Abstract
Some fruits and their anthocyanin-rich extracts have been reported to exhibit chemopreventive activity in the oral cavity. Insights regarding oral metabolism of anthocyanins remain limited. Anthocyanin-rich extracts from blueberry, chokeberry, black raspberry, red grape, and strawberry were incubated ex vivo with human saliva from 14 healthy subjects. All anthocyanins were partially degraded in saliva. Degradation of chokeberry anthocyanins in saliva was temperature dependent and decreased by heating saliva to 80 °C and after removal of cells. Glycosides of delphinidin and petunidin were more susceptible to degradation than those of cyanidin, pelargonidin, peonidin and malvidin in both intact and artificial saliva. Stability of di- and tri-saccharide conjugates of anthocyanidins slightly, but significantly, exceeded that of monosaccharide compounds. Ex vivo degradation of anthocyanins in saliva was significantly decreased after oral rinsing with antibacterial chlorhexidine. These results suggest that anthocyanin degradation in the mouth is structure-dependent and largely mediated by oral microbiota.
Keywords: Anthocyanins, Saliva, Metabolism, Oral cavity, Blueberry, Chokeberry, Black raspberry, Red grape, Strawberry
1. Introduction
Anthocyanins (ACN) are a major class of flavonoids that contribute bright orange, red and blue colours to many vegetables and fruits. These compounds are of interest for use as natural colourants for foods and beverages instead of synthetic food colouring agents such as the dye FD&C Red No. 40 (Giusti & Wrolstad, 2003). Typical US diets contain a range of 12.5–215 mg ACN/day (Kuhnau, 1976; Wu et al., 2006), which is greater than that of many other flavonoids in foods and beverages (Cooke, Steward, Gescher, & Marczylo, 2005; Hertog, Hallman, Katan, & Kromhout, 1993).
In vitro and in vivo studies during the past decade have shown that ACN possess antioxidant, anti-inflammatory and chemopreventive activities (Wang & Stoner, 2008; Zafra-Stone et al., 2007). Chemopreventive activity has been reported in the gastrointestinal tract (Casto et al., 2002; Rodrigo et al., 2006). For example, ACN-rich extract from black raspberry suppressed proliferation of human oral squamous cell carcinoma cells (Rodrigo et al., 2006) and reduced the number of chemically-induced tumours in the hamster cheek pouch (Casto et al., 2002) and the oesophagus and colon in laboratory rodents (Wang & Stoner, 2008). Also, topical application of ACN-rich berry gel reduced heterozygosity indices (Shumway et al., 2008) and COX-2 (Mallery et al., 2008) in premalignant oral lesions in human subjects. Such observations have provided impetus to the development of delivery vehicles for ACN, such as muco-adhesive gel (Mallery et al., 2007), to increase exposure of oral tissues to ACN.
Numerous studies with animals and humans have demonstrated that ingested ACN are poorly absorbed (<1%) (McGhie & Walton, 2007; Stoner et al., 2005) and largely disappear from the GI tract within several hours after consuming a meal (Prior & Wu, 2006). Bioconversion of ACN to phenolic acids and aldehydes by intestinal microbiota has been demonstrated (Keppler & Humpf, 2005). Moreover, cyanidin-3-glucoside was degraded to protocatechuic acid (PCA) and phloroglucinol aldehyde (PGA) in cultures of Caco-2 human intestinal epithelial cells (Kay, Kroon, & Cassidy, 2009). Collectively, these data suggest that metabolites of ACN may be responsible for observed health-promoting activities of ACN in vivo (Forester & Waterhouse, 2010; Vitaglione et al., 2007).
At present, the extent of ACN uptake and metabolism in the oral cavity is unknown. An understanding of the influence of ACN structure on susceptibility to and patterns of metabolism in oral cavity is expected to facilitate the strategic development of formulations of ACN-rich products for the promotion of oral health. Walle, Browning, Steed, Reed, and Walle (2005) reported flavonoids such as quercetin 4′-glucoside and genistein 7-glucoside, but not quercetin- 3-rhamnoside and naringenin 7-rhamnoglucoside, were hydrolysed to their respective aglycones in human saliva ex vivo. They also found that the extent of hydrolysis of genistin in saliva collected from 17 human subjects varied more than 20 fold and hydrolytic activity was inhibited by antibacterial agents, suggesting a key role for oral microbiota in flavonoid metabolism in the oral cavity. We hypothesised that ACN will be metabolised in saliva, as a result of microbial activity, to unstable ACN aglycones that subsequently degrade to phenolic acids. We present results from studies characterising the degradation of ACN with two aims. The first aim was to characterise the ex vivo degradation of ACN extracted from chokeberry in saliva collected at various times from a single participant. The second aim was to investigate the effect of ACN structure on ex vivo susceptibility to degradation in saliva, using anthocyanin-rich extracts from blueberry, chokeberry, black raspberry, red grape and strawberry, as well as the role of oral bacteria in such degradation.
2. Materials and methods
2.1. Standards and reagents
Unless otherwise indicated, all supplies and chemicals were purchased from Sigma-Aldrich and Fisher Scientific. Standard cyanidin chloride was purchased from Indofine Chemical Co., Inc. (Hillsborough, NJ). Blueberry (BluB) (Vaccinium corymbosum), red grape (RdGrp) (Vitis vinifera), and strawberry (StwB) (Fragaria × ananassa) were purchased from a local supermarket. Black raspberries (BlkRB) (Rubus occidentalis) were donated by Maurer’s farm (Wooster, OH). Concentrated juice and powder made from chokeberry (CkB) (Aronia meloncarpa E.) were donated from Artemis International (Fort Wayne, IN). All fruits except CkB concentrate were frozen (−18 °C) upon arrival until use.
2.2. Subject recruitment
Approval for the human study protocol was received from the Institutional Review Board for Human Research of the Ohio State University (IRB#20090058). Subjects (n = 14; 21–55 years of age) who were periodontally healthy (as evidenced by all sites with attachment levels ≤2 mm and probing depths ≤3 mm) and caries- free as evidenced by a low DMF (decayed, missing, filled teeth) Index were recruited. Subjects, who had had antibiotic therapy or professional cleaning within the previous 3 months, use immunosuppressant medications, bisphosphonates or steroids, or report pregnancy, a history of diabetes or HIV, were excluded from this study (Kumar, Brooker, Dowd, & Camerlengo, 2011), since these conditions have been previously shown to affect salivary secretion and oral bacterial profiles (Dawes, 1987; Socransky & Haffajee, 2005).
2.3. Aim 1: Characterisation of ACN degradation using chokeberry extract in saliva from single human subject
2.3.1. Preparation of chokeberry ACN-rich extracts
CkB extract powder was solubilised in 0.01% HCl in water prior to solid phase extraction (Sep-Pak, C-18, SPE Waters®) (Rodriguez-Saona & Wrolstad, 2005). Monomeric ACN content was estimated by the pH differential method (Giusti & Wrolstad, 2005) as cyanidin- 3-glucoside (Cy-3-glu) equivalents using the extinction coefficient of 26,900 L cm−1 mg−1. Profile of ACN species in CkB ACN-rich extract was determined by high-pressure liquid chromatography– photodiode array detection-electrospray ionisation-mass spectrometry (HPLC–PDA–ESI–MS) as described in Section 2.4.3. Purity of ACN in each extract was estimated by dividing collective HPLC– PDA area under curves (AUC) of identified ACN (510–530 nm) by total AUC monitored from 250–600 nm. Stock ACN-rich extracts were stored at −20 °C until use.
