Abstract
BACKGROUND
Mutations in the v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) are present in approximately 30% to 40% of colorectal adenocarcinomas. Wild-type (WT) KRAS mutation status is predictive of tumor response with epidermal growth factor receptor-directed therapies, but the results from studies evaluating the prognostic value of KRAS status in localized disease have been contradictory. The prognostic value of KRAS in metastatic disease, specifically according to whether patients have synchronous or metachronous disease at presentation, is less understood.
METHODS
One-hundred ten consecutive patients with metastatic colorectal adenocarcinoma underwent testing for KRAS exon 2 mutations by polymerase chain reaction amplification and direct nucleotide sequencing. The clinical characteristics, treatments, and outcomes of these patients were then analyzed retrospectively, stratified according to whether patients presented with synchronous or metachronous metastasis and according to KRAS mutation status (WT or mutated).
RESULTS
For the entire cohort, the median overall survival from the date of diagnosis of metastatic disease was 34.3 months (95% confidence interval, 28.3–49.4 months) for patients with WT KRAS (n = 70). The median overall survival for patients with mutated KRAS (n = 40) was 40.3 months (95% confidence interval, 27.9–51.1 months; log-rank P = .91). Kaplan-Meier survival analysis indicated that 3-year overall survival and 5-year overall survival were not statistically different. Within the subgroups of patients with synchronous and metachronous metastatic disease, no significant differences were observed in median overall survival, 3-year overall survival, or 5-year overall survival between the WT KRAS and mutated KRAS groups.
CONCLUSIONS
In this study, KRAS mutation status did not influence overall survival in either synchronous or metachronous metastatic colorectal adenocarcinoma and, as such, had no prognostic role in this disease setting.
Keywords: metastatic colorectal adenocarcinoma, KRAS, mutation, prognosis
INTRODUCTION
The development of colorectal adenocarcinoma is a multistep process characterized by an accumulation of genetic alterations.1 The v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) belongs to a family of oncogenes that includes the v-Ha-ras Harvey rat sarcoma viral oncogene homolog (HRAS) and the neuroblastoma RAS viral oncogene homolog (NRAS). When mutated, these oncogenes have the ability to promote the malignant transformation of normal cells. Mutated KRAS is the most frequently encountered oncogene of the RAS family in colon cancer and is present in 30% to 40% of colorectal adenocarcinomas.2,3 KRAS is a small G-protein with guanine diphosphate (GDP)-binding and guanine triphosphate (GTP)-binding abilities, with GDP binding in its inactive form and GTP binding when activated. In normal function, KRAS is activated transiently in response to extracellular signals, such as growth factors, cytokines, and hormones. Mutations in KRAS usually are point mutations on codons 12 and 13 (exon 2), codon 61 (exon 3),4 and codon 146 (exon 4).5 Mutations result in loss of its GTPase activity, leading to activation and unregulated proliferation of its downstream effects through signal-transduction pathways, including the Raf/mitogen-activated protein kinase/extracellular signal-regulated kinase (Raf/MEK/ERK) pathway and the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathway, thereby promoting cell growth and survival in the absence of external signals.
Epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor involved in the transmission of extracellular signals to KRAS. Ligands bind the extracellular domain with resultant autophosphorylation of the intracellular domain and downstream activation of KRAS. Inhibition of EGFR has become an important pharmacologic target in oncology. Two anti-EGFR monoclonal antibody therapies, panitumumab and cetuximab, are approved for use in metastatic colorectal cancers. Although initial studies of these agents demonstrated modest activity in unselected patients,6,7 it has become clear that KRAS mutation status is predictive of tumor response.8,9 Wild-type (WT) KRAS status is predictive of tumor response to the EGFR-directed antibodies and, conversely, mutated KRAS is negatively predictive of response. The American Society of Clinical Oncology recently released a provisional clinical opinion that patients with metastatic colorectal who harbor KRAS mutations should not receive EGFR-directed therapies.10
The presence of a KRAS mutation also may be prognostic, although studies have produced conflicting results.11–23 Given the limited and conflicting data available on the outcome of metastatic colorectal cancer on the basis of KRAS mutation status, in the current study, we retrospectively analyzed the outcomes of patients with colorectal cancer in the metastatic setting. This was performed irrespective of whether EGFR-directed therapies were received, and we evaluated for the first time patients in both synchronous (metastatic at the time of diagnosis) and metachronous (metastases developing after initial diagnosis of localized disease) metastatic settings. The prognostic implication of the time to disease recurrence remains unclear in medically treated patients; modern chemotherapy trials have not addressed this issue and often exclude patients who develop recurrent disease within 6 to 12 months after completing adjuvant therapy. However, when considering metastatectomy, metachronous presentation of disease is associated with improved survival.24
MATERIALS AND METHODS
Study Design
Patients who had KRAS mutation testing for colorectal adenocarcinoma from a single tertiary care institution (The Ohio State University Medical Center) were included in this study. Only patients with documented metastatic disease were included. Synchronous was defined as metastatic disease at the time of the original colorectal cancer diagnosis. Metachronous was defined as the absence of metastatic disease at the time of initial diagnosis with metastatic disease developing later. Patients were excluded if they died in the immediate postoperative period before receipt of any systemic therapy (n = 3). KRAS testing could be performed at any time during the disease course.
