Abstract
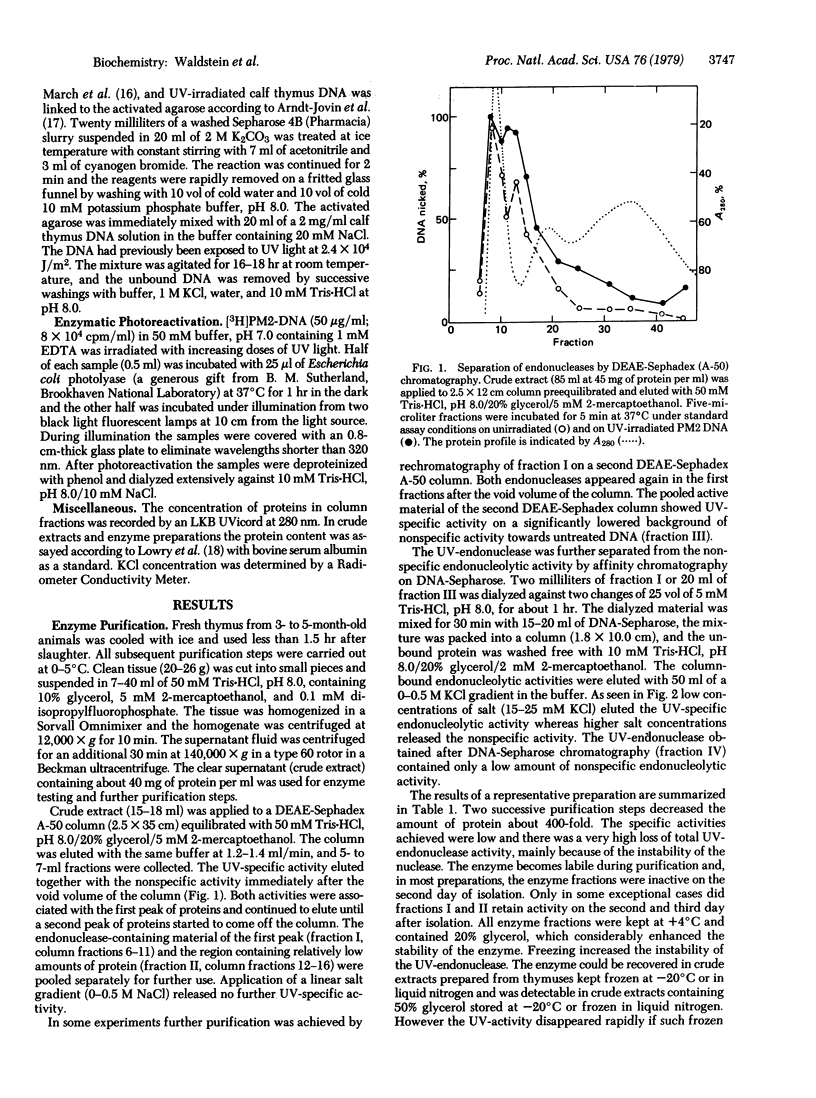
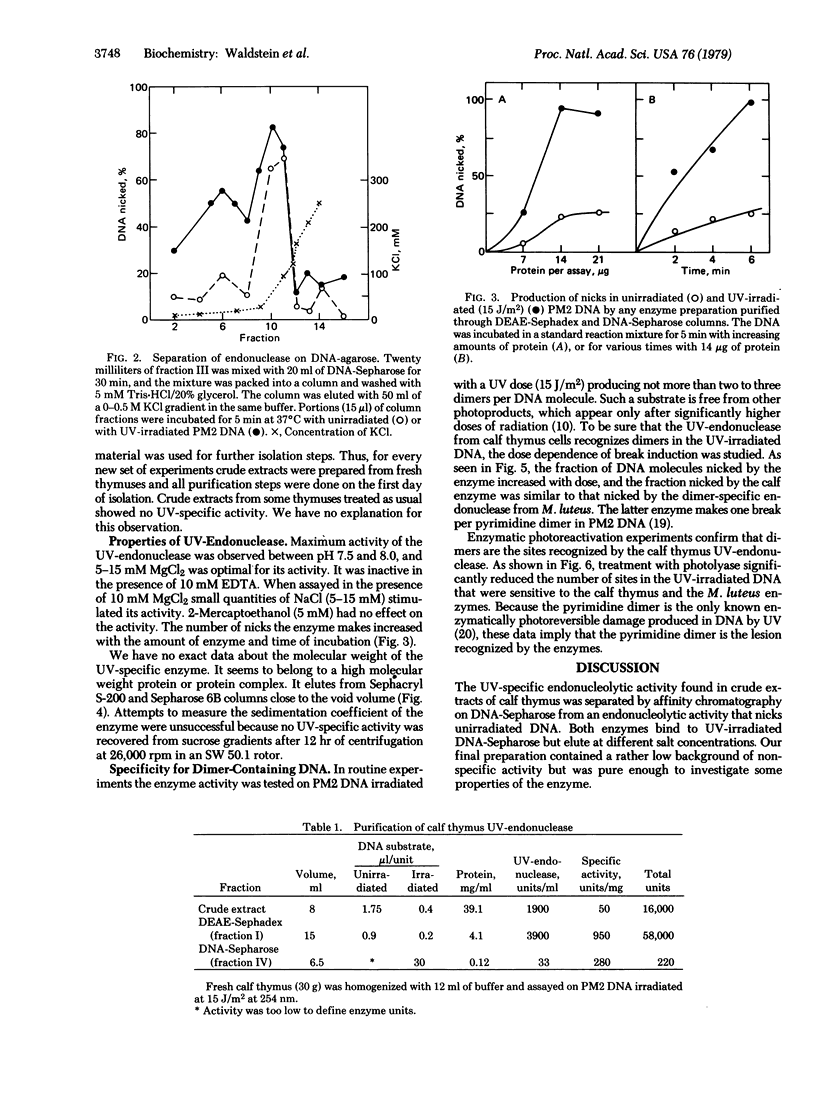
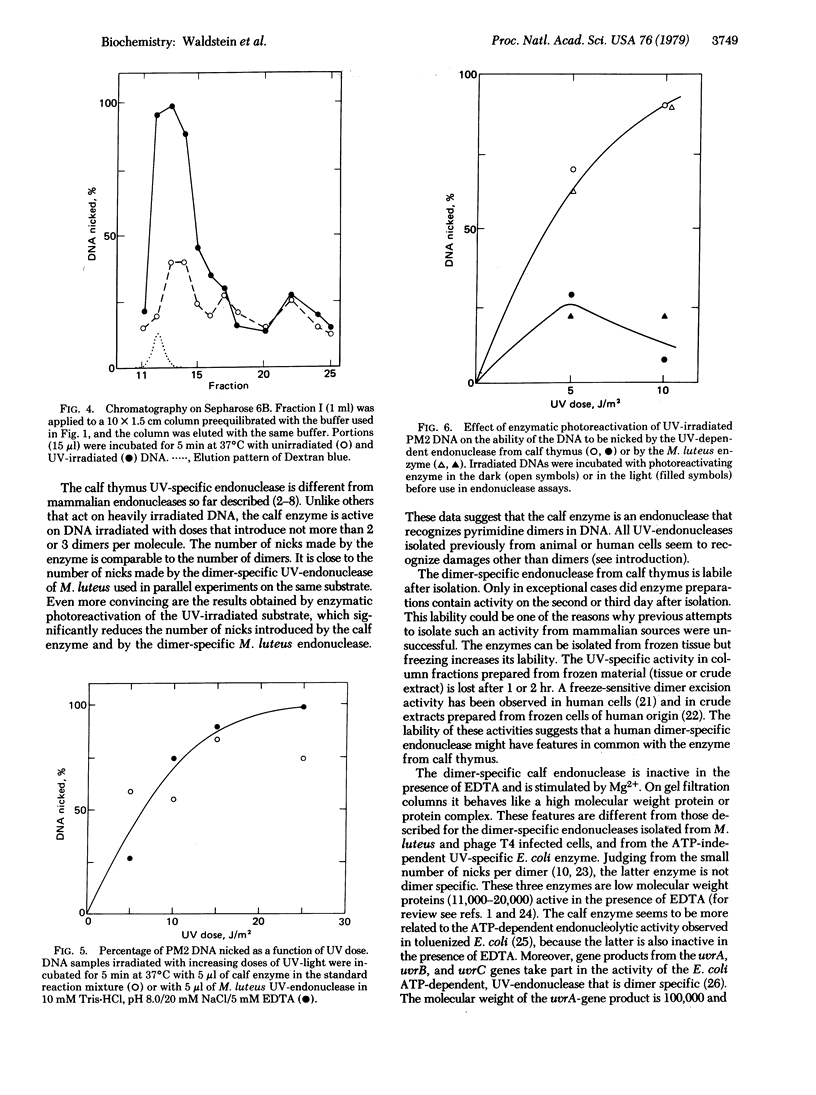
We describe the partial purification of an endonuclease from calf thymus that nicks phage PM2 DNA irradiated with UV doses producing only a few pyrimidine dimers per molecule. It has much less activity on DNA that has been subjected to enzymatic photoreactivation after UV irradiation. The calf thymus endonuclease is different from other mammalian UV-endonucleases so far described in that it seems to be dimer specific. The enzyme is stimulated by Mg2+ and is inactive in the presence of EDTA. It binds to UV-irradiated DNA-Sepharose from which it is released by low concentrations of KCl. Gel filtration data indicate that the endonuclease may belong to a high molecular weight protein or protein complex. The enzyme is very labile and freezing increases its lability.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Jovin T. M., Bähr W., Frischauf A. M., Marquardt M. Covalent attachment of DNA to agarose. Improved synthesis and use in affinity chromatography. Eur J Biochem. 1975 Jun;54(2):411–418. doi: 10.1111/j.1432-1033.1975.tb04151.x. [DOI] [PubMed] [Google Scholar]
- Bacchetti S., Benne R. Purification and characterization of an endonuclease from calf thymus acting on irradiated DNA. Biochim Biophys Acta. 1975 May 16;390(3):285–297. doi: 10.1016/0005-2787(75)90349-4. [DOI] [PubMed] [Google Scholar]
- Bacchetti S., van der Plas A., Veldhuisen G. A UV-specific endonucleolytic activity present in human cell extracts. Biochem Biophys Res Commun. 1972 Aug 7;48(3):662–669. doi: 10.1016/0006-291x(72)90399-3. [DOI] [PubMed] [Google Scholar]
- Braun A. G., Radman M., Grossman L. Enzymatic repair of DNA: SITES OF HYDROLYSIS BY THE Escherichia coli endonuclease specific for pyrimidine dimers (correndonuclease II). Biochemistry. 1976 Sep 7;15(18):4116–4120. doi: 10.1021/bi00663a031. [DOI] [PubMed] [Google Scholar]
