Abstract
PNU-120596 (1-(5-chloro-2,4-dimethoxyphenyl)-3-(5-methylisoxazol-3-yl)urea), a Type-II positive allosteric modulator of α7 nicotinic acetylcholine receptors inhibits α7 desensitization and robustly prolongs openings of α7 channels. However, these effects may render α7 channels more accessible to positively charged molecules and thus, more susceptible to voltage-dependent open-channel-block-like inhibition. To test this hypothesis, choline chloride (i.e., choline), a selective endogenous α7 agonist, and bicuculline methochloride (i.e., bicuculline), a competitive α7 antagonist, were used as membrane voltage-sensitive probes in whole-cell voltage-clamp recordings from hippocampal CA1 interneurons in acute brain slices in the absence and presence of PNU-120596. PNU-120596 enhanced voltage-dependent inhibition of α7 responses by bicuculline and choline. In the presence of PNU-120596, α7 channels favored a burst-like kinetic modality in the presence, but not absence of bicuculline and bursts of α7 openings were voltage-dependent. These results suggest that PNU-120596 alters the pharmacology of α7 channels by making these channels more susceptible to voltage-dependent inhibitory interactions with positively charged drugs at concentrations that do not potently inhibit α7 channels without PNU-120596. This inhibition imitates α7 nicotinic receptor desensitization and compromises the potentiating anti-desensitization effects of PNU-120596 on α7 nicotinic receptors. This unexpected dual action of PNU-120596, and possibly other Type-II positive allosteric modulators of α7 nicotinic receptors, may lead to unanticipated α7 channel-drug interactions and misinterpretation of α7 single-channel data.
Keywords: PNU-120596, PNU120596, α7 nicotinic receptor, desensitization, channel block, choline, bicuculline
1. Introduction
PNU-120596 (i.e., 1-(5-chloro-2,4-dimethoxyphenyl)-3-(5-methylisoxazol-3-yl)urea), a Type-II positive allosteric modulator of α7 nicotinic acetylcholine receptors inhibits α7 receptor desensitization and enhances the potency of nicotinic agonists for activation of α7 nicotinic receptors, but does not activate these receptors when administered alone (Gusev and Uteshev, 2010; Hurst et al., 2005; Kalappa et al., 2010). PNU-120596 robustly increases the open time of α7 ion channels from ~100 µs (Mike et al., 2000) to up to ~1 s (Gusev and Uteshev, 2010; Kalappa et al., 2010). However, by enhancing α7 activation, PNU-120596 may also enhance unanticipated interactions of α7 channels with positively charged molecules. Thus, PNU-120596 may alter the pharmacology of α7 channel-drug interactions by making α7 ion channels more accessible to positively charged molecules and thus, more susceptible to voltage-dependent inhibitory interactions with positively charged drugs at concentrations that may not potently interact with α7 nicotinic receptor-channels in the absence of PNU-120596. This hypothesis was tested in the present study by investigating interactions of α7 channels with voltage-sensitive probes: bicuculline methochloride (i.e., bicuculline), a competitive α7 antagonist of GABAARs and α7 nicotinic receptors (Demuro et al., 2001) and choline chloride (i.e., choline), a selective endogenous α7 agonist (EC50~0.5–1 mM) (Alkondon et al., 1997; Papke and Papke, 2002), using whole-cell voltage-clamp recordings from hippocampal CA1 interneurons in acute brain slices in the presence and absence of PNU-120596. Both bicuculline and choline are commonly used in studies involved α7 nicotinic receptors. These compounds are positively charged and highly ionized at the physiological pH (pKa>10) (Perrin, 1972; Seutin et al., 1997), but do not potently block α7 channels in the absence of PNU-120596 (Demuro et al., 2001). However, choline at high concentrations (i.e., >10 mM) causes α7 channel block (Uteshev et al., 2002).
In the continuous presence of nicotinic agonists, α7-mediated responses are reduced naturally by two independent processes: receptor desensitization and channel block by agonist (Uteshev, 2012a). These processes may not be easily distinguished from one another especially if α7 activation is elicited by high agonist concentrations (>100 µM acetylcholine or >1 mM choline) administered at highly negative membrane voltages (<−60 mV). PNU-120596 reduces α7 desensitization (Hurst et al., 2005), but may not completely eliminate it (Williams et al., 2011). However, at negative membrane potentials in the presence of PNU-120596, the task of separation of α7 desensitization from channel block by positively charged molecules, such as choline, may become quite challenging. In this study, we demonstrate that PNU-120596 enhances both α7 activation and voltage-dependent inhibition of α7 channels by positively charged compounds, bicuculline and choline. These data suggest that in the presence of PNU-120596 the sites of inhibitory action by bicuculline and choline lie near or within the α7 channel.
2. Materials and methods
Chemical compounds studied in this article: 1-(5-chloro-2,4-dimethoxyphenyl)-3-(5-methylisoxazol-3-yl)urea; i.e., PNU-120596 (PubChem CID: 311434); Bicuculline Methochloride (PubChem CID: 44134574); Choline Chloride (PubChem CID: 6209).
2.1. Preparation of brain slices
Experiments were performed using young adult male and female Sprague Dawley rats (P18-P35). The animal use was in accordance with the Guide for the Care and Use of Laboratory Animals (NIH 865-23, Bethesda, MD), and all experimental protocols were approved by the Animal Care and Use Committee of Southern Illinois University School of Medicine, Springfield, IL and the Institutional Animal Care and Use Committee of University of North Texas Health Science Center at Fort Worth, TX. The rats were subjected to rapid decapitation and brains were swiftly removed and transferred to an ice-cold, sucrose-rich solution of the following composition (in mM): sucrose 250, KCl 3, NaH2PO4 1.23, MgCl2 5, CaCl2 0.5, NaHCO3 26, glucose 10 (pH 7.4), when bubbled with carbogen (95% O2 and 5% CO2). Three to four coronal whole brain slices (250–300 µM thick) containing the caudal hippocampus were cut in a sucrose-rich solution at 3° C using Vibratom-1000+ (Vibratom, St Louis, MO) and transferred to a storage chamber containing oxygenated artificial cerebrospinal fluid (aCSF) of the following composition (in mM): NaCl 125, KCl 3, NaH2PO4 1.23, MgCl2 1, CaCl2 2, NaHCO3 26, glucose 10 (pH 7.4), when bubbled with carbogen. The slices were allowed to recover at 30° C for ~30 min and then maintained at room temperature for the subsequent 6 hrs when the slices were used for recordings.
2.2. Drugs
In this study, 1–2 µM PNU-120596 was used. These concentrations lie near the EC50 for potentiating effects of PNU-120596 in heterologous systems (EC50~1.5 µM) (Gronlien et al., 2007; Young et al., 2008). The intravenous administration of 1 mg/kg PNU-120596 has been shown to elevate the concentration of PNU-120596 in the brains of rats to similar values (~1.5 µM) (Hurst et al., 2005). PNU-120596 was supplied by the National Institute of Drug Addiction (NIDA) through the NIDA Research Resources Drug Supply Program or purchased from Tocris Bioscience (Ellisville, MO). Bicuculline methochloride (bicuculline), Gabazine, 6, 7-dinitroquinoxaline-2, 3-dione (DNQX), (2R)-amino-5-phosphonovaleric acid (AP-5) and tetrodotoxin (TTX) were purchased from Ascent Scientific (Bristol, UK). Other chemicals were purchased from Sigma-Aldrich (St Louis, MO). All antagonists (except for bicuculline) were constantly present in aCSF. We have not detected any apparent effects of these antagonists on α7 nicotinic receptors in the absence or presence of PNU-120596. However, a thorough investigation of possible interactions of these compounds with α7 nicotinic receptor-channels has not been conducted in this study.