2.3.2. Ex vivo assessment of ACN degradation in saliva
The method was adapted from Walle et al. (2005). Unstimulated whole human saliva was collected from a subject (49 years) in the morning (7–8 AM) after brushing teeth and eating breakfast. Saliva was stored on ice until use within 2 h. Saliva was diluted with an equal volume of de-ionised water and shaken to reduce viscosity. Aliquots of diluted saliva (0.6 mL) were incubated with CkB extract containing 15 nmol of Cy-3-glu equivalents. At least three separate reaction tubes were incubated at 37 °C in a shaking water bath at 85 rpm for each characteristic being assessed. Reactions were terminated by addition of 0.6 mL acidified methanol to pH of 2.3 after indicated times. Tubes were centrifuged (27,000 g, 10 min, 4 °C) and supernatant was filtered (0.2 μm syringe filter) prior to analysis. Degradation was calculated as:
To estimate the extent of ACN loss due to association with ACN precipitated proteins, ACN in the pellet were re-extracted with acetonitrile. Acetonitrile has been used as solvent to separate polyphenols, such as catechin and theaflavins, from proteins in plasma and saliva (Laurent, Besancon, & Caporiccio, 2007; Lee, Prabhu, Meng, Li, & Yang, 2000). Acetonitrile (200 μL) and acidified water (4.5% formic acid, 200 μL) were added to pellets, mixtures were vortexed for 30 s and sonicated for 1 min. Acidified water (500 μL) was added to the mixture, vortexed and centrifuged (27,000g, 10 min, 4 °C). Supernatant was filtered (0.45 μm) for analysis.
2.3.3. Evaluation of chemical, enzymatic and cell-mediated degradation of ACN
Extent of ACN degradation during incubation (60 min) in intact saliva was tested for the following treatments: intact saliva at 37 °C; 4 °C; artificial saliva at 37 °C; saliva pre-heated to 80 °C for 15 min to destroy enzyme activity and cooled to 37 °C; and cell-free saliva at 37 °C prepared by centrifugation (800g, 5 min, 4 °C) of diluted saliva followed by filtration of the supernatant through sterile Millex® 0.22 μm (Millipore Express® PES membrane) to remove microorganisms and shed epithelial cells. Artificial saliva was prepared according to Oomen et al. (2003) excluding enzymes to evaluate chemical (non-enzymatic) degradation of ACN in saliva.
2.3.4. β-Glycosidase activity in saliva
β-D-glycosidases activity in saliva was examined by incubating (at 37 °C) 25 nmol/mL of either p-nitrophenyl galactopyranoside (pNP-gal) or glucopyranoside (pNP-glu) with intact saliva or centrifuged (800g, 5 min, 4 °C) and filtered (0.2 μm) to remove bacterial cells. Activity was quenched after incubation for 30, 45, and 60 min by placing in ice and centrifugation to remove cells. pNP in supernatant was quantified by monitoring absorbance at 405 nm compared to a five point standard curve prepared with pure pNP.
2.3.5. Stability of predicted phenolic metabolites of cyanidin-3- glycosides in saliva
The stabilities of protocatechuic acid (PCA) and phloroglucinol aldehyde (PGA), two predicted products of cyanidin ring fission (Kay et al., 2009; Keppler & Humpf, 2005), were examined in diluted saliva. PCA and PGA (25 nmol/mL) were added to diluted saliva with and without CkB ACN-rich extract. Tubes were incubated at 37 °C for 60 min before analysis. To investigate the production of PCA and PGA from anthocyanidin aglycone, cyanidin chloride (CyCl) was incubated (37 °C, 60 min) in saliva (25 nmol/mL diluted saliva) before HPLC analysis.
2.3.6. HPLC–PDA analysis
Compounds were analysed by HPLC (Bonerz, Nikfardjam, & Creasy, 2008). The HPLC system consisted of a Waters 2695 separation module (Waters Corp., Milford, MA) and aWaters 2996 photodiode array detector (PDA). Separation was achieved using a Symmetry C-18 reverse-phase column (4.6 × 75 mm; particle size 3.5 μm) with Phenomenex Security Guard™ column (C-18 packing; 4 × 3.0 mm; Phenomenex, Torrance, CA). The mobile phase consisted of (A) water/phosphoric acid (99.5:0.5; v/v) and (B) acetonitrile/ water/phosphoric acid (50:49.5:0.5; v/v/v) as follows: 0– 2 min, 0% B; 2–7 min, 0–20% B; 7–25 min, 20–40% B; 25–31 min, 40%B; 31–35 min, 40–80% B; 35–40 min, 80–100% B; 40–42 min, 100–0% B, 42–45 min, 0% B. Flow rate of mobile phase was 0.4 mL/min. ACN, phloroglucinol aldehyde (PGA), and protocatechuic acid (PCA) were monitored at 520, 292, and 259 nm, respectively. Identification of ACN was accomplished by comparing order of elution, and spectral characteristics of ACN to previous literature and later confirmed by HPLC–PDA–ESI–MS data. Quantification was achieved by comparison with five-point calibration curves obtained with pure cyanidin chloride, PCA and PGA.
2.4. Aim 2: Effects of ACN structure and oral microbiota on extent of salivary degradation in human subjects
2.4.1. Preparation of ACN-rich extracts
Five fruits (BlkRB, BluB, CkB, RdGrp, and StwB) were selected for investigation based on their distinct ACN profiles, to facilitate comparison of the susceptibility of all 6 anthocyanidins and their derivatives conjugated to either mono-, di- or tri-saccharides (Table 1). Frozen fruits were thawed at 4 °C overnight and homogenised in a commercial blender at <10 °C. ACN were extracted from homogenised fruits and CkB juice with an equal volume of acetone and filtered (Whatman #1). After removal of the organic phase, the residual material was extracted two additional times with an equal volume of acetone:water mixture (70:30 v/v).. Hydrophobic compounds in the extract were extracted into chloroform (2:1 v/v) (Rodriguez-Saona & Wrolstad, 2005). Anthocyanins in the crude extracts were further enriched using cation exchange columns: Oasis® MCX SPE cartridge (Waters Corp.) for CkB, and STRATA™ X-C cartridge (Phenomenex), for BlkRB, BluB, RdGrp, and StwB, according to He and Giusti (2011). The columns were conditioned with 10 mL methanol and 10 mL 0.1% (v/v) trifluoroacetic acid (TFA) prior to addition of extracts. Neutral or anionic phenolics were eluted with 10 mL water (0.1% TFA) and 10 mL methanol (0.1% TFA). ACN were then eluted with 5 mL water: methanol (40:60 v/ v) containing 1% NH4OH followed by methanol with 1% NH4OH into a flask containing 500 μL formic acid (88%) to achieve a final pH < 2. Remaining salts were removed by C18 solid-phase extraction as described in Section 2.3.1. Monomeric ACN content was estimated by the differential pH method (Giusti & Wrolstad, 2005) as Cy-3-glu equivalents (extinction coefficient of 26,900 L cm−1 mg−1) for BlkRB, BluB, CkB, and RdGrp, and Pg-3-glu equivalents (extinction coefficient of 15,600 L cm−1 mg−1) for StwB. Profile of ACN species in final extract was determined by HPLC–PDA–ESI–MS as described in Section 2.4.4. Stock ACN rich extracts were stored at −20 °C until use.
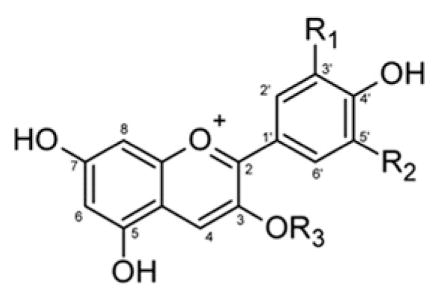
Table 1.