- Brent T. P. Characterization of human enzymes specific for damaged DNA: resolution of endonuclease for irradiated DNA from an apparent N-glycosidase active on alkylated DNA. Nucleic Acids Res. 1977 Jul;4(7):2445–2454. doi: 10.1093/nar/4.7.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent T. P. Partial purification of endonuclease activity from human lymphoblasts. Separation of activities for depurinated DNA and DNA irradiated with ultraviolet light. Biochim Biophys Acta. 1975 Oct 1;407(2):191–199. doi: 10.1016/0005-2787(75)90284-1. [DOI] [PubMed] [Google Scholar]
- Brent T. P. Purification and characterization of human endonucleases specific for damaged DNA. Analysis of lesions induced by ultraviolet or x-radiation. Biochim Biophys Acta. 1976 Nov 12;454(1):172–183. doi: 10.1016/0005-2787(76)90363-4. [DOI] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Endonuclease from Micrococcus luteus which has activity toward ultraviolet-irradiated deoxyribonucleic acid: purification and properties. J Bacteriol. 1970 Apr;102(1):178–186. doi: 10.1128/jb.102.1.178-186.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center M. S., Richardson C. C. An endonuclease induced after infection of Escherichia coli with bacteriophage T7. I. Purification and properties of the enzyme. J Biol Chem. 1970 Dec 10;245(23):6285–6291. [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H., Trosko J. E., Lett J. T. Excision repair (dimer excision, strand breakage and repair replication) in primary cultures of eukaryotic (bovine) cells. Exp Cell Res. 1972 Sep;74(1):67–80. doi: 10.1016/0014-4827(72)90482-x. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Gates F. T., Linn S. Endonuclease from Escherichia coli that acts specifically upon duplex DNA damaged by ultraviolet light, osmium tetroxide, acid, or x-rays. J Biol Chem. 1977 May 10;252(9):2802–2807. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Masamune Y., Fleischman R. A., Richardson C. C. Enzymatic removal and replacement of nucleotides at single strand breaks in deoxyribonucleic acid. J Biol Chem. 1971 Apr 25;246(8):2680–2691. [PubMed] [Google Scholar]
