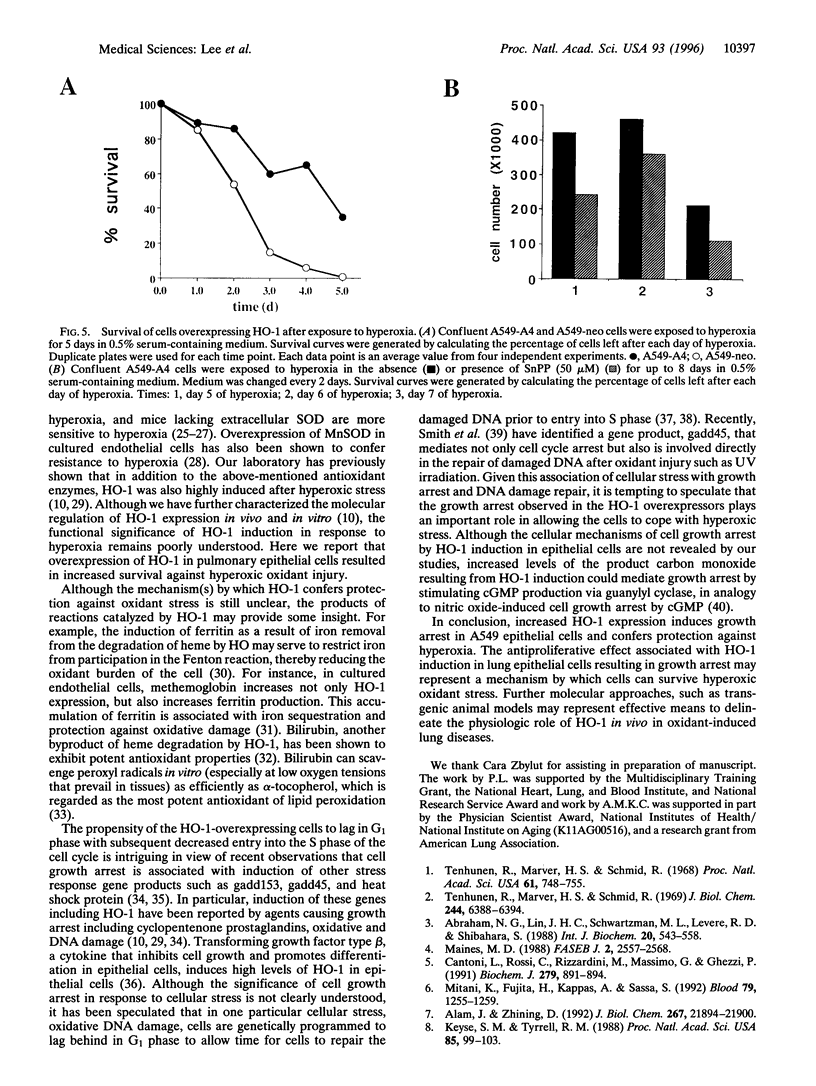
Abstract
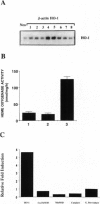
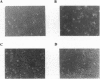
Heme oxygenase (HO) catalyzes the rate-limiting step in the degradation of heme to biliverdin, which is reduced by biliverdin reductase to bilirubin. Heme oxygenase-1 (HO-1) is inducible not only by its heme substrate, but also by a variety of agents causing oxidative stress. Although much is known about the regulation of HO-1 expression, the functional significance of HO-1 induction after oxidant insult is still poorly understood. We hypothesize and provide evidence that HO-1 induction serves to protect cells against oxidant stress. Human pulmonary epithelial cells (A549 cells) stably transfected with the rat HO-1 cDNA exhibit marked increases of HO-1 mRNA levels which were correlated with increased HO enzyme activity. Cells that overexpress HO-1 (A549-A4) exhibited a marked decrease in cell growth compared with wild-type A549 (A549-WT) cells or A549 cells transfected with control DNA (A549-neo). This slowing of cell growth was associated with an increased number of cells in G0/G1 phase during the exponential growth phase and decreased entry into the S phase, as determined by flow cytometric analysis of propidium iodide-stained cells and pulse experiments with bromodeoxyuridine. Furthermore, the A549-A4 cells accumulated at the G2/M phase and failed to progress through the cell cycle when stimulated with serum, whereas the A549-neo control cells exhibited normal cell cycle progression. Interestingly, the A549-A4 cells also exhibited marked resistance to hyperoxic oxidant insult. Tin protoporphyrin, a selective inhibitor of HO, reversed the growth arrest and ablated the increased survival against hyperoxia observed in the A549-A4 cells overexpressing HO-1. Taken together, our data suggest that overexpression of HO-1 results in cell growth arrest, which may facilitate cellular protection against non-heme-mediated oxidant insult such as hyperoxia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N. G., Lavrovsky Y., Schwartzman M. L., Stoltz R. A., Levere R. D., Gerritsen M. E., Shibahara S., Kappas A. Transfection of the human heme oxygenase gene into rabbit coronary microvessel endothelial cells: protective effect against heme and hemoglobin toxicity. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6798–6802. doi: 10.1073/pnas.92.15.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham N. G., Lin J. H., Schwartzman M. L., Levere R. D., Shibahara S. The physiological significance of heme oxygenase. Int J Biochem. 1988;20(6):543–558. doi: 10.1016/0020-711x(88)90093-6. [DOI] [PubMed] [Google Scholar]
- Alam J., Cai J., Smith A. Isolation and characterization of the mouse heme oxygenase-1 gene. Distal 5' sequences are required for induction by heme or heavy metals. J Biol Chem. 1994 Jan 14;269(2):1001–1009. [PubMed] [Google Scholar]
- Alam J., Den Z. Distal AP-1 binding sites mediate basal level enhancement and TPA induction of the mouse heme oxygenase-1 gene. J Biol Chem. 1992 Oct 25;267(30):21894–21900. [PubMed] [Google Scholar]
- Applegate L. A., Luscher P., Tyrrell R. M. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991 Feb 1;51(3):974–978. [PubMed] [Google Scholar]
