulation (28, 31-36). We hypothesized that siRNA targeting of IKKβ in islets prior to transplantation would ameliorate the detrimental effects of cytokine-mediated β cell dysfunction and improve islet survival post transplantation. The current series of experiments demonstrate how these SNA-NCs can be translationally applied to help solve the difficult problem of acute islet injury following intra-portal implantation.
Results
Knockdown of IKKβ by custom-designed siRNA sequence inhibited cytokine-induced NF-κB activation
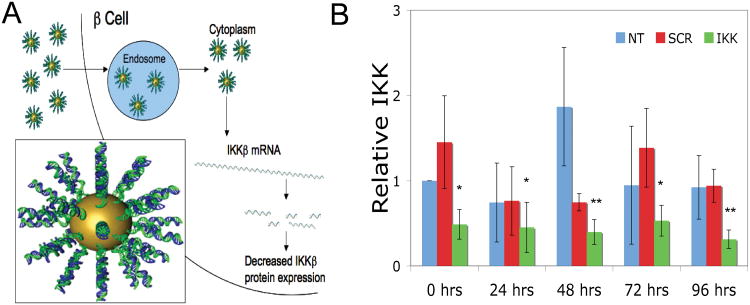
We designed a custom siRNA sequence predicted to knockdown IKKβ expression and validated the siRNA in a murine monocyte cell line (J774) using the lipid-based DharmaFECT 4 reagent. Transfection of J774 cells with the custom-designed siRNA decreased IKKβ expression to 25.2% compared to controls (SDC Table 1). The IKKβ siRNA was then conjugated to SNA-NCs (IKKβ SNA-NC) to knockdown expression of IKKβ in mouse islets. The entry of SNA-NCs into islet cells is shown in Figure 1A. Islets were treated with 10 nM IKKβ SNA-NC, 10 nM SCR SNA-NC (scrambled control siRNA sequence), or were left untreated for 24 hours. Following this incubation, RNA was isolated from the islets (t = 0) or at 24, 48, 72 and 96 hours and analyzed for IKKβ expression by RT-qPCR. Treatment with 10 nM IKKβ SNA-NC decreased IKKβ expression to 48.7% ± 17.4% compared to untreated control at t = 0 (p<0.05; Figure 1B). IKKβ knockdown by IKKβ SNA-NC persisted over the 96-hour time course (45.2% ± 29.5% at 24 hours, p<0.05; 39.8% ± 14.6% at 48 hours, p<0.01; 53.1% ± 18.0% at 72 hours, p<0.05; and 31.3% ± 10.9% at 96 hours, p<0.01). Treatment with SCR SNA-NCs did not significantly decrease IKKβ expression at any time point. Based on these results, we treated islets with 10 nM SNA-NCs for 24 hours prior to use in all subsequent experiments.
Figure 1. Entry of SNA-NC into islet cells and knockdown of IKKβ by IKKβ SNA-NCs.
A. Schematic of IKKβ SNA-NC uptake by host cell and target protein regulation. Inset: Representation of a siRNA-functionalized SNA-NC. B. RT-qPCR results of IKKβ knockdown in isolated mouse islets treated with either 10 nM IKKβ SNA-NC or 10 nM SCR SNA-NC for 24 hours, then cultured for an additional 0, 24, 48, 72 or 96 hours. *p<0.05 when compared to untreated control at t=0. **p<0.01 when compared to untreated control at t=0.
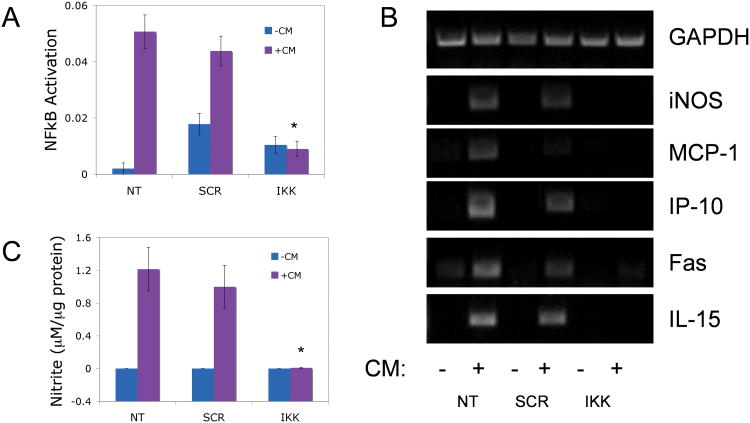
Isolated islets were treated with or without SNA-NCs prior to 1 hour of exposure to a mix of pro-inflammatory cytokines previously shown to activate NF-κB (37-39) (CM: 50 U/mL IL-1β, 1000 U/mL TNF-α and 750 U/mL IFN-γ) and the level of activated NF-κB measured by ELISA. Treatment with IKKβ SNA-NCs decreased cytokine-induced activation of NF-κB to 17.8% of untreated control islets (0.009 ± 0.003 absorbance units/mg protein vs. 0.0507 ± 0.006) and SCR SNA-NC-treated islets (0.0438 ± 0.005; p<0.05; Figure 2A). No statistically significant differences in NF-κB activation were measured when cytokines were not applied.