KRAS Mutation Analysis
For KRAS mutation testing, genomic DNA was extracted from formalin-fixed, paraffin-embedded tumor tissue using the Qiagen DNA preparation kit (Qiagen, Valencia, Calif). The sequences containing the target mutations were amplified by polymerase chain reaction (PCR) with primers flanking exon 2 of the KRAS gene. This included the codons G12 and G13. The PCR products were purified and sequenced bidirectionally using the ABI3130xl DNA analyzer (Applied Biosystems, Foster City, Calif) at the Clinical Laboratory Improvement Act-certified and College of American Pathologists-accredited clinical molecular laboratory at the Pathology Core Facility of The Ohio State University Medical Center.
Statistical Analysis
The demographic and clinical characteristics of patients are summarized in Table 1, in which frequencies/percentages are used for categorical variables, and means, standard deviations, and ranges are used for continuous variables. The Kaplan-Meier method was used to estimate the median overall survival, the 3-year survival rate, and the 5-year survival rate for each patient group. Kaplan-Meier plots also were generated. The log-rank test was used to compare the survival of different patient groups. All P values were from 2-sided tests, and P values < .05 were considered statistically significant. Data analyses were conducted using SAS 9.1 statistical software (SAS Institute Inc., Cary. NC).
Table 1.
Patient Characteristics
| No. of Patients (%)a | ||||||
|---|---|---|---|---|---|---|
| Synchronous Group | Metachronous Group | Total | ||||
| KRAS WT | KRAS Mutant | KRAS WT | KRAS Mutant | KRAS WT | KRAS Mutant | |
| Total no. of patients | 39 | 25 | 31 | 15 | 70 | 40 |
| Men | 17 (44) | 7 (28) | 19 (61) | 9 (60) | 36 (51) | 16 (40) |
| Age: Median [range], yr | 60.2 [22.8–79.1] | 54.6 [32.8–76.6] | 56.0 [33.7–76.6] | 58.1 [45.4–81.1] | 59.6 [22.8–79.1] | 55.4 [32.8–81.1] |
| Primary location | ||||||
| Colon | 34 (87) | 21 (84) | 24 (77) | 11 (73) | 58 (83) | 32 (80) |
| Rectum | 5 (13) | 4 (16) | 7 (23) | 4 (27) | 12 (17) | 8 (20) |
| Neoadjuvant chemoradiotherapy with 5-FU or capecitabine | 1 (3) | 2 (8) | 5 (16) | 1 (7) | 6 (9) | 3 (8) |
| Adjuvant chemotherapy, any | NA | NA | 27 (87) | 8 (53)b | NA | NA |
| 5-FU/leucovorin/oxaliplatin | NA | NA | 13 (42) | 3 (20) | NA | NA |
| 5-FU/leucovorin/irinotecan | NA | NA | 1 (3) | 0 (0) | NA | NA |
| 5-FU or capecitabine alone | NA | NA | 13 (42) | 5 (33) | NA | NA |
| Stage at diagnosis | ||||||
| I | NA | NA | 2 (6) | 0 (0) | NA | 0 (0) |
| II | NA | NA | 6 (19) | 7 (47) | NA | 7 (47) |
| III | NA | NA | 23 (74) | 8 (53) | NA | 8 (53) |
| IV | 39 (100) | 25 (100) | NA | NA | NA | NA |
| Initial liver metastasis alone | 21 (54) | 12 (48) | 10 (32) | 1 (7) | 31 (44) | 13 (33) |
| Metastatectomy or RFA | 12 (31) | 3 (12) | 3 (10) | 1 (7) | 15 (21) | 4 (10) |
| Receipt of each fluoropyrimidine, irinotecan, and oxaliplatin | 26 (67) | 20 (80) | 24 (77) | 11 (73) | 50 (71) | 31 (78) |
| Receipt of EGFR-directed therapy | 25 (64) | 5 (20)b | 20 (65) | 2 (13)b | 45 (64) | 7 (18)b |
| Receipt of bevacizumab | 32 (82) | 23 (92) | 26 (84) | 14 (93) | 58 (83) | 37 (93) |
| Median follow-up from original diagnosis [range], mo | 30.5 [8.0–120.6] | 28.7 [3.7–61.4] | 60.2 [6.6–156.1] | 62.0 [22.6–152.7] | 29.1 [1.8–120.6] | 28.5 [3.7–83.1] |
| Median follow-up from metastatic diagnosis [range], mo | 30.5 [8.0–120.6] | 28.7 [3.7–61.4] | 28.8 [1.8–80.4] | 30.5 [11.3–83.1] | 29.1 [1.8–120.6] | 28.5 [3.7–83.1] |
Abbreviations: 5-FU, 5-fluorouracil; EGFR, epidermal growth factor receptor; KRAS, v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; NA, not applicable; RFA, radiofrequency ablation; WT. wild-type.
Patients in the synchronous group had metastatic disease at the time of diagnosis; patients in the metachronous group developed metastases after their initial diagnosis of localized disease.
P < .05 (Fisher exact test) compared with KRAS WT in the synchronous, metachronous, or combined groups.