2.3. Drug application
To ensure equilibration of concentrations of drugs within the brain slice, PNU-120596 and bicuculline were added to aCSF for at least 50 min and 25 min, respectively, prior to patch-clamp recordings. In experiments investigating α7 single-channel openings, 10 min pre-incubation in bicuculline was used. These rates of equilibration of PNU-120596 (τ~16 min) and choline (τ~5 min) have been estimated from our previous studies, where α7 responses were monitored during the onset and washout of PNU-120596 or choline chloride in hypothalamic and hippocampal acute slices (Gusev and Uteshev, 2010; Kalappa et al., 2010). The equilibration rates of bicuculline and choline chloride were assumed to be similar.
2.4. Patch-clamp recordings
All recordings were conducted at room temperature. For patch-clamp whole-cell recordings, slices were transferred into a recording chamber which was perfused with aCSF at a rate of 1 ml/min using a perfusion pump 2232 Microperpex S (LK.B, Upsalla, Sweden). In the majority of the patch-clamp experiments, aCSF contained 20 µM gabazine, 15 µM DNQX, 50 µM AP-5, 10 µM atropine, 40 µM picrotoxin and 0.3 µM TTX to inhibit γ-aminobutyric acid type A (GABAA), 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid (AMPA), N-methyl-D-aspartate (NMDA), muscarinic acetylcholine, GABAA/glycine receptors and voltage gated Na+ ion channels respectively. An Olympus BX-51WI microscope (Olympus America Inc, Center Valley, PA) was used to select hippocampal CA1 stratum radiatum interneurons for electrophysiological patch-clamp experiments. Recordings were conducted at room temperature using a Multiclamp-700B amplifier equipped with Digidata-1440A A/D converter (Molecular Devices, Sunnyvale, CA). Data were filtered at 2.8–5 kHz, sampled at 10–20 kHz and stored on a hard drive for offline analysis. When necessary, single-channel data were additionally filtered at 0.2–0.5 kHz prior to analysis to improve signal-to-noise ratio. Patch pipettes of ~4–6 MΩ were pulled using a Sutter P-97 horizontal puller (Sutter Instruments, Novato, CA). The intracellular electrode solution contained (in mM): CsMeSO3 140, NaCl 6, MgCl2 2, Mg-ATP (adenosine-5’-triphosphate) 2, Na-GTP 0.3, HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) 10, CsOH 0.3 (pH ~7.4). Membrane voltages were not corrected for the liquid junction potential: VLJ=9.8 mV. Whole-cell configurations were established after the formation of a stable gigaseal (>2 GΩ). Cells with membrane leaks >100 pA were discarded. Picospritzer pipettes identical to those of patch pipettes were used for choline (1 mM) application (pressure 5–8 psi, Parker Hannifin Instrumentation, Cleveland, OH, USA). Application pipettes were positioned 10 µm from the recorded interneurons and brief (100 ms) puffs of 1 mM choline were delivered every 3 min. In experiments where α7 single-channel activity was recorded in whole-cell, 10 µM choline was always present in the aCSF. The aCSF flow rate was maintained at 1 ml/min using perfusion pump 2232 Microperpex S (LK.B, Upsalla, Sweden). PNU-120596 (1–2 µM) and bicuculline methochloride (bicuculline; 1–1000 µM) were added to the aCSF. Choline-containing solutions were prepared fresh each day from a stock solution of 1M stored at −20°C.
2.5. Analysis
The analysis of conventional and single-channel whole-cell recordings was done using Clampfit 10.1 software program (Molecular Devices, Sunnyvale, CA). The effects of bicuculline on synchronous α7 responses were investigated in conventional whole-cell recordings where α7 activity was synchronized by pressure puffs of 1 mM choline in the presence of 2 µM PNU-120596. In these experiments, net charge of whole-cell voltage-clamp responses was measured over 20 s after each 1 mM choline puff. Each final data point was an average of at least 3 consecutive data points recorded every 3 min. In experiments utilizing whole-cell α7 single-channel recordings, the effects of bicuculline on asynchronous α7 activity (i.e., spontaneous α7 single-channel openings elicited by 10 µM choline +1 µM PNU-120596) was investigated. However, bursts of single-channel openings in whole-cell recordings cannot be readily defined because hundreds of α7 nicotinic receptors contribute to generation of asynchronous α7 single-channel events in a given experiment and therefore, α7 single-channel openings generated by different α7 channels may routinely be erroneously defined as intraburst openings generated by the same single channel. In fact, as α7 Popen is extremely small even in the presence of PNU-120596 (an estimate of Popen <0.000027 for α7 channels activated by 10 µM choline+1 µM PNU-120596 was given previously (Gusev and Uteshev, 2010)), it is very likely that many, if not all, α7 single-channel openings/bursts recorded during 20–30 min of our whole-cell experiments were produced by different α7 channels. Nevertheless, the probability of erroneously defining openings from different α7 channels as events belonging to the same burst can be substantially reduced by considering only long (e.g., >500 ms) clearly isolated single-channel openings/bursts (e.g., separated from other similar openings by t>tcrit=150 ms; where tcrit is the burst delimiting interval or critical time). Once a subset of long isolated bursts is collected, a standard analysis of intraburst events can be performed. The main limitation of this approach is that only a subset of α7 single-channel events is analyzed and thus, the sample size is reduced and certain single-channel kinetic parameters (e.g., burst duration) could not be estimated. In this study, subsets of long (i.e., >500 ms) isolated (tcrit=150 ms) α7 single-channel openings/bursts were used to evaluate and determine voltage-dependence of the number of events per second of opening/burst, the apparent intraburst mean open time, the total open time per second of opening/burst, the amount of block time and net charge reduction in the presence of PNU-120596±bicuculline. Block time was estimated as a ratio of the total open times per second of opening/burst in the absence and presence of bicuculline:
topen(−bicuculline)/topen(+bicuculline). This value was then multiplied by a coefficient representing the amount of reduction in the apparent single-channel amplitude by bicuculline:
I(−bicuculline)/I(+bicuculline) and thus, the total reduction of net charge was calculated:
[topen(−bicuculline)*I(−bicuculline)]/[topen(+bicuculline)*I(+bicuculline)]. This value was then compared with net charge reduction obtained from analysis of synchronous α7 activity (i.e., synchronized by pressure puffs of 1 mM choline). For statistical analysis of data, GraphPad Prism statistical software package was used (GraphPad Software, La Jolla, CA). Statistical significance between pairs of experimental data points (*) or between experimental data points and theoretical values calculated from the Ohm’s law (#) were defined by P-values: */#P<0.05, **/##P<0.01, ***/###P<0.001 and ****/####P<0.0001. Results are presented as the mean ± S.E.M.