Structure and identification of ACN in ACN-rich extracts. Numbers correspond to HPLC peak assignments shown in chromatograms presented in Figure S1, Supplementary data.
| ||||||
|---|---|---|---|---|---|---|
| Peak | Identification | R1 | R2 | R3 | Absorbance maxima (nm) | Most abundance mass in SCAN, SIM (m/z) |
| 1 | Cyanidin-3-O-sambubioside | OH | H | D-glucose-D-xylose | 514, 277 | 518, 287 |
| 2 | Cyanidin-3-O-glucoside | OH | H | D-glucose | 514, 278 | 449, 287 |
| 3 | Cyanidin-3-O-(2G-xylosyl rutinoside) | OH | H | D-glucose-L-rhamnose-xylose | 520, 279 | 727, 287 |
| 4 | Cyanidin-3-O-rutinoside | OH | H | D-glucose-L-rhamose | 516, 279 | 595, 287 |
| 5 | Delphinidin-3-O-galactoside | OH | OH | Galactose | 524, 275 | 465, 303 |
| 6 | Delphinidin-3-O-glucoside | OH | OH | D-glucose | 524, 244 | 465, 303 |
| 7 | Cyanidin-3-O-galactoside | OH | H | Galactose | 517, 278 | 449, 287 |
| 8 | Delphinidin-3-O-arabinoside | OH | OH | Arabinose | 524, 275 | 435, 303 |
| 9 | Petunidin-3-O-galactoside | O–Me | OH | Galactose | 526, 275 | 479, 317 |
| 10 | Cyanidin-3-O-arabinoside | OH | H | Arabinose | 518, 278 | 419, 287 |
| 11 | Petunidin-3-O-glucoside | O–Me | OH | D-glucose | 525, 271 | 479, 317 |
| 12 | Peonidin-3-O-galactoside | O–Me | H | Galactose | 518, 276 | 463, 301 |
| 13 | Petunidin-3-O-arabinoside | O–Me | OH | Arabinose | 526, 275 | 449, 317 |
| 14 | Peonidin-3-O-glucoside | O–Me | H | D-glucose | 518, 275 | 463, 301 |
| 15 | Malvidin-3-O-galactoside | O–Me | O–Me | Galactose | 526, 275 | 493, 331 |
| 16 | Peonidin-3-O-arabinoside | O-Me | H | Arabinose | 525, 271 | 433, 301 |
| 17 | Malvidin-3-O-glucoside | O–Me | O–Me | D-glucose | 525, 275 | 493, 331 |
| 18 | Malvidin-3-O-arabinoside | O–Me | O–Me | Arabinose | 528, 275 | 463, 331 |
| 19 | Cyanidin-3-O-xyloside | OH | H | Xylose | 515, 278 | 419, 287 |
| 20 | Pelargonidin-3-O-glucoside | H | H | D-glucose | 501, 268 | 433, 271 |
| 21 | Pelargonidin-3-O-rutinoside | H | H | D-glucose-L-rhamose | 502, 275 | 579, 271 |
2.4.2. Ex vivo assessment of ACN degradation in saliva
Since salivary composition and flow rate are affected by circadian rhythm (Dawes, 1974), brushing teeth (Hoek, Brand, Veerman, & Amerongen, 2002), and consumption of food and beverage (Harthoorn, Brattinga, Van Kekem, Neyraud, & Dransfield, 2009), unstimulated saliva was collected from human subjects (n = 14) at 7–8 a.m. before brushing their teeth and consuming foods or beverages. Samples were placed in ice and delivered to the laboratory in <2 h for treatment and analyses. Ex vivo degradation was evaluated as described in Section 2.3.2. In a subsequent experiment, saliva was collected before and after subjects rinsed the mouth with 30 mL of antibacterial chlorhexidine (Periogard® chlorhexidine gluconate oral rinse, 0.12%) for 5 min to determine the contribution of oral bacteria to the observed degradation of ACN in saliva. Aliquots of diluted saliva were incubated with individual ACN-rich extract (50 nmol of either Cy-3-glu equivalents for BlkRB, BluB, CkB, and RdGrp or Pg-3-glu equivalents for StwB) per mL saliva as outlined in Section 2.3.2.
2.4.3. Chemical degradation of ACN
Three different formulations of enzyme-free artificial saliva (AS) were prepared. These included (1) AS1, as described by Oomen et al. (2003) but excluding enzymes; (2) AS1-org, i.e., AS1 lacking mucin, urea, and uric acid; and (3) AS2 according to Wong and Sissons (2001) with a mucin concentration at 0.3 g/L (Rayment, Liu, Offner, Oppenheim, & Troxler, 2000). The compositions of the three formulations of artificial saliva are shown in Table S1). Each ACN-rich extract was incubated (37 °C, 60 min, 25 nmol/mL saliva) in each of the three preparations of artificial saliva to determine extent of degradation.
2.4.4. HPLC–PDA–ESI–MS analysis of ACN
Separation, identification and quantification of compounds were performed using a Shimadzu LCMS-2010 EV HPLC–MS (Shimadzu Scientific Instruments, Inc., Columbia, MD) connected to an SPD-M20A photodiode array (PDA) detector and single quadrupole electrospray ionisation (ESI) mass spectrometer (MS). Separation was achieved on a Symmetry C-18 reverse-phase column (4.6 × 75 mm; 3.5 μm; Waters Corp.) with a NovaPak® (C-18; 4 × 2.0 mm) guard column. The mobile phase consisted of (A) 4.5% formic acid and (B) acetonitrile and the following gradients were used for the indicated ACN-rich extracts: BlkRB: 0–3 min, 7–8% B; 3–10 min, 8–10% B; BluB: 0–5 min, 7% B; 5–7 min, 7–8% B; 7–18 min, 8–10% B; 18–23 min; 10–15% B; CkB: 0–3 min, 7–10% B; 3–10 min, 10–20% B; RdGrp: 0–3 min, 7% B; 3–4 min, 7–12% B; 4–6 min, 12% B; 6–13 min, 12–35% B; and, StwB: 0–3 min, 7–10% B; 3–10 min, 10–15% B. Flow rate was 0.5 mL/min for all separations and the column was equilibrated for 5 min between runs. Spectroscopic data (200–700 nm) were collected during the whole run.
Flow was partially split to MS to monitor mass of parent and daughter (aglycone) fragments. MS conditions were as follows: nebulising nitrogen gas flow, 1.5 L/min; interface, +4.50 kV; heat block temperature, 200 °C; focus lens, −2.5 V; entrance lens, −50.0 V; pre-rod bias, −3.6 V; main-rod bias, −3.5 V; detector, +1.50 kV. MS spectra were obtained using positive ionisation with full scan (from m/z 100–1200 with scan speed 2000 amu/s) and selective ion monitoring (SIM) modes (6 aglycones pg, cy, pn, dp, pt, and mv at m/z 271, 287, 301, 303, 317, and 331, respectively).
2.5. Data analysis
A minimum of three independent incubations of each ACN-rich extract in saliva was performed for each subject. Data were analysed for statistically significant differences using SPSS Release 17.0 for Windows (SPSS Inc., Chicago, IL). Mixed model analysis of ACN structure (fixed factor) and subject (random factor) with Bonferroni adjustment of mean comparison was used for data with BluB, CkB, and RdGrp. Paired-t test was used for data of BlkRB and StwB. Differences were considered significant at p < 0.05.
3. Results and discussion
3.1. Characterisation of ACN-rich extracts
ACN in the five fruit extracts were identified by comparison of elution order, peak spectra, and molecular weight of parent and daughter ions obtained with published literature [BlkRB (Tian, Giusti, Stoner, & Schwartz, 2005), BluB (Seeram et al., 2006; Tian et al., 2005), CkB (Wu, Gu, Prior, & McKay, 2004; Zheng & Wang, 2003), RdGrp (Cai et al., 2010; Castillo-Muñoz, Fernández-González, Gómez-Alonso, García-Romero, & Hermosín-Gutiérrez, 2009; Frank, Netzel, Strass, Bitsch, & Bitsch, 2003), and StwB (Buendia et al., 2010; Carkeet, Clevidence, & Novotny, 2008; Felgines et al., 2007)] (Table 1 and Fig. S1, Supplementary data). HPLC-PDA-ESI-MS analysis of the 5 ACN-rich extracts confirmed the collective presence of 6 anthocyanidin classes and mono-, di-, and tri-saccharide conjugates. Estimated purity of CkB ACN-rich extract used for Aim 1 was 81.1 ± 0.4% based on HPLC AUC. Estimated purity of ACN-rich extracts used in Aim 2 for BlkRB, BluB, CkB, RdGrp, and StwB was 95.4 ± 1.9, 98.9 ± 1.4, 98.2 ± 0.8, 71.2 ± 6.5, and 85.5 ± 0.7%, respectively, based on AUC.