RESULTS
Demographics
Baseline characteristics of the study population are described in Table 1. In total, 110 patients were identified, of whom 40 had KRAS mutations (36%). This proportion of KRAS-mutant colorectal cancer is consistent with previously reported values.2,3 Sixty four patients(58%) had synchronous metastatic disease, and the other 46 patients (42%) had with metachronousmetastatic disease. In the metachronous group, the diagnosis of metastatic disease occurred at a median of 20 months (range, 4–125 months) from the time of the original, localized diagnosis. Eighteen percent of KRAS mutant patients received EGFR-directed therapy, all of which was prescribed before knowledge that KRAS-mutant tumors do not respond to these agents.
Treatment Efficacy
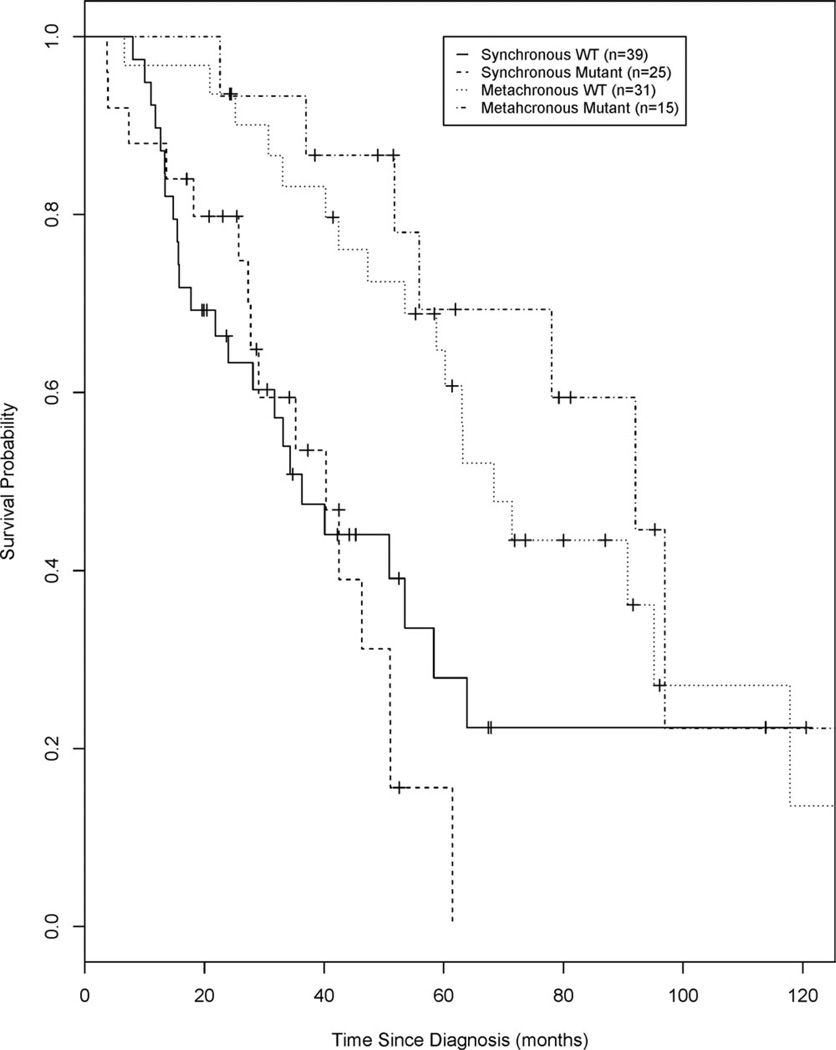
Kaplan-Meier analyses of overall survival from the original date of colorectal cancer diagnosis for synchronous metastatic and metachronous metastatic disease, stratified by KRAS mutation status, are illustrated in Figure 1. The median overall survival (mOS), 3-year, and 5-year survival estimates are presented in Table 2. Analyses of these endpoints did not differ significantly when comparing patients with WT KRAS and mutant KRAS who had either synchronous or metachronous disease.
Figure 1.
Overall survival is illustrated from the original date of colorectal diagnosis for patients with synchronous metastatic and metachronous metastatic disease, stratified by v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation status. WT indicates wild type.
Table 2.
Analysis of Overall Survival Defined From the Date of Original Diagnosis of Colorectal Cancer
| KRAS Statusa | No. of Patients |
Median OS (95% CI), mo |
3-Year Survival Estimate (95% CI) |
5-Year Survival Estimate (95% CI) |
Pb |
|---|---|---|---|---|---|
| Synchronous group | |||||
| WT | 39 | 36.3 (21.8–58.3) | 0.51 (0.33–0.66) | 0.28 (0.12–0.46) | .55 |
| Mutant | 25 | 40.3 (27.3–51.0) | 0.59 (0.36–0.77) | 0.16 (0.03–0.38) | |
| Metachronous group | |||||
| WT | 31 | 68.4 (53.5–95.1) | 0.83 (0.64–0.93) | 0.65 (0.44–0.79) | .37 |
| Mutant | 15 | 92.0 (51.8 to NA) | 0.93 (0.61–0.99) | 0.69 (0.37–0.87) |
Abbreviations: CI, confidence interval; KRAS, v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; NA, not applicable; OS, overall survival; WT, wild type.
Patients in the synchronous group had metastatic disease at the time of diagnosis; patients in the metachronous group developed metastases after their initial diagnosis of localized disease.