- Nes I. F., Nissen-Meyer J. Endonuclease activities from a permanently established mouse cell line that act upon DNA damaged by ultraviolet light, acid and osmium tetroxide. Biochim Biophys Acta. 1978 Aug 23;520(1):111–121. doi: 10.1016/0005-2787(78)90012-6. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Nissen-Meyer J., Strike P. Incision of ultraviolet-irradiated DNA by extracts of E. coli requires three different gene products. Nature. 1976 Oct 7;263(5577):524–526. doi: 10.1038/263524a0. [DOI] [PubMed] [Google Scholar]
- Seeberg E. Reconstitution of an Escherichia coli repair endonuclease activity from the separated uvrA+ and uvrB+/uvrC+ gene products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2569–2573. doi: 10.1073/pnas.75.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slor H., Lev-Sobe T., Friedberg E. C. Evidence for inactivation of DNA repair in frozen and thawed mammalian cells. Mutat Res. 1977 Oct;45(1):137–145. doi: 10.1016/0027-5107(77)90051-3. [DOI] [PubMed] [Google Scholar]
- Teebor G. W., Duker N. J., Becker F. F. Normal endonuclease activities for damaged DNA during hepatocarcinogenesis. Biochim Biophys Acta. 1977 Jul 15;477(2):125–131. doi: 10.1016/0005-2787(77)90228-3. [DOI] [PubMed] [Google Scholar]
- Waldstein E. A., Sharon R., Ben-Ishai R. Role of ATP in excision repair of ultraviolet radiation damage in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2651–2654. doi: 10.1073/pnas.71.7.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lancker J. L., Tomura T. Purification and some properties of a mammalian repair endonuclease. Biochim Biophys Acta. 1974 Jun 14;353(1):99–114. doi: 10.1016/0005-2787(74)90101-4. [DOI] [PubMed] [Google Scholar]