3.2. Aim 1: Characterisation of ex vivo degradation of ACN using chokeberry (CkB) extract
3.2.1. Ex vivo assessment of ACN degradation in saliva
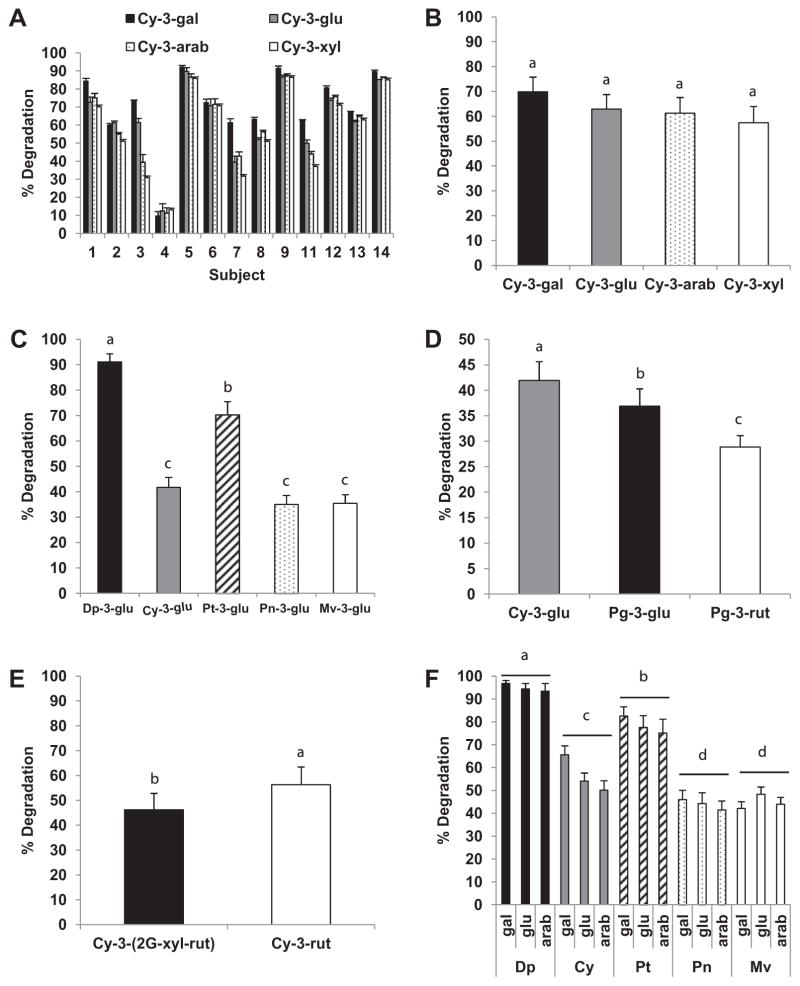
We first examined the extent of degradation of ACN in CkB extract in saliva from a single subject. The extent of degradation of the four cyanidin glycosides increased in a time-dependent manner during the 60 min incubation at 37 °C (Fig. 1A). Approximately 50% of each of the four cyanidin glycosides was degraded after 60 min incubation, suggesting limited impact of type of monosaccharide on extent of ex vivo degradation (Fig. 1B). Variability of extent of CkB ACN degradation in saliva collected on three different days was limited (45.9–52.1% degradation) during 60 min incubation (45.9–52.1% degradation). In all subsequent experiments ACN were incubated in saliva for 60 min to allow more precise assessment of the extent of degradation. Indeed, confectionaries and oral adhesive gels containing bioactive compounds are being developed to prolong oral retention of bioactive compounds (Amoian, Moghadamnia, Barzi, Sheykholeslami, & Rangiani, 2010; Mallery et al., 2007).
Fig. 1.
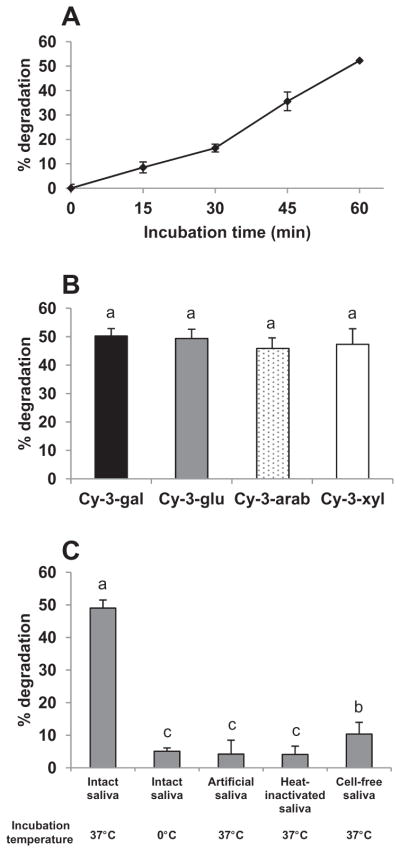
Ex vivo degradation of chokeberry ACN-rich extract in human saliva is primarily enzyme-mediated. Panel A: Time-dependent degradation of total ACN from chokeberry during 60 min incubation. Data are mean ± SD, n = 3. Panel B: Degradation (%) of cyanidin glycosides in chokeberry after incubation (60 min, 37 °C, 85 rpm) with intact saliva from a human subject. Data are mean ± SD, n = 8. There was no significant difference in extent of degradation of the four cyanidin glycosides (p≥0.05). Panel C: Degradation (%) of cyanidin glycosides in chokeberry incubated with saliva from a human subject after indicated treatments. Data are mean ± SD, n = 6. Means not sharing common superscript letters differ significantly (p < 0.05).
Proline-rich proteins, a major class of proteins in saliva, can bind ACN (Laurent et al., 2007; Proctor, Pramanik, Carpenter, & Rees, 2005). This suggested that the loss of ACN following addition to saliva may reflect association of ACN with proline-rich proteins precipitated by addition of acidified methanol at 60 min to terminate reactions. Acetonitrile was used previously to separate polyphenols from more hydrophilic compounds such as sugars and proteins in plasma and saliva (Laurent et al., 2007; Lee et al., 2000). The precipitate resulting from addition of acidic methanol to salivary samples containing ACN lacked evident colour. Mixing the precipitate with acetonitrile yielded an additional 1–3% of initial amount of ACN added to saliva, suggesting that the loss of ACN during incubation was not primarily due to association with precipitated salivary proteins.
ACN have been reported to spontaneously degrade at neutral and alkaline pH in aqueous solutions (Brouillard, 1982) and cell culture media (Kay et al., 2009), and are enzymatically degraded during in vitro digestion (McDougall, Dobson, Smith, Blake, & Stewart, 2005) and incubation with colonic microflora (Keppler & Humpf, 2005) and Caco-2 human intestinal epithelial cells (Kay et al., 2009). To examine the relative extent of spontaneous versus enzymatic degradation of CkB ACN, aliquots of saliva were either incubated with CkB ACN at 0 °C and 37 °C, heated to 80 °C to inactivate enzymes before incubating with CkB ACN at 37 °C, or centrifuged and filtered to remove cells before incubation with CkB ACN extract at 37 °C. ACN degradation in saliva during incubation at 0 °C was approximately 90% less than that during incubation at 37 °C (Fig. 1C). Similarly, loss of cyanidin glycosides during incubation at 37 °C in heat-inactivated saliva, or in cell-free saliva or in enzyme-free artificial saliva was significantly less than that in intact saliva. Collectively, these data suggest that loss of CkB ACN in saliva was primarily enzymatic and dependent on cellular activity rather than secretions from the salivary glands or binding of CkB ACN to salivary proteins.