3. RESULTS
3.1. PNU-120596 significantly enhances inhibition of α7 responses by bicuculline
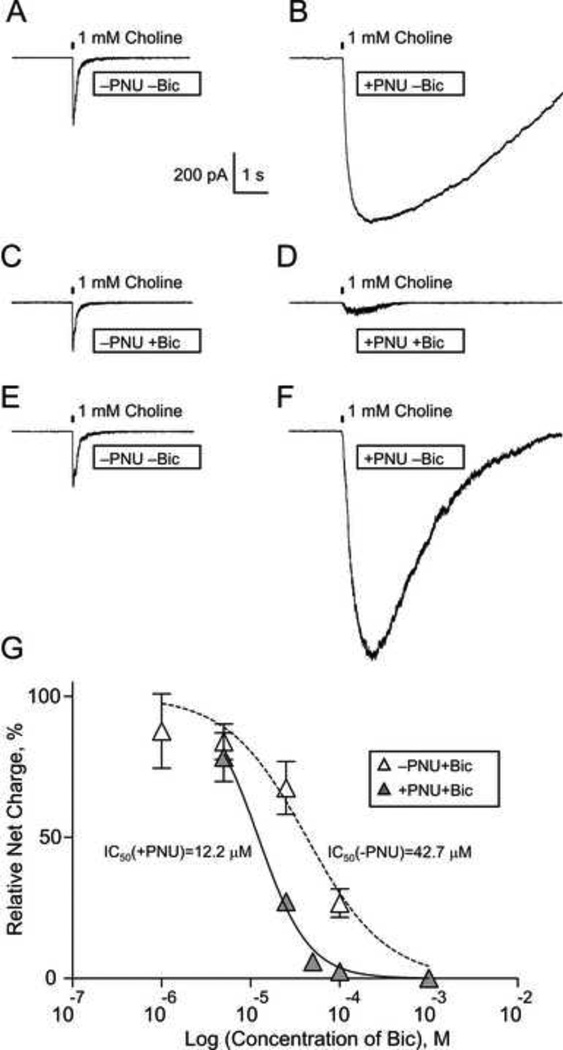
The effect of PNU-120596 on inhibition of α7 nicotinic receptor-mediated responses by bicuculline was determined in whole-cell voltage-clamp experiments using hippocampal CA1 interneurons in acute coronal whole-brain slices. Responses of α7 nicotinic receptors were elicited by brief focal (100 ms) pressure puffs of 1 mM choline. The membrane voltage was held at −60 mV. Control α7 responses were recorded in the absence (i.e., −PNU, Fig. 1A) and presence (i.e., +PNU, Fig. 1B) of 2 µM PNU-120596 in aCSF. Consistent with previous reports (Kalappa et al., 2010), α7 responses of hippocampal CA1 interneurons to 1 mM choline in the presence of 2 µM PNU-120596 were considerably larger than those in the absence of PNU-120596 (Fig. 1A-B). Bicuculline (0.001–1 mM) added to aCSF reversibly inhibited α7 responses in a concentration-dependent manner both in the absence (i.e., −PNU; Fig. 1A, 1C and 1E) and presence (i.e., +PNU; Fig. 1B, 1D and 1F) of 2 µM PNU-120596. However, the inhibitory effects of bicuculline on α7 responses were significantly enhanced [F(1,38)=22.18, P<0.0001; F-test] by 2 µM PNU-120596 (solid vs. dashed lines, Fig. 1G) from IC50(−PNU)=42.7 µM (Hill slope, 0.98) without PNU-120596 to IC50(+PNU)=12.2 µM (Hill slope, 1.53) with PNU-120596. In these experiments, one bicuculline concentration was tested per experiment: at least 3 consecutive responses to 1 mM choline were recorded and averaged. The resulting averaged values were normalized to the corresponding control value obtained from the same neuron prior to bicuculline administration. Recordings in the absence (Fig. 1A, 1C and 1E) and presence (Fig. 1B, 1D and 1F) of PNU-120596 were conducted using different groups of neurons.
Figure 1. Concentration-dependence of the effects of bicuculline on α7 nicotinic receptor-mediated responses in the absence and presence of 2 µM PNU-120596.
Representative α7 nicotinic receptor-mediated whole-cell responses of hippocampal CA1 striatum-radiatum interneurons to focal brief (100 ms) pressure puffs of 1 mM choline were recorded in acute brain slices in the absence (i.e., −bicuculline; A-B and E-F) or presence (i.e., +bicuculline; C-D) of various concentrations of bicuculline in aCSF and in the absence (i.e., −PNU; A, C and E) or presence (i.e., +PNU; B, D and F) of 2 µM PNU-120596 in aCSF. In the examples illustrated in (A-F), 25 µM bicuculline was used. The effects of bicuculline were reversible (E-F) and concentration-dependent (G). 2 µM PNU-120596 significantly (F(1,38)=22.18, P<0.0001, n=2–7; F-test) enhanced the inhibition of α7 responses by bicuculline from IC50(−PNU)=42.7 µM (Hill slope, 0.98) to IC50(+PNU)~12.2 µM (Hill slope, 1.53). One bicuculline concentration was tested per experiment: at least 3 responses to brief puffs of 1 mM choline were recorded and averaged. The resulting averaged values were normalized to the corresponding control value obtained from the same neuron prior to bicuculline administration. Recordings in the absence (A, C and E) and presence (B, D and F) of PNU-120596 were conducted using different groups of neurons.