P values for OS were calculated with the log-rank test.
For the patients with synchronous disease, the mOS for the WT KRAS group was 36.3 months (95% confidence interval [CI], 21.8–58.3 months), and it was 40.3 months (95% CI, 27.3–51.0 months) for the mutant KRAS group (log-rank test; P = .55). Kaplan-Meier analysis indicated that patients with synchronous WT KRAS had a 3-year survival estimate of 0.51 (95% CI, 0.33–0.66) and a 5-year survival estimate of 0.28 (95% CI, 0.12–0.46). Patients with synchronous disease who had mutant KRAS had a 3-year survival estimate of 0.59 (95% CI, 0.36–0.77) and 5-year survival estimate of 0.16 (95% CI, 0.03–0.38).
For the patients with metachronous disease, the mOS for the WT KRAS group was 68.4 months (95% CI, 53.1–95.1 months), and it was 92.0 months (95% CI, 51.8 months to not applicable) for the mutant KRAS group (log-rank P = .37). Kaplan-Meier analysis indicated that patients with metachronous WT KRAS had a 3-year survival estimate of 0.83 (95% CI, 0.64–0.93) and a 5-year survival estimate of 0.65 (95% CI, 0.44–0.79). Patients with metachronous who had mutant KRAS had a 3-year survival estimate of 0.93 (95% CI, 0.61–0.99) and a 5-year survival estimate of 0.69 (95%CI, 0.37–0.87). Patients who were diagnosed with metachronous disease, as would be expected, had a prolonged mOS from the time of original diagnosis compared with those who were diagnosed with synchronous disease (log-rank P = .015 for KRAS WT and P = .001 for KRAS mutant).
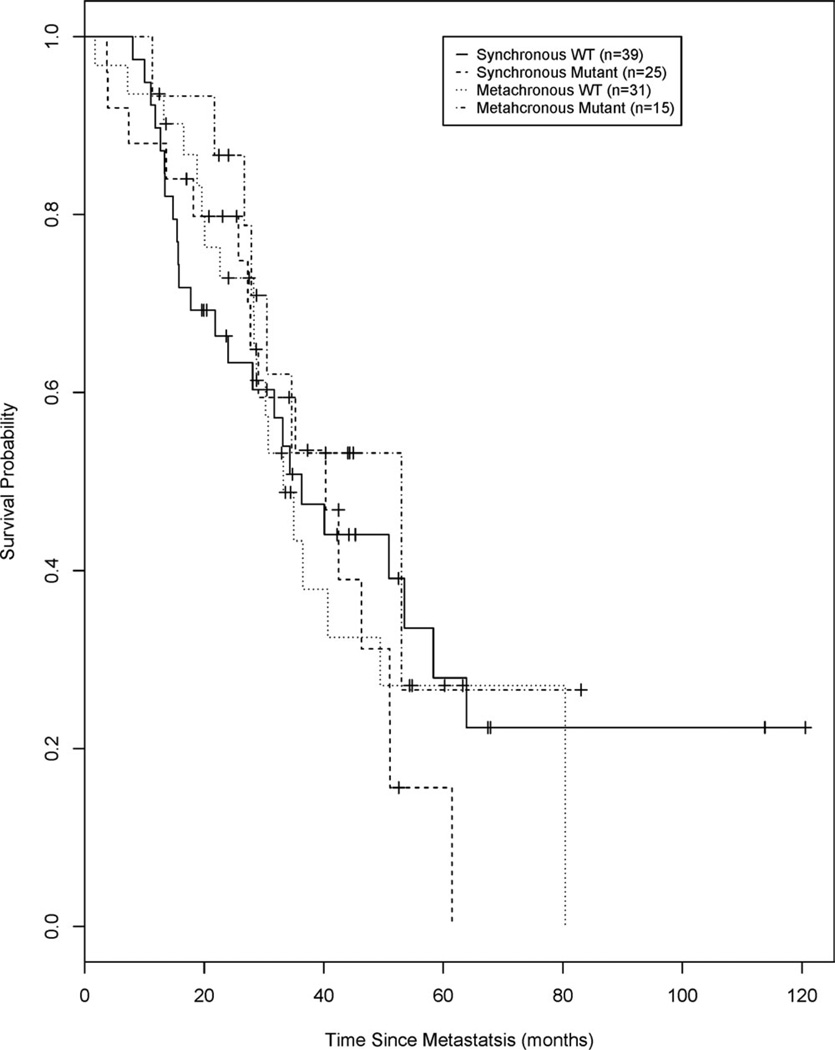
Figure 2 illustrates the shows Kaplan-Meier analyses for overall survival from the date of diagnosis of metastatic disease for patients with synchronous and metachronous disease stratified according to KRAS mutation status. The mOS and the 3-year and 5-year survival estimates are presented in Table 3. No significant differences were observed in OS or in the 3-year or 5-year survival estimates between the WT KRAS group and the mutant KRAS group for those with either synchronous or metachronous disease. By definition, synchronous disease is metastatic at diagnosis; thus, the data in Table 3 are the same as the data from Table 2, described above, and are included in Table 3 for comparative purposes. The mOS for patients with WT KRAS and mutant KRAS from the date of diagnosis of metachronous disease was 33.2 months (95% CI, 28.3–49.4 months) and 53.0 months (95% CI, 26.7 months to not applicable; log-rank P = .33), respectively. In Kaplan-Meier analysis, patients with metachronous disease who had WT KRAS had a 3-year survival estimate of 0.43 (95%CI, 0.24–0.62) and a 5-year survival estimate of 0.27 (95% CI, 0.11–0.47). Patients with metachronous who had mutant KRAS had a 3-year survival estimate of 0.53 (95% CI, 0.23–0.76) and a 5-year survival estimate of 0.27 (95% CI, 0.02–0.65).