3.2.2. Degradation products of chokeberry cyanidin glycosides in saliva
Protocatechuic acid (PCA) and phloroglucinol aldehyde (PGA) have been identified as microbial metabolites accounting for as much as 20% of the observed loss of cyanidin glycosides during incubation with human and pig faeces (Aura et al., 2005; Keppler & Humpf, 2005). It has been proposed that microbial β-D-glycosidase activity generated the anthocyanidin aglycone that was spontaneously cleaved to phenolics PCA and PGA (Kay et al., 2009). We investigated whether the loss of CkB cyanidin glycosides in saliva was associated with generation of cyanidin aglycone and its phenolic products. Neither cyanidin aglycone, PCA or PGA were detected after incubation of CkB ACN in intact saliva at 37 °C (detection limit = 3.6, 2.0 and 1.8 ng/mL, respectively), despite the presence of β-D-galactosidase and β-D-glucosidase activities in intact saliva (data not shown). Cyanidin aglycone (25 nmol/L CyCl) was extensively degraded (>90%) upon addition to intact saliva with further loss during the next 60 min (Fig. 2A). PCA was detected in response to the loss of CyCl, although it only accounted for 15.3% and 9.6% of the degraded CyCl at 0 and 60 min, respectively (Fig. 2B). Greater than 86% of CyCl was recovered immediately after addition to acidified water, although greater than 88% was lost after incubation for 60 min (Fig. 2A). PCA in acidified water accounted for much of the degraded CyCl at 0 min, but only 14.5% of that degraded after 60 min (Fig. 2B). Mallery et al. (2011) reported that a very low level of cyanidin aglycone was detected 5 min after a 3-min oral rinse with 10% black raspberry water and was undetectable at 60 min post rinse. Our detection of some cyanidin aglycone and PCA during ex vivo incubation of cyanidin aglycone, but not cyanidin glycosides, suggests that aglycone formation was not a primary intermediate in the degradation of cyanidin glycosides in saliva. To test the possibility that generated PCA and PGA might be further metabolised in saliva, PCA and PGA were added to intact saliva followed by incubation at 37 °C for 60 min. Recoveries of PCA and PGA were 90% and 43%, respectively (Fig. 2C), which is similar to the observed stability of PCA during in vitro incubation with human faecal microflora (Fleschhut, Kratzer, Rechkemmer, & Kulling, 2006) suggesting that PCA should be readily detected if generated in saliva. Mallery et al. (2011) reported the presence of PCA and its glucuronidated metabolites in saliva from some subjects for as long as several hours after rinsing their mouths with black raspberry preparation suspended in water for 3 min. These authors suggested that PCA and its glucuronidated metabolites may be generated after uptake and metabolism of BlkRB ACN by the oral epithelium.
Fig. 2.
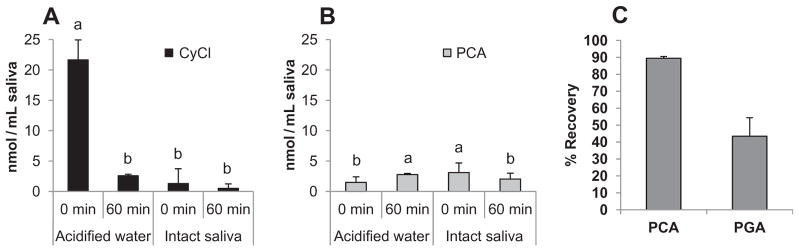
Protocatechuic acid (PCA) and phloroglucinol aldehyde (PGA) are not primary degradation products of cyanidin glycosides in saliva. Panel A: Degradation of cyanidin chloride upon addition to and incubation in acidified water and human saliva. Panel B: Minimal PCA accumulates in acidified water and saliva during degradation of cyanidin chloride. Samples were incubated in saliva at 37 °C for 0 and 60 min. Data are mean ± SD, n≥3. The presence of different superscript above error bars denotes significant (p < 0.05) differences. Panel C: Recovery of exogenous PCA and PGA after incubation in human saliva at 37 °C for 60 min. Data are mean ± SD, n = 6.
Preliminary analysis of metabolic products using high resolution quadrupole time-of-flight mass spectrometry suggested that chalcone glucosides of Cy may account for as much as 30% of the original amount of standard Cy-3-glu incubated in saliva ex vivo. This observation is in line with an alternative degradation pathway proposed by Markakis (1974) that the heterocyclic C-ring of carbinol glycosides can be opened to yield colourless chalcone prior to hydrolysis of the glycosidic bond. It is interesting that several chalcone flavonoids structurally resembling Cy chalcone glycosides have reported bioactivity. For example, butein (3,4,2′,4′-tetrahydroxychalcone) was recently shown to suppress the activity of transcription factor NF-κB in several cancer cell lines (Chua et al., 2010; Moon, Choi, Moon, Kim, & Kim, 2010) and prevent cytokine-induced nitric oxide production in rat pancreatic β cells (Jeong et al., 2011). As a result, it is possible that unidentified metabolites generated in the oral cavity are bioactive, warranting further examination of the degradation products of CkB.
3.3. Aim 2: Effect of ACN structure and oral microbiota on extent of ex vivo salivary degradation
3.3.1. Effect of structure of ACN and inter-subject variation
ACN-rich extracts from BluB, CkB, BlkRB, RdGrp, and StwB were incubated in saliva collected from 14 healthy human subjects. The extracts from these fruits include 6 anthocyanidin aglycones covalently linked to mono-, di-, and tri-saccharides. All 21 identified ACN in the extracts were partially or completely degraded during incubation in saliva from all subjects (Fig. S1, Supplementary data).
The extent of degradation of total ACN incubated in saliva from the different subjects varied considerably, i.e., 45–75% for BluB, 10– 90% for CkB, 10–80% for BlkRB, 8–60% for RdGrp, and 13–60% for StwB. The pattern of degradation of CkB ACN in saliva for individual subjects is presented in Fig. 3A. At least 50% CkB ACN were degraded in saliva from the majority of the subjects. Although mean degradation of the four different cyanidin glycosides was similar in saliva from 13 subjects (p = 0.560; Fig. 3B), the extent of degradation of the galactoside and glucoside conjugates exceeded that of arabinoside and xyloside conjugates in saliva from subjects 3, 7 and 11. These observations suggest inter-subject variability in both extent and pattern of cyanidin glycoside degradation in saliva.
Fig. 3.
Ex vivo degradation of ACN in saliva is variable among donors and affected by ACN structure. Panel A: Inter-subject variability in extent of degradation of chokeberry ACN after incubation (60 min, 37 °C) in saliva (n = 13). Data are mean ± SE of four replicate samples. Salivary degradation (%) of ACN from chokeberry (Panel B, n = 13), red grape (Panel C, n = 14), strawberry (Panel D, n = 14), black raspberry (Panel E, n = 10), and blueberry (Panel F, n = 11). Data are mean ± SE. Means not sharing common letters above bars within a panel differ significantly (p < 0.05).
RdGrp was selected to compare the influence of the structures of anthocyanidins on the extent of ACN degradation in saliva. The five anthocyanidins (i.e., Dp, Cy, Pt, Pn, and Mv) in RdGrp are all conjugated with glucose at the 3′ position (Fig. S1, Supplementary data). Degradation of Dp and Pt glucosides exceeded that of Cy, Pn and Mv (p < 0.001; Fig. 3C). This pattern of degradation was consistent in all 14 subjects. The extent of degradation among subjects for each ACN in RdGrp ranged from 60–100% (Dp), 7–60% (Cy), 65–100% (Pt), 5– 60% (Pn), and 5–55% (Mv) (data not shown).