Figure 2. Effect of IKKβ SNA-NC treatment on cytokine-induced intra-islet NF-κB activity.
A. NF-κB activation in mouse islets treated with 10 nM IKKβ SNA-NC or SCR SNA-NC for 24 hours prior to 1 hour cytokine mix (CM) stimulation. B. Semi-quantitative RT-PCR analysis of cytokine-induced genes in mouse islets treated with SNA-NCs for 24 hours prior to 24 hour CM exposure. C. Nitric oxide production by cytokine-stimulated mouse islets treated with SNA-NCs for 24 hours prior to 24 hour CM exposure, as measured by nitrite production. Data displayed as μM nitrite per μg protein. CM= 50 U/mL IL-1β, 1000 U/mL TNF-α, 750 U/mL IFN-γ. *p<0.05 when compared to NT and SCR treated with CM.
IKKβ SNA-NC treatment decreased cytokine-induced gene expression in mouse islets
Islets were treated in culture for 24 hours with 10 nM IKKβ SNA-NC, 10 nM SCR SNA-NC, or untreated, and subsequently exposed to CM for 24 hours. Cytokine exposure induced expression of iNOS, MCP-1, IP-10, IL-15 and Fas in untreated and SCR SNA-NC treated islets (Figure 2B). Islets treated with IKKβ SNA-NCs substantially diminished expression of the same gene set.
Pro-inflammatory cytokine exposure of islets induced expression of iNOS and the production of nitric oxide, the latter quantified through measurement of the byproduct, nitrite, using the Griess reaction. Treatment of islets with 10 nM IKKβ SNA-NC decreased cytokine-induced nitrite production 135-fold compared to untreated control islets and SCR SNA-NC-treated islets (0.009 ± 0.006 mM nitrite/mg protein vs. 1.241 ± 0.265 vs. 0.999 ± 0.259; p<0.05; Figure 2C).
IKKβ SNA-NC protected β cells from cytokine-induced cell death
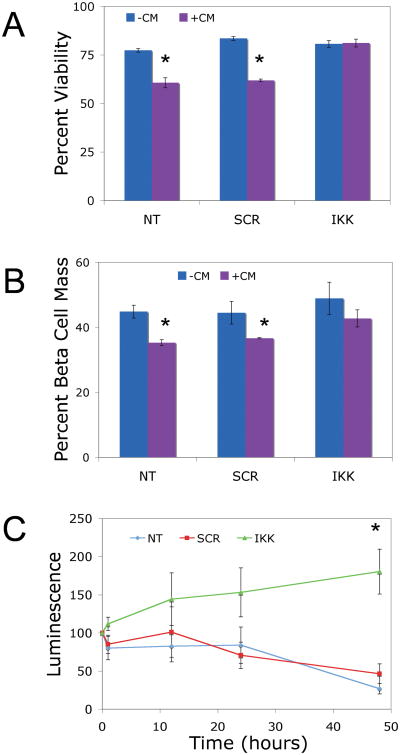
To investigate whether IKKβ knockdown prevented cytokine-induced cell death, islets were treated with SNA-NCs or left untreated prior to cytokine exposure as described above. Cytokine treatment significantly decreased cell viability, measured as the percentage of TMRE (tetramethylrhodamine) positive (viable) cells, in untreated control (60.65% ± 2.57%) and SCR SNA-NC treated (61.9% ± 0.70%) islet cells, but not in IKKβ SNA-NC treated islet cells (81.2% ± 2.03%; p<0.05; Figure 3A). Pro-inflammatory cytokines also specifically reduced the percentage of viable β cells in untreated control (35.25% ± 0.90% Zinbo5 positive cells) and SCR SNA-NC treated (36.60% ± 0.20%) islets compared to IKKβ SNA-NC treated islets (42.73% ± 2.63%; p<0.05; Figure 3B).
Figure 3. Effect of IKKβ SNA-NC treatment islet cell viability and β cell mass in vitro.