Figure 2.
Overall survival is illustrated from the date of diagnosis of metastatic disease for synchronous and metachronous disease, stratified by v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation status. WT indicates wild type.
Table 3.
Analysis of Overall Survival Defined From the Date of Diagnosis of Metastatic Disease
| KRAS Statusa | No. of Patients |
Median OS (95% CI), mo |
3-Year Survival Estimate (95% CI) |
5-Year Survival Estimate (95% CI) |
Pb |
|---|---|---|---|---|---|
| Synchronous group | |||||
| WT | 39 | 36.3 (21.8–58.3) | 0.51 (0.33–0.66) | 0.28 (0.12–0.46) | .55 |
| Mutant | 25 | 40.3 (27.3–51.0) | 0.59 (0.36–0.77) | 0.16 (0.03–0.38) | |
| Metachronous group | |||||
| WT | 31 | 33.2 (28.3–49.4) | 0.43 (0.24–0.62) | 0.27 (0.11–0.47) | .33 |
| Mutant | 15 | 53.0 (26.7 to NA) | 0.53 (0.23–0.76) | 0.27 (0.02–0.65) | |
| Combined groupc | |||||
| WT | 70 | 34.3 (28.3–49.4) | 0.50 (0.37–0.62) | 0.28 (0.16–0.41) | .91 |
| Mutant | 40 | 40.3 (27.9–51.1) | 0.53 (0.35–0.69) | 0.17 (0.04–0.39) | |
| Total | |||||
| WT and mutant | 110 | 35.2 (30.5–49.4) | 0.50 (0.39–0.60) | 0.25 (0.15–0.37) |
Abbreviations: CI, confidence interval; KRAS, v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; NA, not applicable; OS, overall survival; WT, wild type.
Patients in the synchronous group had metastatic disease at the time of diagnosis; patients in the metachronous group developed metastases after their initial diagnosis of localized disease.
P values for OS were calculated with the log-rank test.
The synchronous and metachronous groups combined.
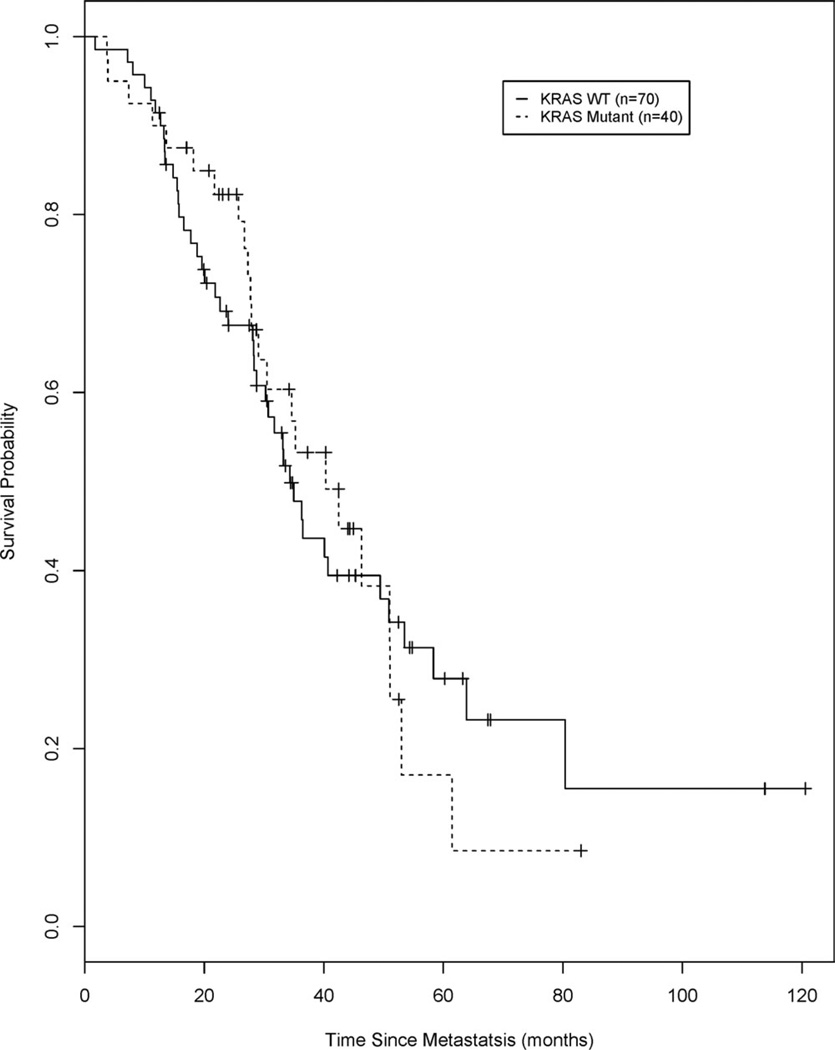
Figure 3 illustrates the Kaplan-Meier analyses of overall survival calculated from the date of diagnosis of metastatic disease for all patients (synchronous and metachronous) stratified according to KRAS mutation status. The mOS and the 3-year and 5-year survival estimates are presented in Table 3. Again, no differences in these endpoints were observed between patients with WT KRAS and patients with mutant KRAS. The mOS was 34.3 months (95% CI, 28.3–49.4 months) for patients with WT KRAS and 40.3 months (95% CI, 27.9–51.1 months) for patients with mutant KRAS (log-rank P = .91). In Kaplan-Meier analysis, patients with WT KRAS had a 3-year survival estimate of 0.50 (95% CI, 0.37–0.62) and a 5-year survival estimate of 0.28 (95% CI, 0.16–0.41), and patients with mutant KRAS had a 3-year survival estimate of 0.53 (95% CI, 0.35–0.69) and a 5-year survival estimate of 0.17 (95% CI, 0.04–0.39).