ACN-rich extract from StwB was investigated to assess the effect of linkage of a monosaccharide (Pg-3-glu) versus a disaccharide (Pg-3-rut) to the anthocyanidin on susceptibility to degradation in saliva. Degradation of Pg-3-rut was significantly (p < 0.001) less than that of Pg-3-glu (Fig. 3D). Slightly, but significantly (p < 0.001), less degradation of Pg-3-glu compared to Cy-3-glu also was observed, further suggesting differential susceptibility associated with anthocyanidin structure (Fig. 3D).
To further examine the possible influence of more complex carbohydrates moieties on susceptibility to degradation in saliva, ACN-rich extract from BlkRB was tested. Although the presence of Cy-3-samb and Cy-3-glu was confirmed by mass spectrometry (Table 1), these compounds were not adequately separated by HPLC (Fig. S1, Supplementary data) and therefore excluded from this comparison. Mean degradation of the trisaccharide (xylosylrotinoside) conjugate of cyanidin for all subjects was significantly (p < 0.001) lower than degradation of the disaccharide rutinose Cy conjugate (Fig. 3E). Similarly, quercetin di- and tri-saccharides and cyanidin di- and tri-saccharides have been reported to be less susceptible to degradation than their respective monosaccharide conjugates (Keppler & Humpf, 2005; Walle et al., 2005).
BluB extract was tested to compare the influence of structure for five anthocyanidins linked to three different monosaccharides on the extent of degradation in saliva (Fig. 3F). A mixed model statistical analysis revealed that degradation was significantly affected by type of anthocyanidin (p < 0.001), but not by type of monosaccharide (p = 0.063) or the interaction between ACN and monosaccharide (p = 0.495). As observed with RdGp (Fig. 3C), degradation of Dp and Pt-3-glucosides was significantly (p < 0.001) greater than that of the 3-glucose conjugates of Cy, Pn and Mv in saliva from all subjects (data not shown). Also, as observed with cyanidin glycosides in CkB extract (Fig. 3A), the extent of degradation of the anthocyanidin in BluB extract was not significantly altered by type of monosaccharide for all but three subjects (numbers 3, 7 and 11). Degradation of the hexose conjugates exceeded that of the pentose conjugates in saliva from these three subjects.
The extent of degradation of a specific ACN in saliva appeared to differ across the different extracts. For example, mean degradation of Cy-3-glu in CkB was 63% as compared to 54% degradation in BluB and only 42% degradation, in RdGrp and StwB (Fig. 3). We suspect that the presence of multiple ACN and possibly contaminating phenolics may affect the stability of ACN via co-pigmentation (Eiro & Heinonen, 2002; Malien-Aubert, Dangles, & Amiot, 2001), i.e., chemical interactions within different extracts.
Glucuronidated derivatives of PCA have been detected in saliva collected 5 min after an oral rinse with 10% BlkRB slurry, suggesting that phenolic metabolites of anthocyanins were conjugated within and effluxed from the oral epithelium (Mallery et al., 2011). We did not detect either PCA or PGA after incubation of ACN extracts in saliva from any subject. Similarly, treatment of post-incubation mixtures in which ACN degradation was high (saliva from subjects 1 and 5 for BluB, subjects 1, 5 and 9 for CkB, subjects 1 and 9 for BlkRB, subjects 1 and 5 for RdGrp, and subjects 5 and 9 for StwB) with H. pomatia enzyme preparation containing glucuronidase and sulfatase activities for 3 h at 37 °C also failed to generate detectable PCA and PGA. As the limit of detection of our analytical system was an order of magnitude less than that of the Mallery team (Mallery et al., 2011), it is possible that low concentrations of these metabolites may have been present. Interestingly, a peak corresponding to 4-hydroxybenzoic acid (retention time and absorbance spectrum) was observed following enzyme treatment of saliva incubated with CkB, BlkRB, BluB and StwB extracts.
3.3.2. Non-enzymatic degradation of Dp and Pt glycosides
To address the basis for the greater susceptibility of Dp and Pt glycosides to degradation in saliva compared to that of the other anthocyanins, ACN-rich extract from RdGrp was first incubated in water and enzyme-free artificial saliva (Fig. 4A). RdGrp ACN degraded to a significantly (p < 0.001) greater extent in artificial saliva (pH 6.4) than in DI water (pH 7.3). This observation is similar to a recent report that Dp-3-glu, Pg-3-glu, and Cy-3-glu were less stable in Na/K phosphate buffer than in water (Woodward, Kroon, Cassidy, & Kay, 2009). Moreover, degradation of Dp and Pt-3-glucosides in enzyme-free artificial saliva was significantly (p < 0.001) greater than that of Cy, Pn and Mv-3-glucosides and further confirms that the losses during incubation were not due to protein binding. This increased susceptibility of Dp and Pt glycosides to non-enzymatic degradation in artificial saliva was also observed with ACN-rich extract from BluB (data not shown) and was aligned with their preferential degradation in intact saliva (Fig. 3C and F). Removal of organic compounds from the enzyme-free artificial saliva did not further affect (p > 0.05) the extent of degradation of RdGrp ACN, suggesting that mucin, urea, and uric acid do not contribute to the relative instability of Dp and Pt glycosides (Fig. 4B). In contrast, differences in the inorganic contents of artificial saliva preparations AS1 (Na2SO4, NaOH, KSCN, and NaH2PO4) and AS2 (MgCl2, CaCl2, NH4Cl, KH2PO4, and K2HPO4) significantly (p < 0.001) affected the extent of degradation of Dp and Pt in RdGrp extracts (Fig. 4B), suggesting that one or more electrolytes (Table S1, Supplementary data) in saliva may affect the stability of the Dp and Pt glucosides. Cabrita et al. reported that Dp and Pt glucosides are less stable than Cy, Mv, and Pn glucosides in aqueous buffer at pH > 7 (Cabrita, Fossen, & Andersen, 2000). Dp-3-glu and Dp-3-rut were also reported to degrade more rapidly than Cy-3-glu and Cy-3-rut in Dulbecco’s modified Eagle’s medium (Steinert, Ditscheid, Netzel, & Jahreis, 2008). Interestingly, susceptibility to degradation appears to be related to superoxide radical scavenging potential of ACN glycosides, as degradation of Dp > Pt > Cy ≈ Mv > Pn > Pg (Rahman, Ichiyanagi, Komiyama, Hatano, & Konishi, 2006). Dp-3-glu also possesses greater antioxidant activity than Cy-3-glu in the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay (Kahkonen & Heinonen, 2003). Such results suggest that oxidative degradation of ACN, and especially Dp glycosides, may contribute to the observed losses in saliva. As Dp and Pt, but not Cy, Pn and Mv, are hydroxylated at the C5′ position of the B-ring, the possible influence of this substitution on stability in saliva merits investigation.
Fig. 4.
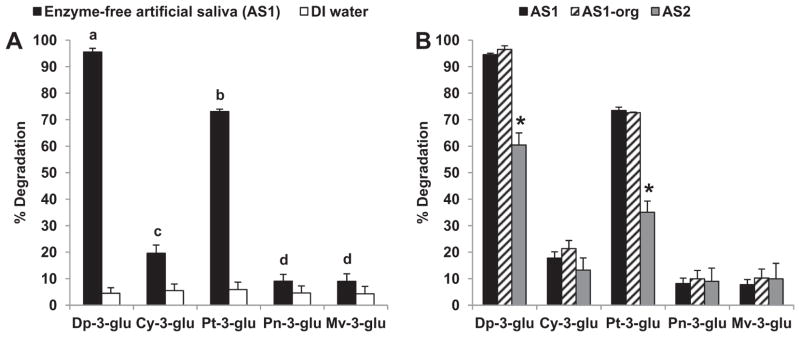
Dp and Pt glucosides are preferentially degraded in artificial saliva. Panel A: Degradation of Dp and Pt is greater than Cy, Pt and Mv in enzyme-free artificial saliva. Data are mean ± SD, n = 6. Presence of different letter above error bars for ACN in enzyme-free artificial saliva are significantly different (p < 0.05). Panel B: The electrolyte composition of artificial saliva affects the stability of Dp and Pt glucosides. Data are mean ± SD, n = 3. The asterisks above error bars denote mean for AS2 differs significantly from AS1 and AS1-org (p < 0.05).