3.2. Inhibition of α7 responses by bicuculline and choline in the presence of PNU-120596 is voltage-dependent
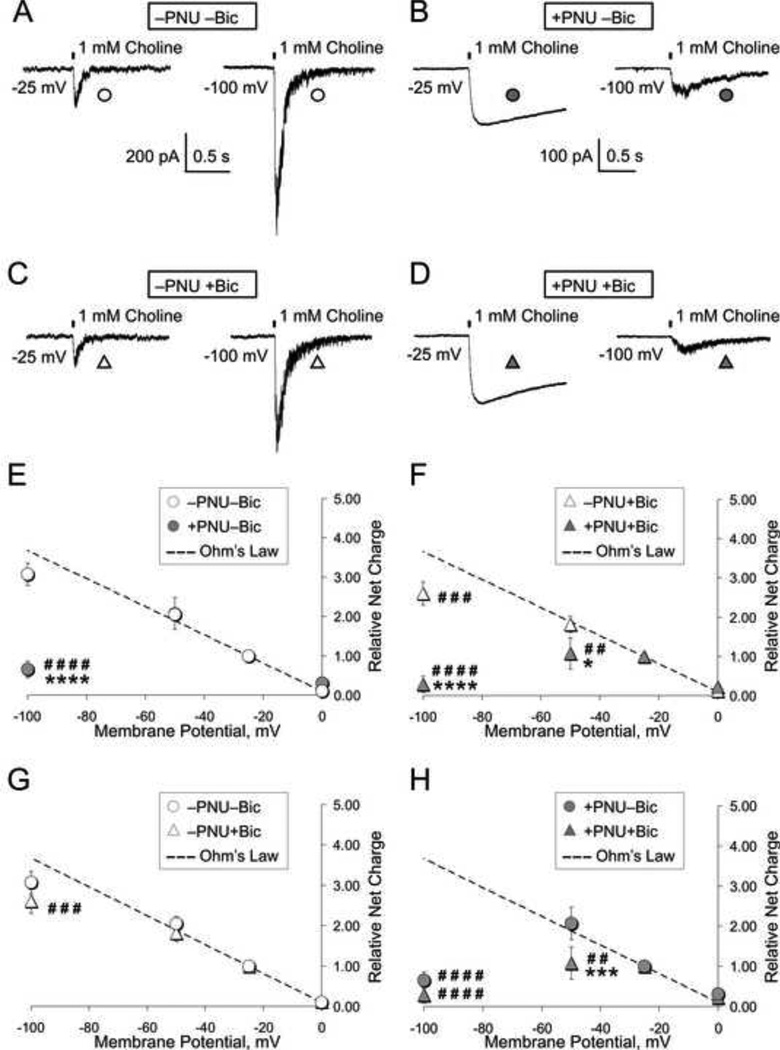
Responses of hippocampal CA1 interneurons to brief pressure puffs of 1 mM choline (Fig. 2A-D) were recorded in whole-cell voltage-clamp experiments in the presence and absence of 2 µM PNU-120596 and 15 µM bicuculline at different membrane voltages: i.e., 0 mV, −25 mV, −50 mV and −100 mV. This concentration of bicuculline was chosen because it falls near the IC50 for the bicuculline inhibition of α7 responses in the presence of PNU-120596 (see Fig. 1G). At each membrane voltage tested, the net charge of α7 responses was measured over the first 20 s after the choline puff. At least 3 responses to 1 mM choline were recorded and averaged at each membrane voltage. The resulting averaged values were normalized to the corresponding values obtained at −25 mV in the same experiment. The normalized charge voltage relationships were then built and compared for four experimental conditions (Fig. 2): −PNU-bicuculline, +PNU-bicuculline, −PNU+bicuculline and +PNU+bicuculline. The experimental data points were also compared to the theoretical points calculated from the Ohm’s law, which was defined by the function, charge(V)=0.1–0.036V (dashed lines, Fig. 2E-H), where charge(V) is the normalized α7 net charge over the first 20 s after choline puffs; and V is the corresponding membrane voltage measured in mV. This function was determined by two normalized data points recorded in the absence of PNU and bicuculline (i.e., −PNU-bicuculline; open circles, Fig. 2E) at the membrane voltages of −25 mV and 0 mV within the assumption that there was only a minimal, if any, voltage-dependent inhibition at the membrane voltages more positive than −25 mV. This assumption appears valid because significant voltage dependence was not detected even at −100 mV in the absence of PNU-120596 and bicuculline (open circles, Fig. 2E). In these experiments, α7 responses in the absence and presence of 15 µM bicuculline were obtained from the same individual neurons tested at all specified membrane voltages. Recordings in the absence (Fig. 2A and 2C) and presence (Fig. 2B and 2D) of PNU-120596 were obtained from different groups of neurons.
Figure 2. Voltage-dependence of the effects of 2 µM PNU-120596 on α7 nicotinic receptor-mediated responses.
A-D) Representative α7 nicotinic receptor-mediated whole-cell responses of hippocampal CA1 interneurons to focal brief (100 ms) pressure puffs of 1 mM choline were recorded in acute brain slices in the absence (i.e., −PNU; A and C) or presence (i.e., +PNU; B and D) of 2 µM PNU-120596 and in the absence (i.e., −bicuculline; A-B) or presence (i.e., +bicuculline; C-D) of 15 µM bicuculline in aCSF. This concentration of bicuculline was chosen because it falls near the IC50 for the bicuculline-elicited inhibition of α7 responses in the presence of PNU-120596 (see Fig. 1G). E-F) A summary of experiments obtained in the absence and presence of 2 µM PNU-120596 and 15 µM bicuculline. The PNU-120596-induced inhibition of α7 responses by bicuculline and choline was found to be voltage-dependent. The effects of treatments and membrane potentials on α7 charge-voltage relationships as well as deviations from the Ohm’s law (dashed lines) were found to be highly significant (a two-way ANOVA with repeated measurements and a post-hoc Bonferroni tests): F(4,20)=13.06, P<0.0001 (treatments) and F(2,40)=75.19, P<0.0001 (membrane potentials). A significant inhibition of α7 channel responses in the absence of bicuculline (i.e., by choline puffs alone) was observed only at −100 mV in the presence of 2 µM PNU-120596 (closed circles, 2E and 2H). The levels of significance for inhibition of α7 responses at various membrane potentials are defined by asterisks: *P<0.05, ***P<0.001 and ****P<0.0001. Significant deviations from the Ohm’s law (dashed line) are marked by number signs: #P<0.05, ##P<0.01, ###P<0.001 and ###P<0.0001.
A two-way ANOVA with repeated measurements was applied to determine the levels of statistical significance of the effects of treatments and membrane voltages on α7 charge-voltage relationships as well as the statistical significance of deviations from the Ohm’s law as a function of different treatments and membrane voltages. The results indicated the presence of extremely significant effects of treatments [F(4,20)=13.06, P<0.0001] and membrane voltages [F(2,40)=75.19, P<0.0001] on the charge-voltage relationships of α7 ion channels. A post-hoc Bonferroni test detected significant effects of 2 µM PNU-120596 on α7-mediated responses at −100 mV in the absence (circles, Fig. 2E; ****P<0.0001, n=5) and presence (triangles, Fig. 2F; ****P<0.0001, n=5) of 15 µM bicuculline; as well as at −50 mV in the presence of 15 µM bicuculline (triangles, Fig. 2F; *P<0.05, n=5). Significant effects of 15 µM bicuculline on α7-mediated responses were detected at −50 mV in the presence of 2 µM PNU-120596 (closed circle and triangle, Fig. 2H; ***P<0.001, n=5). Note that open and closed circles illustrating α7 net charge measurements at the membrane voltages −25 mV and −50 mV in Fig. 2E are completely overlaid.
Moreover, 2 µM PNU-120596 caused significant deviations from the Ohm’s law and thus, significant response inhibition at −100 mV both in the absence (closed circles, Fig. 2E and 2H; ####P<0.0001, n=5) and presence (closed triangles, Fig. 2F and 2H; ####P<0.0001, n=5) of 15 µM bicuculline, as well as at ™50 mV in the presence of 15 µM bicuculline (closed triangles, Fig. 2F and 2H; ##P<0.01, n=5). By contrast, in the absence of PNU-120596, 15 µM bicuculline caused significant deviations from the Ohm’s law only at ™100 mV (open triangles, Fig. 2F-and 2G; ###P<0.001, n=5). These results support the hypothesis that PNU-120596 significantly enhances voltage-dependent inhibition of α7 channels by bicuculline and choline. However, in these experiments, significant inhibition in the absence of bicuculline (presumably, by choline puffs alone) was observed only at −100 mV in the presence of 2 µM PNU-120596 (closed circles, Fig. 2E and 2H).
