Abstract
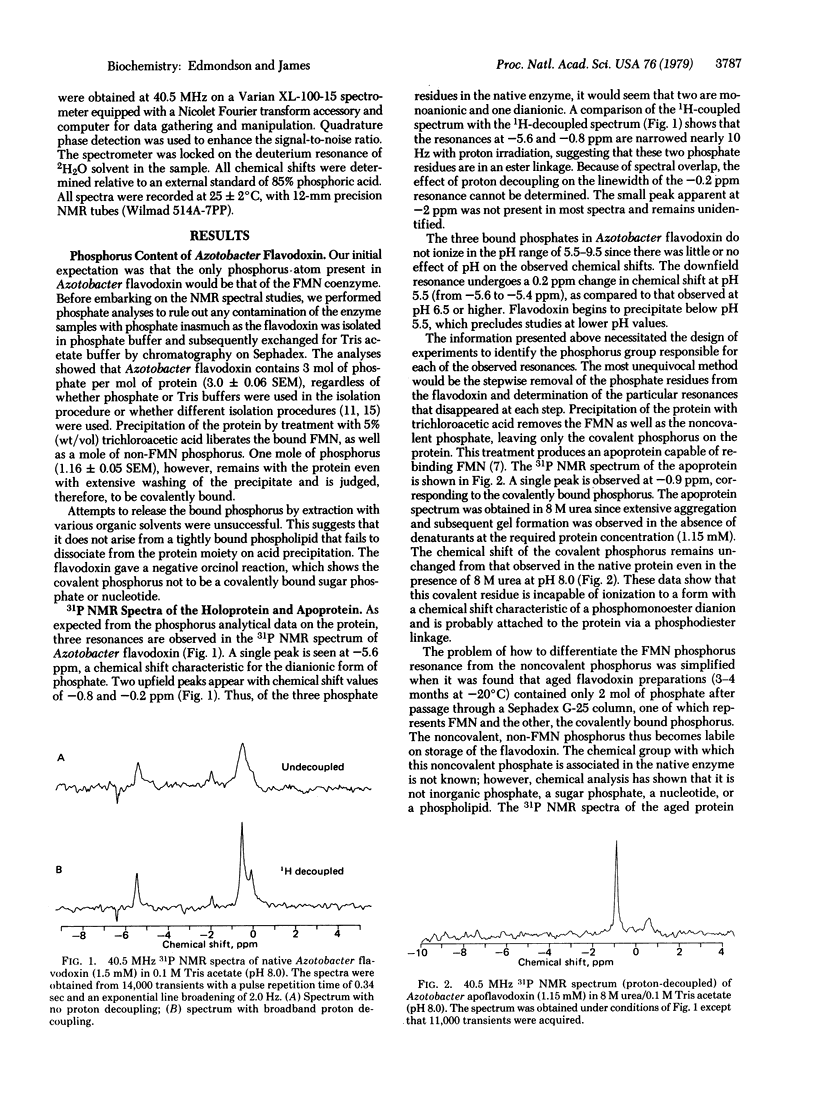
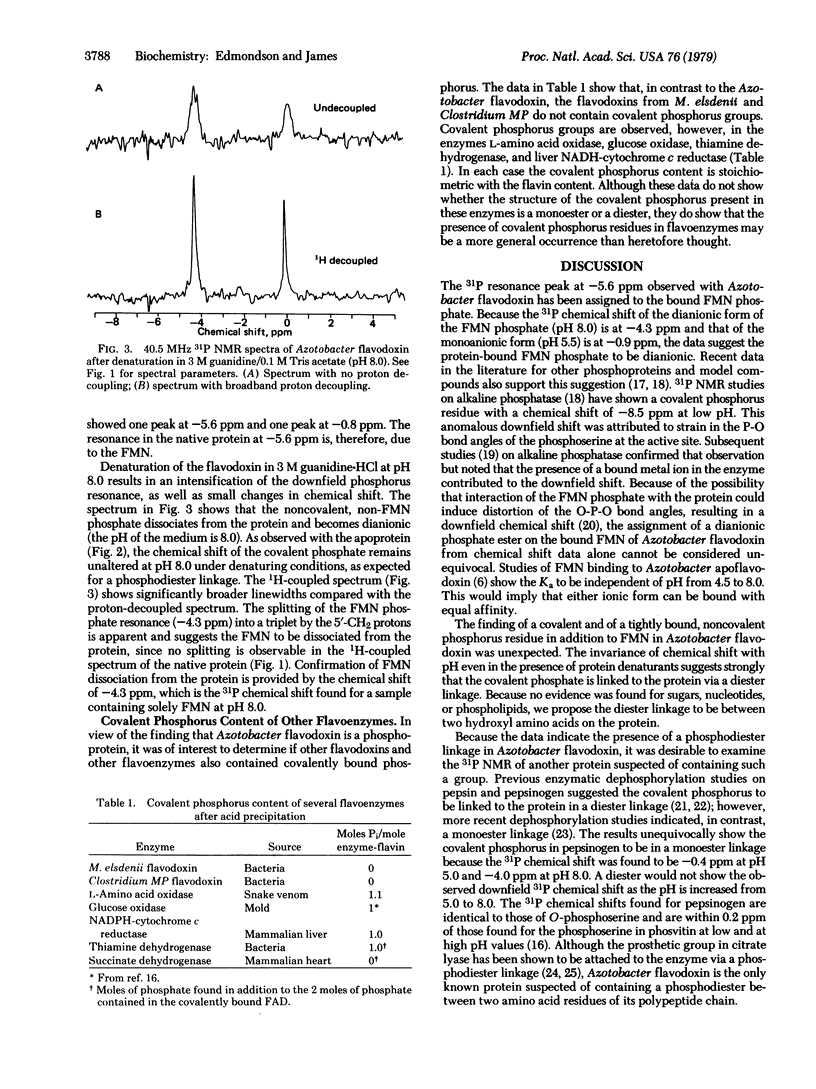
In addition to the 5′-phosphate ester on its flavin mononucleotide (FMN) moiety, flavodoxin from Azotobacter vinelandii contains 2 moles of tightly bound phosphate. One non-coenzyme phosphate group is covalently bound to the protein, as it remains with the protein on acid precipitation, whereas the other phosphate is released. The invariance of the 31P nuclear magnetic resonance chemical shift of the covalently bound phosphate (-0.8 ppm relative to 85% phosphoric acid) with pH, even in the presence of protein denaturants, implies it is in a diester linkage to the protein. Because no evidence could be found for the presence of covalently bound sugars, nucleotides, or phospholipids, it is suggested that the phosphate residue forms a diester linkage with two hydroxyl amino acids in the protein. The only other suggestion of a phosphodiester linkage in proteins is from previous studies on pepsin and pepsinogen [Perlmann, G. E. (1955) Adv. Prot. Chem. 10, 1-30]. The observed changes in 31P chemical shift with pH show that the covalent phosphorus in pepsinogen has ionization properties of a monoester rather than a diester. The 31P resonance of the FMN phosphate occurs at -5.6 ppm in native Azotobacter flavodoxin. No ionization of the protein-bound FMN phosphate is observed since the chemical shift does not change appreciably in the pH range of 5.5-9.5. The chemical shift data suggest, but do not prove, that the coenzyme phosphate in its protein-bound form is dianionic. Chemical analysis of several other flavoenzymes from a variety of sources shows the presence of covalently bound phosphorus in quantities stoichiometric with the flavin content in most of the enzymes tested. Thus, the presence of covalent phosphorus in flavoenzymes may be a general phenomenon with currently unknown catalytic significance.
Keywords: phosphodiester linkage, covalent phosphorus in flavoproteins, pepsinogen, flavin mononucleotide phosphate
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Barman B. G., Tollin G. Flavine-protein interactions in flavoenzymes. Temperature-jump and stopped-flow studies of flavine analog binding to the apoprotein of Azotobacter flavodoxin. Biochemistry. 1972 Dec 5;11(25):4746–4754. doi: 10.1021/bi00775a018. [DOI] [PubMed] [Google Scholar]