3.3.3. Effect of oral microbiota on salivary degradation of ACN
To investigate the possible role of oral microbiota in the ex vivo degradation of ACN, saliva was collected before and after oral rinse with antibacterial chlorhexidine. Antibacterial treatment significantly (p < 0.001) decreased the degradation of ACN extracted from RdGrp, StwB, and CkB, in saliva by 49%, 60% and 80%, respectively (Fig. 5). The degree of inhibition was not affected (p > 0.05) by type of conjugated monosaccharide (Fig. 5A) or number of saccharide units of Pg (Fig. 5C). In contrast, type of aglycone in RdGrp ACN significantly (p < 0.001) affected the impact of treatment with the antibacterial chlorhexidine on the extent of degradation (Fig. 5B). Degradation of Pt-3-glu in saliva after antibacterial treatment decreased by 10%, which was significantly (p < 0.05) less than that of Dp (30%), Cy (40%), Pn, and Mv (50%). Thus, degradation of glucosides of Dp, Cy, Pn, and Mv was more closely associated with microbial activity than was that of Pt glucosides. Our observation in saliva contrasts with the report of Forester and Waterhouse (2008), who did not observe preferential degradation of Dp, Pt, Pn, and Mv glucosides during incubation with large intestinal contents from swine. Given the unique profile of microbiota residing in the various sections of GI tract (Kerckhoffs et al., 2006), further characterisation of oral microbial activity on ACN metabolism by the oral microbial community merits future investigation.
Fig. 5.
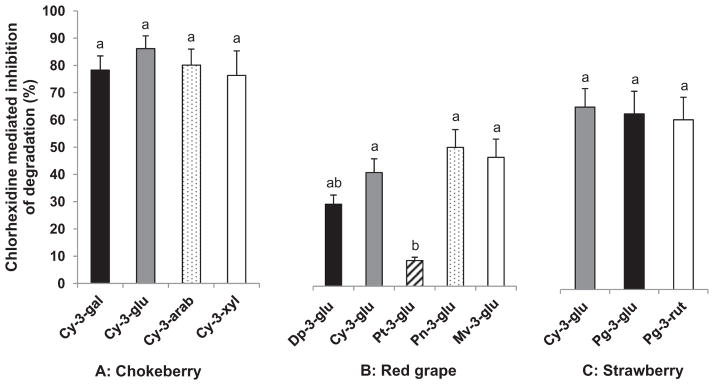
Chlorhexidine oral rinse suppresses ex vivo degradation of ACN in saliva. Data are mean ± SE for eight human subjects with four replicate samples per ACN extract. Means not sharing common letters above bars within same extract are significantly different at p < 0.05.
4. Conclusions
To the best of our knowledge, this is the first examination of the effect of ACN structure on susceptibility to degradation in saliva. ACN from five dietary sources containing a total of 6 anthocyanidins conjugated to mono-, di- and tri-saccharides were partially degraded during incubation in human saliva at 37 °C. Degradation was in part due to microbial activity, although non-enzymatic degradation of Dp and Pt exceeded that of Cy, Pn and Mv. Disappearance of Cy glycosides or Cy aglycone was not coupled with generation of detectable amounts of PCA or PGA. Preliminary detection of chalcone glycosides of Cy suggests a degradation pathway of ACN in oral cavity that may differ from that in the large intestine. Moderate inter-subject variation in the extent of degradation was observed, but the general patterns of degradation were rather consistent among individuals. While types of monosaccharide did not affect the extent of degradation, anthocyanidins with di- and trisaccharide conjugates were slightly, but significantly, less susceptible to degradation in saliva. These data collectively suggest that ACN structure affects susceptibility to degradation and perhaps the relative bioaccessibility and efficacy of these compounds in the oral cavity. Studies to assess this possibility have been initiated.
Supplementary Material
Acknowledgments
The project was supported in part by The Ohio Agricultural Research and Development Center (OARDC). We thank Dr. Joseph Scheerens of the OARDC for obtaining black raspberries from Maurer’s farm. We also thank Artemis International for the gift of chokeberry samples. We are grateful to Dr. Steven Schwartz of Department of Food Science and Technology for providing access to the HPLC–TOF–MS in the Nutrient and Phytochemical Analytic Shared Resource Core of the OSU Comprehensive Cancer Center (NPASR).
Abbreviations
- Arab
arabinoside
- AUC
area under curve
- BlkRB
black raspberry
- BluB
blueberry
- CkB
chokeberry
- Cy
cyanidin
- CyCl
cyanidin chloride
- ESI
electron spray ionisation
- Gal
galactoside
- Glu
glucoside
- HPLC
high performance liquid chromatography
- m/z
mass-to-charge ratio
- MS
mass spectrometer
- PCA
protocatechuic acid
- PDA
photodiode array
- PGA
phloroglucinol aldehyde
- Pg
pelargonidin
- pNP
para-nitrophenol
- RdGrp
red grape
- Rham
rhamnoside
- Rut
rutinoside
- SIM
selective ion monitoring
- StwB
strawberry
- Xyl
xyloside
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.foodchem.2012.04.110.
Contributor Information
Kom Kamonpatana, Email: kkamonpatana@ehe.osu.edu.
M. Mónica Giusti, Email: giusti.6@osu.edu.
Chureeporn Chitchumroonchokchai, Email: chitchumroonchok.1@ehe.osu.edu.
Maria MorenoCruz, Email: morenocruz.1@osu.edu.
Ken M. Riedl, Email: riedl.7@osu.edu.
Purnima Kumar, Email: kumar.83@osu.edu.
Mark L. Failla, Email: failla.3@osu.edu.