3.3. PNU-120596 fails to enhance inhibition by bicuculline at positive membrane voltages
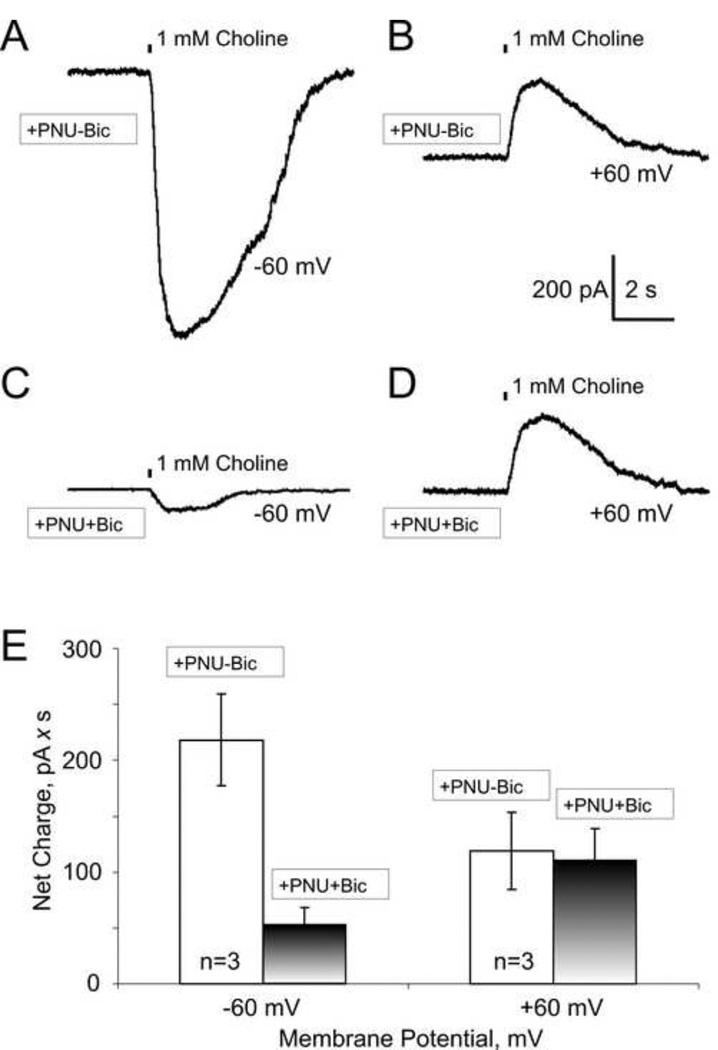
As we have shown earlier in this study, inhibition of α7 nicotinic receptor-mediated responses by PNU+bicuculline was not observed at depolarized membrane potentials (i.e., more positive than −25 mV; Fig. 2). To extend this conclusion, similar experiments were conducted at a positive membrane potential, +60 mV (Fig. 3). In these experiments, α7 nicotinic receptor-mediated responses to 1 mM choline puffs were recorded from the same neuron at −60 mV and +60 mV in the presence of 2 µM PNU-120596 in the absence (Fig. 3A-B) and presence (Fig. 3C-D) of 15 µM bicuculline. To generate outward α7-mediated responses at +60 mV, Mg2+ ions were removed from both the internal and external solutions because of a strong inward rectification of the current-voltage relationship of α7 nicotinic receptor-mediated responses (Uteshev, 2010; Uteshev et al., 1996). Bicuculline inhibited α7 responses only at −60 mV (Fig. 3A vs. Fig. 3C), but not +60 mV (Fig. 3B vs. Fig. 3D) further supporting the hypothesis of inhibitory interactions between bicuculline and α7 channels in the presence of PNU-120596. A summary of results is shown in Fig. 3E.
Figure 3. PNU-120596 fails to enhance inhibition by bicuculline at positive membrane voltages.
Typical α7 nicotinic receptor-mediated responses of hippocampal CA1 striatum radiatum interneurons to brief (100 ms) puffs of 1 mM choline in the presence of 2 µM PNU-120596 and absence (A-B) or presence (C-D) of 25 µM bicuculline at negative (−60 mV; A and C) and positive (+60 mV; B and D) membrane potentials. PNU-120596 significantly enhanced inhibition by bicuculline at −60 mV, but not +60 mV. E) A summary of the effects obtained from five interneurons. The same set of neurons was tested in all experimental conditions.
3.4. Evaluation of α7 single-channel activity in whole-cell recordings in the presence of PNU±bicuculline
We have previously reported that asynchronous α7 single-channel activity can be detected in voltage-clamp and current-clamp whole-cell recordings as a result of synergistic action of 1–2 µM PNU-120596 and physiological concentrations of choline (i.e., 5–10 µM) on α7 nicotinic receptors (Gusev and Uteshev, 2010; Kalappa et al., 2010; Uteshev, 2012b). This experimental approach was used in the present study to detect bicuculline-induced bursts of α7 single-channel openings expected to result from interactions of positively charged molecules, like bicuculline, with α7 channels and quantify bicuculline-induced α7 single-channel intraburst events and their voltage-dependence in whole-cell recordings.
3.4.1. Whole-cell single-channel activity is α7 nAChR-mediated
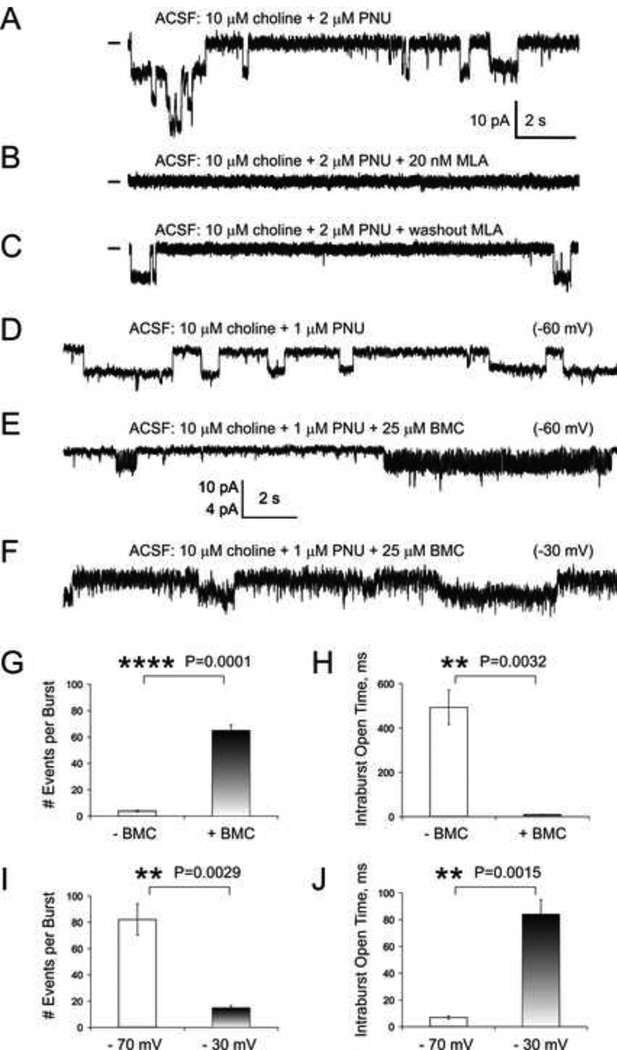
In the presence of 10 µM choline +2 µM PNU-120596 in aCSF, α7 nicotinic receptor-mediated single-channel openings were recorded in hippocampal CA1 interneurons in whole-cell voltage-clamp patch-clamp experiments in acute whole-brain slices (Fig. 4A) as previously described (Kalappa et al., 2010). These openings were completely and reversibly blocked by 20 nM methyllycaconitine (MLA), a selective α7 nicotinic receptor antagonist (n=5, Fig. 4B-C) supporting the involvement of functional α7 nicotinic receptors. The membrane voltage was held at −60 mV.