A. Flow cytometric analysis of viability of islets treated with 10 nM IKKβ SNA-NC or 10 nM SCR SNA-NC for 24 hours prior to 24 hour CM exposure. Data presented as percent viable cells. B. Flow cytometric analysis of β cell percentage of islets treated with10 nM IKKβ SNA-NC or 10 nM SCR SNA-NC for 24 hours prior to 24 hour CM exposure. Data are presented as percent of total cell population. C. Tg(PRIP-luc) islets treated with 10 nM IKKβ SNA-NC or 10 nM SCR SNA-NC for 24 hours prior to 48 hour CM exposure. Data are presented as percentage of luminescent signal at time 0 of CM exposure. CM= 50 U/mL IL-1β, 1000 U/mL TNF-α, 750 U/mL IFN-γ. *p<0.05
To measure decreases in the functional β cell mass, islets isolated from transgenic mice expressing luciferase under the control of the rat insulin II promoter (Tg(PRIP-luc)) were treated with or without the SNA-NCs for 24 hours and then subjected to bioluminescence imaging ex vivo before and after cytokine treatment. As shown in Figure 3C, cytokine exposure decreased the luminescent signal of untreated control and SCR SNA-NC-treated islets over the 48 hour time course to 26.8% ± 6.9% and 46.5% ± 12.7%, respectively, compared to that of time 0. Treatment with IKKβ SNA-NCs prevented the cytokine-induced decrease in luminescence, with the islet luminescent signal intensity at 48 hours of cytokine exposure at 180.4% ± 29.5% (p<0.05) of that at t=0.
IKKβ SNA-NC treatment enhanced islet engraftment in a syngeneic marginal mass islet transplant model
To investigate whether IKKβ SNA-NC treatment had a beneficial effect on islet graft function in a transplant setting, the syngeneic marginal islet mass transplant model was used. Previous work has defined 50 islets as a marginal mass since that number of isolated islets that permanently correct hyperglycemia after being transplanted intra-portally to streptozotocin-induced diabetic mice (19, 40).
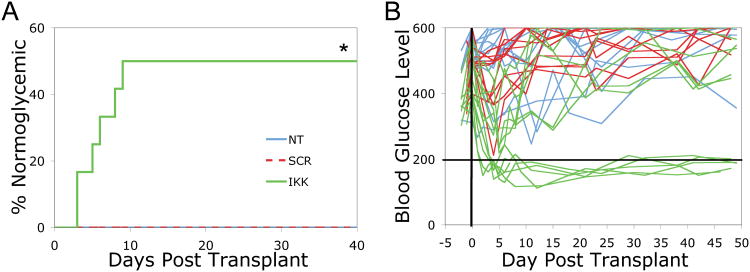
Islets were isolated from donors and treated in culture with 10 nM IKKβ SNA-NCs, 10 nM SCR SNA-NCs, or untreated, for 24 hours prior to transplantation into streptozotocin-induced diabetic mice. Time to amelioration of diabetes was defined as the first day post-transplant that the recipient achieved 2 consecutive blood glucose readings below 200 mg/dL. In untreated control islet (N=12) and SCR SNA-NC treated islet (N=11) recipients, none of the diabetic mice reverted to normoglycemia. In contrast, treatment of islets with IKKβ SNA-NC resulted in 6 of 12 mice reverting to normoglycemia at a mean (± S.D.) of 5.67 ± 2.50 days (p<0.05; Figure 4A). Additionally, the IKKβ SNA-NC treated islet recipients demonstrated improved blood glucose control compared to the SCR SNA-NCs and untreated islet recipients (Figure 4B, SDC Tables 2-4). These results demonstrated that knockdown of IKKβ expression by siRNA-based SNA-NCs enhanced islet engraftment and function post transplantation.
Figure 4. Syngeneic marginal mass intra-portal islet transplantation to STZ-induced diabetic mice.
Isolated mouse islets were treated with 10 nM IKKβ SNA-NC or 10 nM SCR SNA-NC for 24 hours prior to intra-portal transplantation to streptozotocin-induced diabetic recipients. A total of 50 islets were transplanted per recipient. Normoglycemia is defined as 2 consecutive blood glucose readings below 200 mg/dL. A. Data presented as percent of recipients reverting to normoglycemia. B. Blood glucose control (mg/dL) of STZ-induced diabetic mice pre- and post transplant. *p<0.05
IKKβ SNA-NC treatment prevents islet graft infiltration by host immune cells
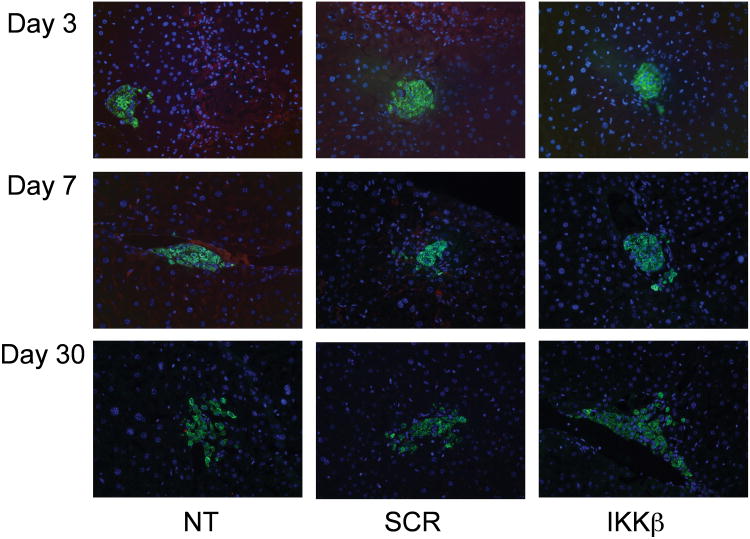
To investigate the effect of IKKβ SNA-NC treatment on marginal mass islet graft function in vivo, histological analyses were conducted on day 3, 7 and 30 post-transplant. H & E staining revealed no obvious differences in islet morphology across the three treatment groups (SDC Figure 1). Mild infiltration of grafts in untreated and SCR SNA-NC-treated islet recipients by CD4+ cells (SDC Figure 2) and CD8+ cells (SDC Figure 3) were present on Day 7 but not on Day 3 or 30. CD11b+ cells were present on Days 3 and 7 in the untreated and SCR SNA-NC-treated islet recipients, but diminished by Day 30 (Figure 5). Little, if any, CD11b+ staining was observed in the IKKβ SNA-NC-treated islet recipients.