- Balla G., Jacob H. S., Balla J., Rosenberg M., Nath K., Apple F., Eaton J. W., Vercellotti G. M. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992 Sep 5;267(25):18148–18153. [PubMed] [Google Scholar]
- Balla J., Jacob H. S., Balla G., Nath K., Eaton J. W., Vercellotti G. M. Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9285–9289. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. M. Proposed structure for the zinc-binding domains from transcription factor IIIA and related proteins. Proc Natl Acad Sci U S A. 1988 Jan;85(1):99–102. doi: 10.1073/pnas.85.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camhi S. L., Alam J., Otterbein L., Sylvester S. L., Choi A. M. Induction of heme oxygenase-1 gene expression by lipopolysaccharide is mediated by AP-1 activation. Am J Respir Cell Mol Biol. 1995 Oct;13(4):387–398. doi: 10.1165/ajrcmb.13.4.7546768. [DOI] [PubMed] [Google Scholar]
- Cantoni L., Rossi C., Rizzardini M., Gadina M., Ghezzi P. Interleukin-1 and tumour necrosis factor induce hepatic haem oxygenase. Feedback regulation by glucocorticoids. Biochem J. 1991 Nov 1;279(Pt 3):891–894. doi: 10.1042/bj2790891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson L. M., Jonsson J., Edlund T., Marklund S. L. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi A. M., Sylvester S., Otterbein L., Holbrook N. J. Molecular responses to hyperoxia in vivo: relationship to increased tolerance in aged rats. Am J Respir Cell Mol Biol. 1995 Jul;13(1):74–82. doi: 10.1165/ajrcmb.13.1.7598940. [DOI] [PubMed] [Google Scholar]
- Choi A. M., Tucker R. W., Carlson S. G., Weigand G., Holbrook N. J. Calcium mediates expression of stress-response genes in prostaglandin A2-induced growth arrest. FASEB J. 1994 Oct;8(13):1048–1054. doi: 10.1096/fasebj.8.13.7926370. [DOI] [PubMed] [Google Scholar]
- Clerch L. B., Massaro D. Tolerance of rats to hyperoxia. Lung antioxidant enzyme gene expression. J Clin Invest. 1993 Feb;91(2):499–508. doi: 10.1172/JCI116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell T. L., Arnold E., Boerth N. J., Lincoln T. M. Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1994 Nov;267(5 Pt 1):C1405–C1413. doi: 10.1152/ajpcell.1994.267.5.C1405. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981 Nov 10;256(21):10986–10992. [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook N. J., Carlson S. G., Choi A. M., Fargnoli J. Induction of HSP70 gene expression by the antiproliferative prostaglandin PGA2: a growth-dependent response mediated by activation of heat shock transcription factor. Mol Cell Biol. 1992 Apr;12(4):1528–1534. doi: 10.1128/mcb.12.4.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Keyse S. M., Applegate L. A., Tromvoukis Y., Tyrrell R. M. Oxidant stress leads to transcriptional activation of the human heme oxygenase gene in cultured skin fibroblasts. Mol Cell Biol. 1990 Sep;10(9):4967–4969. doi: 10.1128/mcb.10.9.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball R. E., Reddy K., Peirce T. H., Schwartz L. W., Mustafa M. G., Cross C. E. Oxygen toxicity: augmentation of antioxidant defense mechanisms in rat lung. Am J Physiol. 1976 May;230(5):1425–1431. doi: 10.1152/ajplegacy.1976.230.5.1425. [DOI] [PubMed] [Google Scholar]