Figure 4. The effects of 25 µM bicuculline on α7 single-channel responses in the presence of 1 µM PNU-120596.
A-C) Typical examples of current traces obtained in whole-cell α7 single-channel recordings conducted in voltage-clamp patch-clamp experiments using hippocampal CA1 striatum radiatum interneurons in acute coronal brain slices (A). This single-channel activity was completely (B), but reversibly (C) blocked by 20 nM MLA supporting the involvement of α7 nicotinic receptors. In this (D) and previous studies (Gusev and Uteshev, 2010; Kalappa et al., 2010; Uteshev, 2012b), in the presence of PNU-120596, α7 single-channel openings did not exhibit strong bursts in the absence of bicuculline. However, upon an addition of 25 µM bicuculline to aCSF (see text), α7 channels appear to favor a burst-like kinetic modality (compare D and E) and these effects were fully reversible (not shown). Horizontal bars in front of current traces indicate the current baseline. This burst-like kinetic was strongly voltage-dependent and significantly reduced at a depolarized (i.e., −30 mV) membrane potential (F). Results of single-channel analysis applied to very long (>500 ms) clearly isolated (tcrit=150 ms) openings/bursts (see Methods) are shown for various experimental conditions: ±bicuculline (G-H) and hyperpolarized (i.e., −70 mV) vs. depolarized (i.e., −30 mV) membrane potentials (I-J).
3.4.2. Evaluation of bicuculline-induced bursts of α7 single-channel openings in the presence of PNU-120596
In this and previous studies conducted using α7-expressing hypothalamic and hippocampal neurons (Gusev and Uteshev, 2010; Kalappa et al., 2010; Uteshev, 2012b), we have not observed strong consistent bursts of α7 single-channel openings in whole-cell single-channel recordings in the absence of bicuculline (Fig. 4A, 4C and 4D). By contrast, in the presence of bicuculline, α7 channel openings favored a burst-like kinetic modality (Fig. 4E). To evaluate the effects of 25 µM bicuculline on α7 single-channel intraburst activity elicited by 10 µM choline +1 µM PNU-120596 and its voltage-dependence, subsets of long (>500 ms) isolated (tcrit=150 ms) openings/bursts were used (see Methods).
These experiments demonstrated that 25 µM bicuculline significantly (paired, two-tailed, Student’s t-test) decreased the apparent amplitude of α7 single-channel intraburst events, the apparent mean intraburst open time and the apparent total open time per second of opening/burst and significantly (paired, two-tailed, Student’s t-test) increased the number of events per second of opening/burst (Table 1 and Fig. 4). Moreover, 25 µM bicuculline nearly doubled the amount of block time and reduced net charge associated with α7 activity by nearly 3-fold (Table 1) (see Methods). The ~2.6-fold reduction in net charge caused by 25 µM bicuculline in experiments with asynchronous α7 activity (Table 1) is similar to the 2–3-fold decrease in net charge of synchronous α7 responses caused by 25 µM bicuculline (Fig. 1G) suggesting that bicuculline inhibits synchronous and asynchronous α7 activity equally and this inhibition may involve similar mechanisms. These results support the hypothesis that bicuculline inhibits α7-mediated currents via direct inhibitory interactions with α7 channels. An example of histograms of apparent intraburst open times obtained from a typical CA1 hippocampal interneuron before (Supplementary Fig. 1A) and 10 min after (Supplementary Fig. 1B) the addition of 25 µM bicuculline to aCSF is shown in Supplementary Fig. 1. In this and other similar experiments, single-channel data were collected for 10 min before and 10 min after administration of 25 µM bicuculline. Additional 10 min were given in between these recordings for equilibration of bicuculline within the slice (see Methods).
Table 1.
The effects of 25 µM bicuculline on α7 single-channel openings.
| Parameters/Conditions | −bicuculline | +25 µM bicuculline | P-values (n=5) |
|---|---|---|---|
| Apparent event amplitude (pA) | 4.9±0.3 | 3.4±0.2 | 0.0045 |
| # Events per second of opening/burst | 3.9±0.5 | 53.4±4.9 | 0.0001 |
| Apparent mean intraburst open time (ms) | 493.1±76.6 | 12.1±2.5 | 0.0032 |
| Apparent total open time per second of opening/burst (ms) | 964.2±9.4 | 618.0±32.8 | 0.0007 |
| Amount of block time ratio (−bicuculline/+bicuculline) | 1.8±0.2 (n=5) | ||
| Net charge reduction by 25 µM bicuculline | 2.6±0.4 (n=5) |
In the presence of 1 µM PNU-120596 +10 µM choline, bicuculline (25 µM) significantly (paired, two-tailed, Student’s t-test) decreased the apparent amplitude of α7 single-channel events, the apparent mean intraburst open time and the apparent total open time per second of opening/burst and significantly (paired, two-tailed, Student’s t-test) increased the number of events per second of opening/burst. Moreover, 25 µM bicuculline nearly doubled the amount of block time and reduced net charge associated with α7 activity by nearly 3-fold (see Methods). Subsets of long (>500 ms) isolated (tcrit=150 ms) α7 single-channel openings/bursts were used in this analysis.
3.4.3. Voltage-dependence of the effects of bicuculline on α7 single-channel activity in the presence of PNU-120596
To evaluate the voltage-dependence of the effects of 25 µM bicuculline on asynchronous α7 single-channel intraburst activity elicited by 10 µM choline +1 µM PNU-120596, subsets of long (>500 ms) isolated (tcrit=150 ms) openings/bursts were used (see Methods). The effects of bicuculline on the number of events per second of opening/burst, the apparent mean intraburst open time, the apparent total open time per second of opening/burst and the amount of block time were measured and compared at depolarized (i.e., −30 mV) and hyperpolarized (i.e., −70 mV) membrane potentials to confirm the voltage-dependence of bicuculline-induced inhibition observed in experiments with pressure-puffed 1 mM choline (Fig. 2) where the effects of bicuculline on synchronized activity of α7 nicotinic receptor-channels were investigated. The selection of membrane potentials (i.e., −30 mV and −70 mV) was dictated by the results illustrated in Fig. 2 that suggested only a minimal, if any, current inhibition by bicuculline at membrane voltages near −30 mV and a significant inhibition at −70 mV.
These experiments demonstrated that the effects of 25 µM bicuculline on asynchronous α7 activity was strongly voltage-dependent (Table 2 and Fig. 4) as depolarization from −70 mV to −30 mV significantly (paired, two-tailed, Student’s t-test) reduced the number of events per second of opening/burst and significantly (paired, two-tailed, Student’s t-test) increased the apparent mean intraburst open time and the apparent total open time per second of opening/burst. Moreover, depolarization from −70 mV to −30 mV halved the amount of block time (Table 2) (see Methods). These results are consistent with those obtained in experiments where the effects of bicuculline on synchronous α7 activity were studied (Fig. 1–3) and further support the hypothesis that in the presence of PNU-120596, bicuculline enhances the bursting modality of α7 activity in a strongly voltage-dependent manner and thus, the site of bicuculline-elicited inhibition is likely located near or within the α7 channel.