Figure 5. Presence of CD11b+ cells in intra-portally transplanted islet grafts over time.
Isolated mouse islets were treated with 10 nM IKKβ SNA-NC, 10 nM SCR SNA-NC, or untreated for 24 hours prior to intra-portal transplantation to STZ-induced diabetic recipients. A minimum of 200 islets were transplanted per recipient. Livers were harvested on days 3, 7 and 30, fixed with 4% paraformaldehyde, sectioned and stained for insulin (green), CD11b (red) and DAPI (blue). Images were taken under 20× magnification.
Apoptotic cells (TUNEL+) were apparent in the Day 7 untreated and SCR SNA-NC-treated islet recipients but not in the IKKβ SNA-NC islet recipients (SDC Figure 4). No apoptotic cells were observed in any of the Day 30 samples. Due to the dispersion of the islets throughout the liver, quantification of the percentage of islet cells undergoing apoptosis was not possible.
Discussion
Intra-portal islet grafts are susceptible to the detrimental effects of early host innate immune responses associated with inflammatory effects mediated by pro-inflammatory cytokines. Proof-of-principle data obtained in transgenic animals suggest that inhibition of NF-κB activation would enhance islet engraftment and function (19). Thus, genetic manipulation of NF-κB activity in isolated islets, is a promising approach (41, 42); however, current methods to deliver oligonucleotides based upon lipoplexes and viruses have severe drawbacks, including toxicity, an inability to reach the cells at the core of the islets and, in the case of viral vectors, a potential to trigger an immune response to latent viral vector protein expression (21-23, 43).
We have previously demonstrated that SNA-NCs are capable of crossing cell membranes and regulating target gene expression in isolated islets (24). DNA-based SNA-NCs are nontoxic to islets, distribute throughout the cells of isolated islets including those cells at the islet core, and regulate target gene expression. SNA-NCs, therefore, are promising gene-regulatory agents for intact islets, and provide a means for testing the hypothesis that siRNA targeting of β cell IKKβ would reduce cytokine-mediated dysfunction and improve islet survival post transplantation.
We have prepared SNA-NCs from a custom-designed siRNA sequence against IKKβ, a crucial kinase subunit in the NF-κB activation pathway. IKKβ SNA-NC effectively diminished constitutive IKKβ expression over a 96-hour time course and resulted in significant decreases of cytokine-mediated intra-islet NF-κB activation and of NF-κB-dependent iNOS, MCP-1, IP-10, Fas and IL-15 expression, prevented cytokine-induced cell death and maintained the β cell mass compared to controls. The increase of the bioluminescence signal intensity in islets treated with IKKβ SNA-NCs with time may represent improved islet function (an increase in insulin promoter activity) through inhibition of NF-κB activation, since islet cell NF-κB can be activated by the islet isolation procedure and results in islet cell demise. Taken together, these results demonstrated knockdown of IKKβ by IKKβ SNA-NCs protected the islet cells from the detrimental effects of cytokines in vitro.