Figure 3.
Overall survival is illustrated from the date of diagnosis of metastatic disease for all patients (synchronous and metachronous), stratified by v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation status. WT indicates wild type.
Outcomes after the diagnosis of metastatic disease for all 110 patients who were included in this study are provided in Table 3. The mOS was 35.2 months (95% CI, 30.5–49.4), the 3-year survival estimate was 0.50 (95% CI, 0.39–0.60), and the 5-year survival estimate was 0.25 (95% CI, 0.15–0.37).
DISCUSSION
KRAS mutations are observed commonly in colorectal cancer, and WT status is predictive of response to EGFR-directed therapy. The prognostic implications of KRAS mutation status are less well defined, and various studies in both localized and metastatic disease have produced conflicting results. The current study demonstrates that, with modern chemotherapy regimens, patients who have metastatic colorectal cancer with mutant KRAS tumors have an overall survival similar to that of patients who have metastatic colorectal cancer with WT KRAS tumors. This finding was observed consistently in patients who were diagnosed with synchronous as well as metachronous metastatic disease.
Our findings are in overall concordance with other studies of metastatic disease. The recently published follow-up analysis of the phase 3 Australian Gastrointestinal Trials Group (AGITG) (the AGITG MAX trial12) assessed the predictive and prognostic value of KRAS and v-raf murine sarcoma viral oncogene homolog B1 (BRAF) mutation status in patients who were receiving capecitabine with or without mitomycin and bevacizumab in the first-line setting. Mutation status was determined in 315 patients (67%) in the original study population, and KRAS mutations were observed in 28.8% of the population. KRAS was not prognostic of patient outcomes, and the mOS was 18.4 months in the mutant KRAS group compared with 20.0 months in the WT KRAS group (P = .82). Two other studies that assessed the efficacy of EGFR inhibitors added to chemotherapy and bevacizumab confirmed that KRAS mutation status had no prognostic significance. The Panitumumab Advanced Colorectal Cancer Evaluation (PACCE) study assessed the efficacy of panitumumab added to first-line 5-fluorouracil–based doublet chemotherapy plus bevacizumab.12 There was no difference in overall survival when patients were stratified according to KRAS mutation status. The second Capecitabine, Irinotecan, and Oxaliplatin (CAIRO2) study assessed the addition of cetuximab to capecitabine, oxaliplatin, and bevacizumab.13 Seven hundred fifty-five patients were enrolled, and 520 underwent KRAS mutation assessment. Sixty percent of those patients had WT KRAS. There was no significant difference in overall survival when the patients were stratified according to the presence of KRAS mutation in either the control arm or the experimental arm, which was receiving cetuximab. An assessment of 394 specimens for KRAS mutation status in the CO.17 trial confirmed the predictive role in WT KRAS tumors and response to cetuximab.9 Despite its predictive value for cetuximab, KRAS mutation status lacked prognostic significance.