Table 2.
The effects of depolarization on α7 single-channel openings in the presence of 25 µM bicuculline.
| Parameters/Conditions | −70 mV (+25 µM bicuculline) |
−30 mV (+25µM bicuculline) |
P-values (n=5) |
|---|---|---|---|
| # Events per second of opening/burst | 82.2±12.0 | 14.9±1.9 | 0.0029 |
| Apparent mean intraburst open time (ms) | 6.9±1.3 | 84.2±10.7 | 0.0015 |
| Apparent total open time per second of opening/burst (ms) | 492.5±53.4 | 936.5±9.9 | 0.0009 |
| Amount of block time ratio (−30 mV/−70 mV) | 2.0±0.3 (n=5) |
In the presence of 1 µM PNU-120596 +10 µM choline +25µM bicuculline, depolarization from −70 mV to −30 mV in voltage-clamp significantly (paired, two-tailed, Student’s t-test) decreased the number of events per second of opening/burst, but significantly (paired, two-tailed, Student’s t-test) increased the apparent mean intraburst open time and the apparent total open time per second of opening/burst. Moreover, depolarization from −70 mV to −30 mV halved the amount of block time (see Methods). Subsets of long (>500 ms) isolated (tcrit=150 ms) α7 single-channel openings/bursts were used in this analysis.
4. DISCUSSION
The key finding of this study is the existence of a previously unanticipated inhibitory component in the effects of PNU-120596 on α7 nicotinic receptor-channels. PNU-120596 is a potent inhibitor of α7 desensitization and enhancer of α7 activation (Gronlien et al., 2007; Gusev and Uteshev, 2010; Hurst et al., 2005; Kalappa et al., 2010; Young et al., 2008). However, the results of this study demonstrate that in addition to enhancing α7 channel activity, PNU-120596 also enhances voltage-dependent inhibition of α7 channels by positively charged compounds, bicuculline and choline. PNU-120596 robustly prolongs openings of α7 channels from ~100 µs (Mike et al., 2000) to >1 s (Gusev and Uteshev, 2010). In this study, we propose that this increase in Popen by PNU-120596 makes α7 channels more accessible to positively charged molecules and thus, more susceptible to open-channel-block-like voltage-dependent inhibitory interactions with these molecules. This unanticipated enhancement of α7 response inhibition in the presence of a drug designed to potentiate α7-mediated responses may compromise this very potentiation and may provide new insights into the mechanisms of PNU-120596 action and α7 channel-drug interactions. Therefore, the pharmacology of α7 ion channels in the presence and absence of PNU-120596 appears to be different: drugs and concentrations not known to potently interact with α7 channels in the absence of PNU-120596 may interact with these channels in the presence of PNU-120596.
The observation that in the presence of PNU+bicuculline, α7 ion channels favor voltage-dependent burst-like kinetics (Fig. 4D-L) suggests that the site of PNU+bicuculline action is near or within the α7 channel. Additional support for this hypothesis arises from the strong voltage-dependence of PNU+bicuculline-induced inhibition of both synchronous and asynchronous α7 responses at negative (Fig. 2) or hyperpolarized (i.e., −70 mV; Fig. 4J-L) membrane potentials and the lack of such inhibition at positive (Fig. 3) or depolarized (i.e., −30 mV; Fig. 4J-L) membrane potentials. However, alternative hypotheses are possible. For example, PNU-120596 may create or reveal an allosteric binding site with affinity for bicuculline and this modification of the α7 nicotinic receptor-channel structure by PNU-120596 can be voltage-sensitive. In that event, the observed voltage-dependence of the effects of PNU+bicuculline would reflect voltage-dependence of the bicuculline access to the inhibitory allosteric site which may not necessarily locate in the channel pore. Furthermore, bicuculline may augment α7 channel block by choline in the presence of PNU-120596. However, PNU-120596 also enhances voltage-dependent inhibition of α7 channels by choline alone, i.e., without bicuculline (Fig. 2E), suggesting that it is PNU-120596 and not bicuculline that enhances α7 channel block by choline. This however, does not exclude a possibility that bicuculline provides an additional enhancement to α7 channel block by choline. However, given that both bicuculline and choline are positively charged and highly ionized molecules, the fact that PNU-120596 enhances α7 channel block by choline creates a rational basis to expect that PNU-120596 also enhances α7 channel block by bicuculline. In addition to increasing the potency of nicotinic agonists for activation of α7 nicotinic receptors, PNU-120596 may also increase the potency of competitive antagonists, such as bicuculline. In that case, a certain component of the observed inhibition of α7-mediated currents by bicuculline in the presence of PNU-120596 may not be related to interactions of bicuculline with the α7 channel. However, the fact that PNU-120596-induced inhibition is strongly voltage-dependent (Fig. 2–4) points to the α7 ion channel as being the primary site of interactions between α7 nicotinic receptor/channel complex and charged molecules because interactions of charged molecules with binding sites located outside of the channel (e.g., orthosteric sites) would be expected to be voltage-insensitive. Moreover, PNU-120596 enhances voltage-dependent inhibition of α7 channels by choline alone, i.e., a selective α7 nicotinic receptor agonist (Fig. 2E) further supporting the hypothesis of interactions between charged molecules and the α7 ion channel in the presence of PNU-120596.
In the continuous presence of nicotinic agonists, α7-mediated responses are reduced naturally by two independent processes: α7 receptor desensitization and α7 channel block (Uteshev, 2012a). This study demonstrates that these processes are differentially affected by PNU-120596: PNU-120596 reduces α7 desensitization, as reported previously (Hurst et al., 2005) and enhances voltage-dependent inhibition of α7 channels by bicuculline and choline (Fig. 2–4), positively charged compounds that do not potently block α7 channels in the absence of PNU-120596 (Demuro et al., 2001; Uteshev et al., 2002). Since PNU-120596 reduces α7 desensitization (Hurst et al., 2005), but may not completely eliminate it (Williams et al., 2011), the results of this study caution that in the presence of PNU-120596, the task of separation of the putative PNU-independent component of α7 desensitization from the PNU-enhanced open-channel-block-like voltage-dependent inhibition of α7 channels by positively charged molecules may be quite challenging, especially if these effects are investigated at hyperpolarized membrane voltages (e.g., <−50 mV, Fig. 2) in the presence of high concentrations of PNU-120596 (i.e., >1 µM) and a strong α7 receptor stimulation (e.g., >100 µM acetylcholine, concentrations analogous to >1 mM choline in terms of relative potencies for α7 nicotinic receptor activation (Alkondon et al., 1999)). One could speculate that in experiments utilizing conditions promoting α7 channel block (i.e., strong α7 receptor stimulation), recordings at positive (e.g., +60 mV; Fig. 3) and/or depolarized (e.g., −30 mV; Fig. 4E) membrane potentials could be quite valuable (see also (Uteshev et al., 2002)) because these experimental conditions may facilitate separation of α7 channel block from other possible sources of α7 nicotinic receptor inhibition, such as putative PNU-independent components of α7 desensitization (Williams et al., 2011). Indeed, in our experiments, PNU+bicuculline-induced block of α7 responses was significantly reduced at positive (+60 mV; Fig. 3) or depolarized (−30 mV; Fig. 4F and 4J-L) membrane potentials further supporting direct inhibitory interactions between bicuculline and α7 channels in the presence of PNU-120596.