Transplantation of a marginal mass of islets intra-portally to syngeneic recipients demonstrated that IKKβ SNA-NC treatment enhanced islet survival and function, post-transplant compared to controls. The intra-portal transplantation site was chosen over other sites in order more accurately represent the transplant microenvironment islets are exposed to when transplanted into human recipients. When determining the mass of isologous islets to transplant per recipient, we selected a mass that would not cause reversion to normoglycemia in all of the recipients in order to allow for measurement of enhanced islet survival when pre-treated with the IKKβ SNA-NCs. Based on our previous work using a transgenic mouse model to specifically and conditionally inhibit NF-κB activity in β cells(19), 50 islets were chosen as an appropriate marginal mass for these experiments. For histological analyses, we increased the number of islets transplanted to at least 200 per recipient in order to improve the chances of finding the islets in the liver tissue sections. Histological examination of the islets post intra-portal transplant demonstrated abrogation of CD11b+ cell infiltration. The histological analyses and the observations of significant decreases of cytokine-mediated intra-islet NF-κB activation and of NF-κB-dependent iNOS, MCP-1, IP-10, Fas and IL-15 expression, suggest that decreased chemokine production by the transplanted islets may play a major role in promoting early graft infiltration by host immune cells. These results are consistent with the hypothesis that inhibition of intra-islet NF-κB activation in freshly isolated islets would ameliorate the detrimental effects of host cytokine exposure immediately post-transplant. The findings also provide some insights into the mechanisms of islet cell protection and demonstrated proof-of-principle that knockdown of constitutive intra-islet IKKβ expression using IKKβ SNA-NCs is an effective approach to protect islets from the detrimental effects of the pro-inflammatory cytokines generated by early host non-specific innate immune responses in a transplant setting.
Materials and Methods
siRNA-based SNA-NC synthesis
Gold nanoparticles (13 nm diameter) were synthesized according to published procedures (44). For conjugation to the surface of AuNPs, siRNAs targeting IKKβ and control duplexes required terminal modification with thiol groups on the sense RNA strands (28). Detailed information regarding the design of the custom IKKβ siRNA sequence and the synthesis of the siRNA-based SNA-NCs can be found in the SDC Methods section. In vitro and in vivo treatments were conducted for 24 hours with 10 nM IKKβ SNA-NCs, 10 nM SCR SNA-NCs or were left untreated unless otherwise indicated.
Culture and transfection of J774 cells
See SDC Methods for details.
Mice and islet isolation
FVB/NJ background (H-2q) and the transgenic mouse line Tg(PRIP-luc), with the FVB/NJ background and which express the firefly luciferase gene under the regulation of the rat insulin promoter II, were used as islet donors. For syngeneic islet transplant experiments, FVB/NJ mice were used as recipients. Mice were housed in a barrier facility at Northwestern University and used at ages of 12-16 weeks. All procedures relating to the mice were approved by the Center for Comparative Medicine at Northwestern University and followed guidelines set by the American Veterinary Medical Association. Islets were isolated as described previously (37, 40). Isolated islets were cultured in Roswell Park Memorial Institute (RPMI) media (RPMI 1640; Sigma-Aldrich) containing 10% FBS, 100 U/mL penicillin G and 100 μg/mL streptomycin sulfate at 37°C, 5% CO2.
Real time PCR (RT-qPCR) analysis of IKKβ expression
Detailed information can be found in the SDC Methods section.
Cytokine treatment
Post SNA-NC treatment, islets were exposed to cytokine mixture (CM: 50 U/mL IL-1β, 1000 U/mL TNF-α, 750 U/mL IFN-γ). Concentrations of cytokines were chosen based on previously reported results (37-39).
NF-κB activation assay
After treatment with siRNA-based SNA-NCs, islets were stimulated with CM for 1 hour. Nuclear lysates were prepared using the Nuclear Extract kit (Active Motif, Carlsbad, CA). Activated NF-κB was quantified using an ELISA assay (NF-κB p65 TransAM kit; Active Motif). Protein concentrations of nuclear lysates were determined using the ProStain Protein Quantification Kit (Active Motif). Data are presented as absorbance units per μg of protein.
Gene expression changes in response to cytokine treatment using semiquantitative reverse transcriptase-PCR (RT-PCR)
Islets were isolated and treated with siRNA-based SNA-NCs as described above, and then subject to 24 hour CM treatment. RNA was isolated and reverse transcribed to cDNA (Promega). Reaction conditions can be found in the SDC Methods section.
Nitric oxide measurement
Detailed information can be found in the SDC Methods section.
Viability and β cell percentage by flow cytometry
The zinc binding dye Zinbo5 was used to label the zinc-rich β cells, while the dyes tetramethylrhodamine (TMRE) and 7-aminoactomycin D (7-AAD) were used to label viable and dead cells, respectively(24). The cells were analyzed using flow cytometry (BD Fortessa Flow Cytometer) to determine the percentage of β cells and the viability of the islet cells. Details can be found in the SDC Methods section.
In vitro luciferase measurements
Detailed information can be found in the SDC Methods section.
Syngeneic marginal islet mass transplants
Isolated islets were treated with 10 nM IKKβ SNA-NCs, 10 nM SCR SNA-NCs or left untreated for 24 hours prior to transplantation. Recipient mice were made diabetic via a single injection of streptozotocin (220 mg/kg body weight). Mice exhibiting blood glucose levels greater than 300 mg/dL for at least two consecutive days, as measured using a One Touch Ultra blood glucose meter (Lifescan; Milpitas, CA), were considered diabetic and were used as transplant recipients. A total of 50 islets (IKKβ SNA-NC (N=12), SCR SNA-NC (N=11), or untreated (N=12)) of similar sizes were counted by hand and transplanted intra-portally as described previously (19). Mice were considered normoglycemic when 2 consecutive blood glucose readings were below 200 mg/dL. Data are presented as the percentage of mice achieving normoglycemia in each treatment group versus days post-transplant.