- Barman B. G., Tollin G. Flavine-protein interactions in flavoenzymes. Thermodynamics and kinetics of reduction of Azotobacter flavodoxin. Biochemistry. 1972 Dec 5;11(25):4755–4759. doi: 10.1021/bi00775a019. [DOI] [PubMed] [Google Scholar]
- Benemann J. R., Yoch D. C., Valentine R. C., Arnon D. I. The electron transport system in nitrogen fixation by Azotobacter. I. Azotoflavin as an electron carrier. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1079–1086. doi: 10.1073/pnas.64.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J. L., Sheard B. 31P NMR of alkaline phosphatase. Biochem Biophys Res Commun. 1975 Sep 2;66(1):24–30. doi: 10.1016/s0006-291x(75)80289-0. [DOI] [PubMed] [Google Scholar]
- Burnett R. M., Darling G. D., Kendall D. S., LeQuesne M. E., Mayhew S. G., Smith W. W., Ludwig M. L. The structure of the oxidized form of clostridial flavodoxin at 1.9-A resolution. J Biol Chem. 1974 Jul 25;249(14):4383–4392. [PubMed] [Google Scholar]
- Chlebowski J. F., Armitage I. M., Tusa P. P., Coleman J. E. 31P NMR of phosphate and phosphonate complexes of metalloalkaline phosphatases. J Biol Chem. 1976 Feb 25;251(4):1207–1216. [PubMed] [Google Scholar]
- Clement G. E., Rooney J., Zakheim D., Eastman J. The pH dependence of the dephosphorylated pepsin-catalyzed hydrolysis of N-acetyl-L-phenylalanyl-L-tyrosine methyl ester. J Am Chem Soc. 1970 Jan 14;92(1):186–189. doi: 10.1021/ja00704a031. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Jr, Tollin G. Studies of flavin-protein interaction in flavoproteins using protein fluorescence and circular dichroism. Biochemistry. 1972 Mar 14;11(6):1073–1080. doi: 10.1021/bi00756a020. [DOI] [PubMed] [Google Scholar]
- Edmondson D. E., Tollin G. Chemical and physical characterization of the Shethna flavoprotein and apoprotein and kinetics and thermodynamics of flavin analog binding to the apoprotein. Biochemistry. 1971 Jan 5;10(1):124–132. doi: 10.1021/bi00777a019. [DOI] [PubMed] [Google Scholar]
- Edmondson D. E., Tollin G. Flavin-protein interactions and the redox properties of the Shethna flavoprotein. Biochemistry. 1971 Jan 5;10(1):133–145. doi: 10.1021/bi00777a020. [DOI] [PubMed] [Google Scholar]
- Hinkson J. W., Bulen W. A. A free radical flavoprotein from Azotobacter. Isolation, crystallization, and properties. J Biol Chem. 1967 Jul 25;242(14):3345–3351. [PubMed] [Google Scholar]
- Ho C., Magnuson J. A., Wilson J. B., Magnuson N. S., Kurland R. J. Phosphorus nuclear magnetic resonance studies of phosphoproteins and phosphorylated molecules. II. Chemical nature of phosphorus atoms in alpha S-casein B and phosvitin. Biochemistry. 1969 May;8(5):2074–2082. doi: 10.1021/bi00833a044. [DOI] [PubMed] [Google Scholar]