In this study, α7 nicotinic receptor channels did not exhibit strong bursts in the absence of bicuculline (Fig. 4D). These observations were in conflict with those reported by Williams et al., 2011. This discrepancy may be explained by differences in the expression systems (i.e., native expression in acute slices in this study vs. heterologous expression in Xenopus oocytes in Williams et al., 2011) and/or drug concentrations (i.e., 10 µM choline +1 µM PNU-120596 in this study vs. 100–300 µM acetylcholine +10 µM PNU-120596 in Williams et al., 2011) used in these two studies.
All experiments in this study were conducted at room temperature (~23° C). Higher, more physiological temperatures have been demonstrated to inhibit α7-mediated responses in the presence of PNU-120596 (Sitzia et al., 2011). The effects of more physiological temperatures on α7 single ion channel kinetics in the presence of PNU-120596 have not yet been reported. At higher temperatures, the kinetics of α7 single-channel responses may retain some of the important properties described in this study and are currently under investigation in this laboratory.
In conclusion, at the time of this study, PNU-120596 was the only Type-II positive allosteric modulator of α7 nicotinic receptors available on the market. It is therefore of interest to determine whether other members of Type-II positive allosteric modulator family facilitate similar voltage-dependent interactions between α7 nicotinic receptor-mediated ion channels and charged compounds including those (i.e., choline and bicuculline) tested in this study. It is equally interesting to determine the list of positively charged compounds that initiate voltage-dependent inhibition of α7 channels in the presence of PNU-120596 and possibly, other Type-II positive allosteric modulators. This list may include endogenous compounds at effective concentrations that cannot be readily predicted because these compounds may not exhibit significant affinity for α7 channels in the absence of PNU-120596. This previously unexpected dual action of PNU-120596, and likely other Type-II positive allosteric modulators of α7 nicotinic receptors, needs to be acknowledged and further tested because it imitates α7 desensitization and may lead to unanticipated α7 channel-drug interactions and misinterpretation of α7 single-channel data.
Supplementary Material
Acknowledgements
This work was supported by the NIH grant DK082625 to VU. We thank the NIH NIDA Research Resources Drug Supply Program for PNU-120596; Dr. Nathalie Sumien for advice on statistical analysis and Dr. Eric Gonzales for discussion of mechanisms of open channel block.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur.J.Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1-interneurons in rat hippocampal slices. J.Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuro A, Palma E, Eusebi F, Miledi R. Inhibition of nicotinic acetylcholine receptors by bicuculline. Neuropharmacology. 2001;41:854–861. doi: 10.1016/s0028-3908(01)00137-x. [DOI] [PubMed] [Google Scholar]
- Gronlien JH, Hakerud M, Ween H, Thorin Hagene K, Briggs CA, Gopalakrishnan M, Malysz J. Distinct profiles of alpha7 nicotinic receptor positive allosteric modulation revealed by structurally diverse chemotypes. Mol.Pharmacol. 2007;72:715–724. doi: 10.1124/mol.107.035410. [DOI] [PubMed] [Google Scholar]
- Gusev AG, Uteshev VV. Physiological concentrations of choline activate native alpha7-containing nicotinic acetylcholine receptors in the presence of PNU-120596-[1-(5-chloro-2,4-dimethoxyphenyl)-3-(5-methylisoxazol-3-yl)-urea] J. Pharmacol. Exp. Ther. 2010;332:588–598. doi: 10.1124/jpet.109.162099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, Groppi VE, Allaman G, Ogier R, Bertrand S, Bertrand D, Arneric SP. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J.Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalappa BI, Gusev AG, Uteshev VV. Activation of functional alpha7-containing nicotinic receptors in hippocampal CA1 pyramidal neurons by physiological levels of choline in the presence of PNU 120596. PloS one. 2010;5:e13964. doi: 10.1371/journal.pone.0013964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mike A, Castro NG, Albuquerque EX. Choline and acetylcholine have similar kinetic properties of activation and desensitization on the alpha7 nicotinic receptors in rat hippocampal neurons. Brain Res. 2000;882:155–168. doi: 10.1016/s0006-8993(00)02863-8. [DOI] [PubMed] [Google Scholar]
- Papke RL, Papke JKP. Comparative pharmacology of rat and human alpha7 nicotinic receptor conducted with net charge analysis. Br J of Pharmacol. 2002;137:49–61. doi: 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin DD. Dissociation constants of organic bases in aqueous solution. Butterworths, London: 1972. [Google Scholar]
- Seutin V, Scuvee-Moreau J, Dresse A. Evidence for a non-GABAergic action of quaternary salts of bicuculline on dopaminergic neurones. Neuropharmacology. 1997;36:1653–1657. doi: 10.1016/s0028-3908(97)00147-0. [DOI] [PubMed] [Google Scholar]
- Sitzia F, Brown JT, Randall AD, Dunlop J. Voltage- and Temperature-Dependent Allosteric Modulation of alpha7 Nicotinic Receptors by PNU-120596. Front Pharmacol. 2011;2:81. doi: 10.3389/fphar.2011.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev VV. Evaluation of Ca2+ permeability of nicotinic acetylcholine receptors in hypothalamic histaminergic neurons. Acta Biochim Biophys Sin (Shanghai) 2010;42:8–20. doi: 10.1093/abbs/gmp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev VV. alpha7 Nicotinic ACh Receptors as a Ligand Gated Source of Ca(2+) Ions: The Search for a Ca (2+) Optimum. Adv. Exp. Med. Biol. 2012a;740:603–638. doi: 10.1007/978-94-007-2888-2_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev VV. Somatic Integration of Single Ion Channel Responses of alpha7 Nicotinic Acetylcholine Receptors Enhanced by PNU-120596. PloS one. 2012b;7:e32951. doi: 10.1371/journal.pone.0032951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, Papke RL. Activation and inhibition of native neuronal alpha-bungarotoxin-sensitive nicotinic ACh receptors. Brain Res. 2002;948:33–46. doi: 10.1016/s0006-8993(02)02946-3. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Stevens DR, Haas HL. alpha-Bungarotoxin-sensitive nicotinic responses in rat tuberomammillary neurons. Pflugers Archiv-European Journal of Physiology. 1996;432:607–613. doi: 10.1007/s004240050176. [DOI] [PubMed] [Google Scholar]
- Williams DK, Wang J, Papke RL. Investigation of the molecular mechanism of the alpha7-nicotinic acetylcholine receptor positive allosteric modulator PNU 120596 provides evidence for two distinct desensitized states. Mol. Pharmacol. 2011;80:1013–1032. doi: 10.1124/mol.111.074302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GT, Zwart R, Walker AS, Sher E, Millar NS. Potentiation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14686–14691. doi: 10.1073/pnas.0804372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.