Histology of transplanted islet grafts
Detailed information of the histological analyses can be found in the SDC Methods section.
Statistical analyses
The student's t-test, one-way ANOVA and log rank statistical tests were used to analyze the data. p < 0.05 was considered statistically significant. Results represent a combination of at least 3 independent experiments.
Supplementary Material
Acknowledgments
The authors would like to thank Lennell Reynolds of the Northwestern University Nikon Imaging Facility for his assistance with the preparation and imaging of the TEM samples. This work was supported by the Northwestern University Flow Cytometry Facility and a Cancer Center Support Grant (NCI CA060553). This work was funded by the Comprehensive Transplant Center at Northwestern University. CST is grateful to the Howard Hughes Medical Institute (HHMI) for a Physician-Scientist Early Career Award and to the Juvenile Diabetes Research Foundation (JDRF) for an Innovation Award (5-2011-219). CAM is grateful for an NSSEF Fellowship and support from the Northwestern Cancer Center for Nanotechnology Excellence (CCNE).
Abbreviations
- 7-AAD
7-Aminoactomycin D
- AuNP
gold nanoparticle
- CM
cytokine mixture
- ELISA
enzyme-linked immunosorption assay
- IFN-γ
interferon-gamma
- IκBα
inhibitor of kappaB alpha
- IKKβ
IκB Kinase beta
- iNOS
inducible nitric oxide synthase
- IP-10
IFN-γ-inducible protein-10
- luc
luciferase
- MCP-1
monocyte chemoattractant protein-1
- NF-κB
nuclear factor kappa B
- RIP
rat insulin promoter II
- RT-PCR
reverse transcriptase-polymerase chain reaction
- RT-qPCR
reverse transcriptase- quantitative polymerase chain reaction
- SNA-NC
Spherical Nucleic Acid-Nanoparticle Conjugate
- siRNA
short, interfering RNA
- SCR
scrambled siRNA sequence
- SCR SNA-NC
SNA-NC conjugated with a scrambled siRNA sequence
- IKKβ SNA-NC
SNA-NC conjugated with siRNA targeted against IKKβ
- STZ
streptozotocin
- Tg
transgenic
- TMRE
tetramethylrhodamine
- TNF-α
tumor necrosis factor-alpha
Footnotes
Author Contributions: J.S.R. participated in research design, performance of the research, analysis of the data and writing of the manuscript. J.S.R. was supported by the Comprehensive Transplant Center at Northwestern University.
K.M.M. participated in performance of the research, synthesis of spherical nucleic acid nanoparticle conjugates and writing of the manuscript.
X.Z. participated in performance of the research and was supported by the Comprehensive Transplant Center at Northwestern University.
X.C. and C.A.M. participated in writing the manuscript. X.C. was supported by the Comprehensive Transplant Center at Northwestern University. C.A.M. was supported by an NSSEF Fellowship and by the Northwestern Cancer Center for Nanotechnology Excellence.
C.S.T. and D.B.K. participated in research design, analysis of the data and writing of the manuscript. C.S.T. was supported by a Howard Hughes Medical Institute Physician-Scientist Early Career Award and a Juvenile Diabetes Research Foundation (JDRF) Innovation Award (5-2011-219). D.B.K. was supported by the Comprehensive Transplant Center at Northwestern University.