- Kutty R. K., Nagineni C. N., Kutty G., Hooks J. J., Chader G. J., Wiggert B. Increased expression of heme oxygenase-1 in human retinal pigment epithelial cells by transforming growth factor-beta. J Cell Physiol. 1994 May;159(2):371–378. doi: 10.1002/jcp.1041590221. [DOI] [PubMed] [Google Scholar]
- Lautier D., Luscher P., Tyrrell R. M. Endogenous glutathione levels modulate both constitutive and UVA radiation/hydrogen peroxide inducible expression of the human heme oxygenase gene. Carcinogenesis. 1992 Feb;13(2):227–232. doi: 10.1093/carcin/13.2.227. [DOI] [PubMed] [Google Scholar]
- Lee P. J., Alam J., Sylvester S. L., Inamdar N., Otterbein L., Choi A. M. Regulation of heme oxygenase-1 expression in vivo and in vitro in hyperoxic lung injury. Am J Respir Cell Mol Biol. 1996 Jun;14(6):556–568. doi: 10.1165/ajrcmb.14.6.8652184. [DOI] [PubMed] [Google Scholar]
- Lindau-Shepard B., Shaffer J. B., Del Vecchio P. J. Overexpression of manganous superoxide dismutase (MnSOD) in pulmonary endothelial cells confers resistance to hyperoxia. J Cell Physiol. 1994 Nov;161(2):237–242. doi: 10.1002/jcp.1041610207. [DOI] [PubMed] [Google Scholar]
- Maines M. D. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988 Jul;2(10):2557–2568. [PubMed] [Google Scholar]
- Mitani K., Fujita H., Kappas A., Sassa S. Heme oxygenase is a positive acute-phase reactant in human Hep3B hepatoma cells. Blood. 1992 Mar 1;79(5):1255–1259. [PubMed] [Google Scholar]
- Murray A. W. Creative blocks: cell-cycle checkpoints and feedback controls. Nature. 1992 Oct 15;359(6396):599–604. doi: 10.1038/359599a0. [DOI] [PubMed] [Google Scholar]
- Nath K. A., Balla G., Vercellotti G. M., Balla J., Jacob H. S., Levitt M. D., Rosenberg M. E. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992 Jul;90(1):267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbein L., Sylvester S. L., Choi A. M. Hemoglobin provides protection against lethal endotoxemia in rats: the role of heme oxygenase-1. Am J Respir Cell Mol Biol. 1995 Nov;13(5):595–601. doi: 10.1165/ajrcmb.13.5.7576696. [DOI] [PubMed] [Google Scholar]
- Prestera T., Talalay P., Alam J., Ahn Y. I., Lee P. J., Choi A. M. Parallel induction of heme oxygenase-1 and chemoprotective phase 2 enzymes by electrophiles and antioxidants: regulation by upstream antioxidant-responsive elements (ARE). Mol Med. 1995 Nov;1(7):827–837. [PMC free article] [PubMed] [Google Scholar]
- Smith M. L., Chen I. T., Zhan Q., Bae I., Chen C. Y., Gilmer T. M., Kastan M. B., O'Connor P. M., Fornace A. J., Jr Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994 Nov 25;266(5189):1376–1380. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- Stocker R., Glazer A. N., Ames B. N. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C. W., Avraham K. B., Shanley P. F., Groner Y. Transgenic mice with expression of elevated levels of copper-zinc superoxide dismutase in the lungs are resistant to pulmonary oxygen toxicity. J Clin Invest. 1991 Jun;87(6):2162–2168. doi: 10.1172/JCI115249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wispé J. R., Warner B. B., Clark J. C., Dey C. R., Neuman J., Glasser S. W., Crapo J. D., Chang L. Y., Whitsett J. A. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J Biol Chem. 1992 Nov 25;267(33):23937–23941. [PubMed] [Google Scholar]