In contrast, a retrospective analysis of the Medical Research Council (MRC) Fluorouracil, Oxaliplatin, and Irinotecan (FOCUS) trial evaluated several chemotherapy strategies in patients with previously untreated advanced colorectal cancers.14 It is noteworthy that none of those patients received EGFR-directed therapies. A retrospective KRAS assessment of 711 tumor specimens identified mutations in codons 12 and 13 in 288 patients (40.5%) along with 3 patients (0.6%) who had an additional mutation in codon 61. Progression-free survival was comparable between the mutant KRAS and WT KRAS groups, but overall survival was shorter for patients with mutant KRAS, suggesting the possible prognostic relevance of the mutation.14 The MRC COIN (COntinuous versus INtermittent chemotherapy) trial assessed the benefit of adding cetuximab to oxaliplatin and 5-fluorouracil or capecitabine in 1650 patients with untreated, metastatic colon cancer.15 Patients in that trial were not stratified according to KRAS mutation status before entry of the study. Eighty percent of the patients enrolled underwent KRAS, NRAS, and BRAF mutation assessment. Five hundred sixty-five patients (43%) in that study had a KRAS mutation, 50 patients (4%) had an NRAS mutation, and 102 patients (8%) had a BRAF mutation. In the patients with WT KRAS who received cetuximab, there was no improvement in overall survival compared with those who received chemotherapy alone. The prognostic value of KRAS also was assessed. Patients with any of the aforementioned 3 mutations had worse survival compared with patients who had WT KRAS (14.4 months vs 20.1 months for patients who did not receive cetuximab and 12.7 months vs 19.9 months for patients who did receive cetuximab). There was a strong prognostic effect for KRAS, NRAS, and BRAF mutation status independent of the receipt of cetuximab. Like what was observed in the third Pan-European Trial in Adjuvant Colon Cancer (PETACC-3) study,16 patients who had BRAF mutations fared the poorest in this study. Finally, an updated analysis of the Cetuximab Combined With Irinotecan in First-Line Therapy for Metastatic Colorectal Cancer (CRYS-TAL) study using combined folic acid, 5-fluorouracil, and irinotecan (FOLFIRI) with or without cetuximab examined the prognostic significance of KRAS mutations.17 Of the initial intent-to-treat population of 1198, 1063 patients had tissue available for KRAS assessment. Six hundred sixty-six patients (62.7%) had WT KRAS, and 397 patients (37.3%) had mutant KRAS in codons 12 and 13. Patients with mutated KRAS appeared to have worse overall survival than those with WT tumors (16.7 months vs 20.0 months for who received FOLFIRI, respectively; and 16.2 months vs 23.5 months for those who received FOLFIRI plus cetuximab, respectively). Other studies, such as the German Association of Medical Oncology (AIO) study KRK-1014,18 again suggested a prognostic role for the presence of KRAS mutations.
Studies evaluating the prognostic value of KRAS in earlier stage, resected disease also are conflicting. A large, retrospective analysis of 3439 patients from the KRAS Mutations in Colorectal Cancer Collaborative Group (RASCAL) II study identified 12 different mutations on codons 12 and 13.19 Multivariate analyses identified only a glycine-to-valine substitution on codon 12 (identified in 8.6% of patients) that had a statistically significant impact on failure-free survival. There was a more pronounced influence in patients who had Duke C disease compared with patients who had Duke B disease. Drawbacks to that analysis included the absence of a microsatellite instability assessment as well heterogeneity in assay methodology, because it was not standardized.19 The N0147 study, which evaluated the addition of cetuximab to 5-fluorouracil and oxaliplatin (FOLFOX) in patients with resected, stage III colon cancer, suggested a prognostic role for the mutation.20 In the absence of cetuximab, patients with WT KRAS tumors who received FOLFOX had an improved prognosis with a 75.8% 3-year disease-free survival rate compared with 67.2% of patients who harbored a KRAS mutation. In contrast, investigators from PETACC-2 trial evaluated the prognostic role of KRAS and BRAF mutations in 493 patients with resected stage III colon cancer who received adjuvant 5-fluorouracil.21 KRAS mutations were detected by direct sequencing. After 3 years, 63% of patients with mutant KRAS, 68% of patients with WT KRAS/BRAF, and 65% of patients with BRAF mutations were still alive, indicating no prognostic value of KRAS/BRAF status in that study. In addition, the PETACC-3 trial retrospectively analyzed archival tissue for multiple molecular markers, including KRAS mutations (exon 2, codons 12 and 13) in 1564 patients with resected, stage II and stage III disease.16 KRAS mutations were identified in 36% of patients with stage II colon cancer and in 37% of patients with stage III colon cancers. Multivariate and univariate analyses, again, identified no clear association between KRAS mutation status and relapse-free survival or OS in either stage II or III cancers. In a retrospective analysis of 508 patients with stage III colon cancer who were treated on the Cancer and Leukemia Group B (CALGB) clinical trial CALGB 89803, 35% had KRAS mutations identified. KRAS mutation status did not influence overall survival.22
Overall, as discussed above, the data relating to the prognostic value of KRAS mutation status is conflicting across all stages in patients with colon cancer. In our study, the majority of patients with WT KRAS received anti-EGFR therapies, usually with irinotecan or FOLFIRI when combined with chemotherapy. In patients with metastatic disease, who were the focus of our current study, the receipt of anti-EGFR therapy may have affected the measured outcome. The most representative data set evaluating the influence of KRAS mutation status on prognosis in the metastatic setting ideally would exclude the use of EGFR-inhibitor therapy. For example, the MRC FOCUS study,14 which included only 0.5% and 1.5% of patients in each respective arm received any EGFR-inhibitor therapy, suggested a prognostic role for KRAS mutation status. Whereas the CO.17 study demonstrated no difference in outcome between patients with WT and mutant KRAS in the best supportive care arm, suggesting a lack of prognostic significance for the mutation.9
To our knowledge, this is the first study to specifically investigate differential survival in patients with synchronous and metachronous metastatic colorectal cancer according to KRAS mutation status. Like all retrospective analyses, our study has several limitations. The sample size, although fairly representative, was relatively small. The KRAS testing performed in this study using PCR and direct nucleotide sequencing accounts only for mutations in codons 12 and 13, and not for less common mutations, like those in codons 61 and 146.4,5 In addition, a breakdown of codon 12 mutations versus codon 13 mutations was not available. Mutations in KRAS codon 13 may have more prognostic significance than mutations in KRAS codon 12, although this remains somewhat controversial. 24,25 Finally, data on BRAF mutation status, which may be associated with a worse prognosis in colorectal cancer,14–17,26,27 were not available in our study. Given the rarity of this mutation, this is unlikely to affect the outcome results of our study. One strength of our study is the availability of survival data from the date of first diagnosis for all included patients, allowing a more longitudinal analysis. In conclusion, our study suggests the absence of a prognostic role for KRAS mutation in colon cancer regardless of the presence of synchronous or metachronous metastatic disease.