C.A.M. and C.S.T. are shareholders in AuraSense Therapeutics, the company that licensed the spherical nucleic acid technology from Northwestern University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bassi R, Fiorina P. Impact of islet transplantation on diabetes complications and quality of life. Curr Diab Rep. 2011;11(5):355–63. doi: 10.1007/s11892-011-0211-1. [DOI] [PubMed] [Google Scholar]
- 2.Alejandro R, Cutfield RG, Shienvold FL, et al. Natural history of intrahepatic canine islet cell autografts. J Clin Invest. 1986;78(5):1339–48. doi: 10.1172/JCI112720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77(5):587–97. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 4.Nagata M, Mullen Y, Matsuo S, Herrera M, Clare-Salzler M. Destruction of islet isografts by severe nonspecific inflammation. Transplant Proc. 1990;22(2):855–6. [PubMed] [Google Scholar]
- 5.Debray-Sachs M, Assan R, Bailey D, Hamburger J. Functional inhibition of isolated pancreatic cells, new technic for the detection of macrophage cytotoxicity. C R Acad Sci Hebd Seances Acad Sci D. 1978;287(12):1161–4. [PubMed] [Google Scholar]
- 6.Kaufman DB, Platt JL, Rabe FL, Dunn DL, Bach FH, Sutherland DE. Differential roles of Mac-1+ cells, and CD4+ and CD8+ T lymphocytes in primary nonfunction and classic rejection of islet allografts. J Exp Med. 1990;172(1):291–302. doi: 10.1084/jem.172.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwizer RW, Leiter EH, Evans R. Macrophage-mediated cytotoxicity against cultured pancreatic islet cells. Transplantation. 1984;37(6):539–44. doi: 10.1097/00007890-198406000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 9.Farney AC, Xenos E, Sutherland DE, et al. Inhibition of pancreatic islet beta cell function by tumor necrosis factor is blocked by a soluble tumor necrosis factor receptor. Transplant Proc. 1993;25(1 Pt 2):865–6. [PubMed] [Google Scholar]
- 10.Kaufman DB, Naidu Y, Norman JG, et al. Functional significance of donor islet interleukin-1 receptor type 1 (IL-1Rt1) expression in islet transplantation. Transplant Proc. 1997;29(1-2):772–3. doi: 10.1016/s0041-1345(96)00479-4. [DOI] [PubMed] [Google Scholar]
- 11.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293(7):830–5. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto S, Takita M, Chaussabel D, et al. Improving Efficacy of Clinical Islet Transplantation with Iodixanol Based Islet Purification, Thymoglobulin Induction and Blockage of IL-1-beta and TNF-alpha. Cell Transplant. 2011 doi: 10.3727/096368910X564058. [DOI] [PubMed] [Google Scholar]
- 13.Baker MS, Chen X, Cao XC, Kaufman DB. Expression of a dominant negative inhibitor of NF-kappaB protects MIN6 beta-cells from cytokine-induced apoptosis. J Surg Res. 2001;97(2):117–22. doi: 10.1006/jsre.2001.6121. [DOI] [PubMed] [Google Scholar]
- 14.Baker MS, Chen X, Rotramel A, Nelson J, Kaufman DB. Proinflammatory cytokines induce NF-kappaB-dependent/NO-independent chemokine gene expression in MIN6 beta cells. J Surg Res. 2003;110(1):295–303. doi: 10.1016/s0022-4804(03)00027-1. [DOI] [PubMed] [Google Scholar]
- 15.Cardozo AK, Heimberg H, Heremans Y, et al. A comprehensive analysis of cytokine-induced and nuclear factor-kappa B-dependent genes in primary rat pancreatic beta-cells. J Biol Chem. 2001;276(52):48879–86. doi: 10.1074/jbc.M108658200. [DOI] [PubMed] [Google Scholar]
- 16.Cardozo AK, Proost P, Gysemans C, Chen MC, Mathieu C, Eizirik DL. IL-1beta and IFN-gamma induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice. Diabetologia. 2003;46(2):255–66. doi: 10.1007/s00125-002-1017-0. [DOI] [PubMed] [Google Scholar]
- 17.Sandberg JO, Eizirik DL, Sandler S, Tracey DE, Andersson A. Treatment with an interleukin-1 receptor antagonist protein prolongs mouse islet allograft survival. Diabetes. 1993;42(12):1845–51. doi: 10.2337/diab.42.12.1845. [DOI] [PubMed] [Google Scholar]
- 18.Mathis D, Vence L, Benoist C. beta-Cell death during progression to diabetes. Nature. 2001;414(6865):792–8. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 19.Rink JS, Chen X, Zhang X, Kaufman DB. Conditional and specific inhibition of NF-kappaB in mouse pancreatic beta cells prevents cytokine-induced deleterious effects and improves islet survival posttransplant. Surgery. 2012;151(2):330–9. doi: 10.1016/j.surg.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi T, Matsumoto S, Matsushita M, et al. Donor pretreatment with DHMEQ improves islet transplantation. J Surg Res. 2010;163(1):e23–34. doi: 10.1016/j.jss.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 21.Leibowitz G, Beattie GM, Kafri T, et al. Gene transfer to human pancreatic endocrine cells using viral vectors. Diabetes. 1999;48(4):745–53. doi: 10.2337/diabetes.48.4.745. [DOI] [PubMed] [Google Scholar]
- 22.Mahato RI, Henry J, Narang AS, et al. Cationic lipid and polymer-based gene delivery to human pancreatic islets. Mol Ther. 2003;7(1):89–100. doi: 10.1016/s1525-0016(02)00031-x. [DOI] [PubMed] [Google Scholar]