- MacKnight M. L., Gillard J. M., Tollin G. Flavine-protein interactions in flavoenzymes. pH dependence of the binding of flavine mononucleotide and riboflavine to Azotobacter flavodoxin. Biochemistry. 1973 Oct 9;12(21):4200–4206. doi: 10.1021/bi00745a025. [DOI] [PubMed] [Google Scholar]
- Mayhew S. G., Howell L. G. Chromatography of proteins on diethylaminoethyl-cellulose in concentrated ammonium sulfate. Anal Biochem. 1971 Jun;41(2):466–470. doi: 10.1016/0003-2697(71)90166-7. [DOI] [PubMed] [Google Scholar]
- Oppenheimer N. J., Singh M., Sweeley C. C., Sung S. J., Srere P. A. The configuration and location of the ribosidic linkage in the prosthetic group of citrate lyase (Klebsiella aerogenes). J Biol Chem. 1979 Feb 25;254(4):1000–1002. [PubMed] [Google Scholar]
- PERLMANN G. E. Enzymic dephosphorylation of pepsin and pepsinogen. J Gen Physiol. 1958 Jan 20;41(3):441–450. doi: 10.1085/jgp.41.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERLMANN G. E. The nature of phosphorus linkages in phosphoproteins. Adv Protein Chem. 1955;10:1–30. doi: 10.1016/s0065-3233(08)60103-5. [DOI] [PubMed] [Google Scholar]
- Robinson J. B., Jr, Singh M., Srere P. A. Structure of the prosthetic group of Klebsiella aerogenes citrate (pro-3S)-lyase. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1872–1876. doi: 10.1073/pnas.73.6.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watenpaugh K. D., Sieker L. C., Jensen L. H., Legall J., Dubourdieu M. Structure of the oxidized form of a flavodoxin at 2.5-Angstrom resolution: resolution of the phase ambiguity by anomalous scattering. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3185–3188. doi: 10.1073/pnas.69.11.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates M. G. Electron transport to nitrogenase in Azotobacter chroococcum: Azotobacter flavodoxin hydroquinone as an electron donor. FEBS Lett. 1972 Oct 15;27(1):63–67. doi: 10.1016/0014-5793(72)80410-1. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Arnon D. I. Two biologically active ferredoxins from the aerobic nitrogen-fixing bacteriu, Azotobacter vinelandii. J Biol Chem. 1972 Jul 25;247(14):4514–4520. [PubMed] [Google Scholar]
- Yoch D. C. Dimerization of Azotobacter vinelandii flavodoxin (azotoflavin). Arch Biochem Biophys. 1975 Sep;170(1):326–333. doi: 10.1016/0003-9861(75)90124-1. [DOI] [PubMed] [Google Scholar]