Acknowledgments
FUNDING SOURCES
No specific funding was disclosed.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. Bekaii-Saab is a consultant for Amgen, Bristol-Meyer Squibb, and Genentech.
REFERENCES
- 1.Vogelstein B, Fearon E, Hamilton S, et al. Genetic alterations during colorectal tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 2.Linardou H, Briasoulis E, Dahabreh IJ, et al. All about KRAS for clinical oncology practice: gene profile, clinical implications and laboratory recommendations for somatic mutational testing in colorectal cancer. Cancer Treat Rev. 2011;37:221–233. doi: 10.1016/j.ctrv.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 3.McDermott U, Longley DB, Johnston PG. Molecular and biochemical markers in colorectal cancer. Ann Oncol. 2002;13(suppl 4):235–245. doi: 10.1093/annonc/mdf665. [DOI] [PubMed] [Google Scholar]
- 4.Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4882–4689. [PubMed] [Google Scholar]
- 5.Edkins S, O’Meara S, Parker A, et al. Recurrent KRAS codon 146 mutations in human colorectal cancer. Cancer Biol Ther. 2006;5:928–932. doi: 10.4161/cbt.5.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care in patients with chemotherapy-refractory colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 8.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 9.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. KRAS mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 10.Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 11.Price TJ, Hardingham JE, Chee K, et al. Impact of KRAS and BRAF gene mutation status on outcomes from the phase III AGITG MAX trial of capecitabine alone or in combination with bevacizumab and mitomycin in advanced colorectal cancer. J Clin Oncol. 2001;29:2675–2682. doi: 10.1200/JCO.2010.34.5520. [DOI] [PubMed] [Google Scholar]
- 12.Hecht JR, Mitchell E, Chidiak T, et al. A randomized phase IIIb trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2008;27:672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 13.Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 14.Richman SD, Seymour MT, Chambers P, et al. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27:5931–5937. doi: 10.1200/JCO.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- 15.Maughan TS, Adams R, Smith CG, et al. Identification of potentially responsive subsets when cetuximab is added to oxaliplatin-fluoropyrimidine chemotherapy (CT) in first-line advanced colorectal cancer: mature results of the MRC COIN trial [abstract] J Clin Oncol. 2010;28(15s) Abstract 3502. [Google Scholar]
- 16.Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, and SAKK 60-00 trial. J Clin Oncol. 2010;28:466–474. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 17.Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 18.Moosmann N, von Weikersthal LF, Vehling-Kaiser U, et al. Cetuximab plus capecitabine and irinotecan compared with cetuximab plus capecitabine and oxaliplatin as first-line treatment for patients with metastatic colorectal cancer: AIO KRK-0104-a randomized trial of the German AIO CRC Study Group. J Clin Oncol. 2011;29:1050–1058. doi: 10.1200/JCO.2010.31.1936. [DOI] [PubMed] [Google Scholar]
- 19.Andreyev HJN, Norman AR, Cunningham D, et al. Kirsten ras mutations in patients with colorectal cancer: the “RASCAL II” study. Br J Cancer. 2001;85:692–696. doi: 10.1054/bjoc.2001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberts SR, Thibodeau SN, Sargent DJ, et al. Influence of KRAS and BRAF mutational status and rash on disease-free survival in patients with resected stage III colon cancer receiving cetuximab: results from NCCTG N0147 [abstract] J Clin Oncol. 2011;29(suppl) Abstract 3607. [Google Scholar]
- 21.Aust DE, Lutz MP, Mauer M, et al. Lessons from PETACC-2: no prognostic impact of KRAS-/BRAF-status in stage II colon cancer treated with adjuvant 5-FU monotherapy [abstract] J Clin Oncol. 2010;28(15s) Abstract 3591. [Google Scholar]
- 22.Ogino S, Meyerhardt JA, Irahara N, et al. KRAS mutation in stage III colon cancer and clinical outcome following Intergroup Trial CALGB 89803. Clin Cancer Res. 2009;15:7322–7329. doi: 10.1158/1078-0432.CCR-09-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Roock W, Jonker DJ, Di Nicolantonio F, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812–1820. doi: 10.1001/jama.2010.1535. [DOI] [PubMed] [Google Scholar]
- 25.Peeters M, Douillard JY, Van Cutsem E, et al. Mutant (MT) KRAS codon 12 and 13 alleles in patients (pts) with metastatic colorectal cancer (mCRC): assessment as prognostic and predictive biomarkers of response to panitumumab (pmab) [abstract] J Clin Oncol. 2012;30(suppl 4) doi: 10.1200/JCO.2012.45.1492. Abstract 383. [DOI] [PubMed] [Google Scholar]
- 26.Fransen K, Klintenas M, Osterstrom A, et al. Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis. 2004;25:527–533. doi: 10.1093/carcin/bgh049. [DOI] [PubMed] [Google Scholar]
- 27.Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]