- 23.Narang AS, Mahato RI. Biological and biomaterial approaches for improved islet transplantation. Pharmacol Rev. 2006;58(2):194–243. doi: 10.1124/pr.58.2.6. [DOI] [PubMed] [Google Scholar]
- 24.Rink JS, McMahon KM, Chen X, Mirkin CA, Thaxton CS, Kaufman DB. Transfection of pancreatic islets using polyvalent DNA-functionalized gold nanoparticles. Surgery. 2010;148(2):335–45. doi: 10.1016/j.surg.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cutler JI, Auyeung E, Mirkin CA. Spherical nucleic acids. J Am Chem Soc. 2012;134(3):1376–91. doi: 10.1021/ja209351u. [DOI] [PubMed] [Google Scholar]
- 26.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold nanoparticles for biology and medicine. Angew Chem Int Ed Engl. 2010;49(19):3280–94. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giljohann DA, Seferos DS, Patel PC, Millstone JE, Rosi NL, Mirkin CA. Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett. 2007;7(12):3818–21. doi: 10.1021/nl072471q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giljohann DA, Seferos DS, Prigodich AE, Patel PC, Mirkin CA. Gene regulation with polyvalent siRNA-nanoparticle conjugates. J Am Chem Soc. 2009;131(6):2072–3. doi: 10.1021/ja808719p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AK, Han MS, Mirkin CA. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science. 2006;312(5776):1027–30. doi: 10.1126/science.1125559. [DOI] [PubMed] [Google Scholar]
- 30.Seferos DS, Giljohann DA, Hill HD, Prigodich AE, Mirkin CA. Nano-flares: probes for transfection and mRNA detection in living cells. J Am Chem Soc. 2007;129(50):15477–9. doi: 10.1021/ja0776529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao L, Patel PC, Alhasan AH, Giljohann DA, Mirkin CA. Nucleic acid-gold nanoparticle conjugates as mimics of microRNA. Small. 2011;7(22):3158–62. doi: 10.1002/smll.201101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massich MD, Giljohann DA, Schmucker AL, Patel PC, Mirkin CA. Cellular response of polyvalent oligonucleotide-gold nanoparticle conjugates. ACS Nano. 2010;4(10):5641–6. doi: 10.1021/nn102228s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massich MD, Giljohann DA, Seferos DS, Ludlow LE, Horvath CM, Mirkin CA. Regulating immune response using polyvalent nucleic acid-gold nanoparticle conjugates. Mol Pharm. 2009;6(6):1934–40. doi: 10.1021/mp900172m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel PC, Giljohann DA, Daniel WL, Zheng D, Prigodich AE, Mirkin CA. Scavenger receptors mediate cellular uptake of polyvalent oligonucleotide-functionalized gold nanoparticles. Bioconjug Chem. 2010;21(12):2250–6. doi: 10.1021/bc1002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel PC, Hao L, Yeung WS, Mirkin CA. Duplex end breathing determines serum stability and intracellular potency of siRNA-Au NPs. Mol Pharm. 2011;8(4):1285–91. doi: 10.1021/mp200084y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seferos DS, Prigodich AE, Giljohann DA, Patel PC, Mirkin CA. Polyvalent DNA nanoparticle conjugates stabilize nucleic acids. Nano Lett. 2009;9(1):308–11. doi: 10.1021/nl802958f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker MS, Chen X, Rotramel AR, Nelson JJ, Kaufman DB. Interferon regulatory factor-1 down-regulates cytokine-induced IP-10 expression in pancreatic islets. Surgery. 2003;134(2):134–41. doi: 10.1067/msy.2003.236. [DOI] [PubMed] [Google Scholar]
- 38.Corbett JA, Sweetland MA, Wang JL, Lancaster JR, Jr, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci U S A. 1993;90(5):1731–5. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Southern C, Schulster D, Green IC. Inhibition of insulin secretion by interleukin-1 beta and tumour necrosis factor-alpha via an L-arginine-dependent nitric oxide generating mechanism. FEBS Lett. 1990;276(1-2):42–4. doi: 10.1016/0014-5793(90)80502-a. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Zhang X, Larson C, Chen F, Kissler H, Kaufman DB. The epididymal fat pad as a transplant site for minimal islet mass. Transplantation. 2007;84(1):122–5. doi: 10.1097/01.tp.0000266909.58117.e3. [DOI] [PubMed] [Google Scholar]
- 41.Giannoukakis N, Rudert WA, Trucco M, Robbins PD. Protection of human islets from the effects of interleukin-1beta by adenoviral gene transfer of an Ikappa B repressor. J Biol Chem. 2000;275(47):36509–13. doi: 10.1074/jbc.M005943200. [DOI] [PubMed] [Google Scholar]
- 42.Heimberg H, Heremans Y, Jobin C, et al. Inhibition of cytokine-induced NF-kappaB activation by adenovirus-mediated expression of a NF-kappaB super-repressor prevents beta-cell apoptosis. Diabetes. 2001;50(10):2219–24. doi: 10.2337/diabetes.50.10.2219. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994;91(10):4407–11. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Storhoff JJ, Elghanian R, Mucic RC, Mirkin CA, Letsinger RL. One-Pot Colorimetric Differentiation of Polynucleotides with Single Base Imperfections Using Gold Nanoparticle Probes. J Am Chem Soc. 1998;120(9):1959–64. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.