References
- Amoian B, Moghadamnia AA, Barzi S, Sheykholeslami S, Rangiani A. Salvadora persica extract chewing gum and gingival health: Improvement of gingival and probe-bleeding index. Complementary Therapies in Clinical Practice. 2010;16(3):121–123. doi: 10.1016/j.ctcp.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Aura AM, Martin-Lopez P, O’Leary KA, Williamson G, Oksman-Caldentey KM, Poutanen K, et al. In vitro metabolism of anthocyanins by human gut microflora. European Journal of Nutrition. 2005;44(3):133–142. doi: 10.1007/s00394-004-0502-2. [DOI] [PubMed] [Google Scholar]
- Bonerz DPM, Nikfardjam MSP, Creasy GL. A new RP-HPLC method for analysis of polyphenols, anthocyanins, and indole 3-acetic acid in wine. American Journal of Enology and Viticulture. 2008;59(1):106–109. [Google Scholar]
- Brouillard R. Chemical structure of anthocyanins. In: Markakis P, editor. Anthocyanins as food colors. New York: Academic Press; 1982. pp. 1–40. [Google Scholar]
- Buendia B, Gil MI, Tudela JA, Gady AL, Medina JJ, Soria C, et al. HPLC-MS analysis of proanthocyanidin oligomers and other phenolics in 15 strawberry cultivars. Journal of Agricultural and Food Chemistry. 2010;58(7):3916–3926. doi: 10.1021/jf9030597. [DOI] [PubMed] [Google Scholar]
- Cabrita L, Fossen T, Andersen ØM. Colour and stability of the six common anthocyanidin-3-glucosides in aqueous solutions. Food Chemistry. 2000;68(1):101–107. [Google Scholar]
- Cai H, Marczylo TH, Teller N, Brown K, Steward WP, Marko D, et al. Anthocyanin-rich red grape extract impedes adenoma development in the apc(min) mouse: Pharmacodynamic changes and anthocyanin levels in the murine biophase. European Journal of Cancer. 2010;46(4):811–817. doi: 10.1016/j.ejca.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Carkeet C, Clevidence B, Novotny JA. Anthocyanin excretion by humans increases linearly with increasing strawberry dose. The Journal of Nutrition. 2008;138(5):897–902. doi: 10.1093/jn/138.5.897. [DOI] [PubMed] [Google Scholar]
- Castillo-Muñoz N, Fernández-González M, Gómez-Alonso S, García-Romero E, Hermosín-Gutiérrez I. Red-color related phenolic composition of Garnacha Tintorera (Vitis vinifera L.) grapes and red wines. Journal of Agricultural and Food Chemistry. 2009;57(17):7883–7891. doi: 10.1021/jf9002736. [DOI] [PubMed] [Google Scholar]
- Casto BC, Kresty LA, Kraly CL, Pearl DK, Knobloch TJ, Schut HA, et al. Chemoprevention of oral cancer by black raspberries. Anticancer Research. 2002;22(6C):4005–4015. [PubMed] [Google Scholar]
- Chua AW, Hay HS, Rajendran P, Shanmugam MK, Li F, Bist P, et al. Butein downregulates chemokine receptor CXCR4 expression and function through suppression of NF-κB activation in breast and pancreatic tumor cells. Biochemical Pharmacology. 2010;80(10):1553–1562. doi: 10.1016/j.bcp.2010.07.045. [DOI] [PubMed] [Google Scholar]
- Cooke D, Steward WP, Gescher AJ, Marczylo T. Anthocyans from fruits and vegetables – Does bright colour signal cancer chemopreventive activity? European Journal of Cancer. 2005;41(13):1931–1940. doi: 10.1016/j.ejca.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Dawes C. Rhythms in salivary flow rate and composition. International Journal of Chronobiology. 1974;2(3):253–279. [PubMed] [Google Scholar]
- Dawes C. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. Journal of Dental Research. 1987;66:648–653. doi: 10.1177/00220345870660S107. [DOI] [PubMed] [Google Scholar]
- Eiro MJ, Heinonen M. Anthocyanin color behavior and stability during storage: Effect of intermolecular copigmentation. Journal of Agricultural and Food Chemistry. 2002;50(25):7461–7466. doi: 10.1021/jf0258306. [DOI] [PubMed] [Google Scholar]
- Felgines C, Texier O, Besson C, Lyan B, Lamaison J, Scalbert A. Strawberry pelargonidin glycosides are excreted in urine as intact glycosides and glucuronidated pelargonidin derivatives in rats. British Journal of Nutrition. 2007;98(6):1126–1131. doi: 10.1017/S0007114507764772. [DOI] [PubMed] [Google Scholar]
- Fleschhut J, Kratzer F, Rechkemmer G, Kulling SE. Stability and biotransformation of various dietary anthocyanins in vitro. European Journal of Nutrition. 2006;45(1):7–18. doi: 10.1007/s00394-005-0557-8. [DOI] [PubMed] [Google Scholar]
- Forester SC, Waterhouse AL. Identification of cabernet sauvignon anthocyanin gut microflora metabolites. Journal of Agricultural and Food Chemistry. 2008;56(19):9299–9304. doi: 10.1021/jf801309n. [DOI] [PubMed] [Google Scholar]
- Forester SC, Waterhouse AL. Gut metabolites of anthocyanins, gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde, inhibit cell proliferation of caco-2 cells. Journal of Agricultural and Food Chemistry. 2010;58(9):5320–5327. doi: 10.1021/jf9040172. [DOI] [PubMed] [Google Scholar]
- Frank T, Netzel M, Strass G, Bitsch R, Bitsch I. Bioavailability of anthocyanidin-3-glucosides following consumption of red wine and red grape juice. Canadian Journal of Physiology. 2003;81(5):423–435. doi: 10.1139/y03-038. [DOI] [PubMed] [Google Scholar]
- Giusti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV–visible spectroscopy. In: Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, et al., editors. Handbook of food analytical chemistry. Hoboken, New Jersey: John Wiley & Sons, Inc; 2005. pp. F1.2.1–F1.2.13. [Google Scholar]
- Giusti MM, Wrolstad RE. Acylated anthocyanins from edible sources and their applications in food systems. Biochemical Engineering Journal. 2003;14(3):217–225. [Google Scholar]
- Harthoorn LF, Brattinga C, Van Kekem K, Neyraud E, Dransfield E. Effects of sucrose on salivary flow and composition: Differences between real and sham intake. International Journal of Food Sciences and Nutrition. 2009;60(8):637–646. doi: 10.3109/09637480802039814. [DOI] [PubMed] [Google Scholar]
- He J, Giusti MM. High-purity isolation of anthocyanins mixtures from fruits and vegetables – A novel solid-phase extraction method using mixed mode cation-exchange chromatography. Journal of Chromatography A. 2011;1218(44):7914–7922. doi: 10.1016/j.chroma.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Hertog MG, Hallman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in the Netherlands. Nutrition and Cancer. 1993;20(1):21–29. doi: 10.1080/01635589309514267. [DOI] [PubMed] [Google Scholar]
- Hoek GH, Brand HS, Veerman EC, Amerongen AV. Toothbrushing affects the protein composition of whole saliva. European Journal of Oral Sciences. 2002;110(6):480–481. doi: 10.1034/j.1600-0722.2002.21370.x. [DOI] [PubMed] [Google Scholar]
- Jeong GS, Lee DS, Song MY, Park BH, Kang DG, Lee HS, et al. Butein from rhus verniciflua protects pancreatic β cells against cytokine-induced toxicity mediated by inhibition of nitric oxide formation. Biological and Pharmaceutical Bulletin. 2011;34(1):97–102. doi: 10.1248/bpb.34.97. [DOI] [PubMed] [Google Scholar]
- Kahkonen MP, Heinonen M. Antioxidant activity of anthocyanins and their aglycons. Journal of Agricultural and Food Chemistry. 2003;51(3):628–633. doi: 10.1021/jf025551i. [DOI] [PubMed] [Google Scholar]
- Kay CD, Kroon PA, Cassidy A. The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products. Molecular Nutrition & Food Research. 2009;53(Suppl 1):S92–101. doi: 10.1002/mnfr.200800461. [DOI] [PubMed] [Google Scholar]
- Keppler K, Humpf HU. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorganic & Medicinal Chemistry. 2005;13(17):5195–5205. doi: 10.1016/j.bmc.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Kerckhoffs APM, Samsom M, van Berge Henegouwen GP, Akkermans LMA, Nieuwenhuijs VB, Visser MR. Sampling micribiota in the human gastrointestinal tract. In: Ouwehand AC, Vaughan EE, editors. Gastrointestinal microbiology. New York: Taylor & Francis; 2006. pp. 25–50. [Google Scholar]
- Kuhnau J. The flavonoids. A class of semi-essential food components: Their role in human nutrition. World Review of Nutrition and Dietetics. 1976;24:117–191. [PubMed] [Google Scholar]
- Kumar PS, Brooker MR, Dowd SE, Camerlengo T. Target region selection is a critical determinant of community fingerprints generated by 16S pyrosequencing. PLoS ONE. 2011;6(6):e20956. doi: 10.1371/journal.pone.0020956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent C, Besancon P, Caporiccio B. Flavonoids from a grape seed extract interact with digestive secretions and intestinal cells as assessed in an in vitro digestion/Caco-2 cell culture model. Food Chemistry. 2007;100:1704–1712. [Google Scholar]
- Lee M, Prabhu S, Meng X, Li C, Yang C. An improved method for the determination of green and black tea polyphenols in biomatrices by high-performance liquid chromatography with coulometric array detection. Analytical Biochemistry. 2000;279(2):164–169. doi: 10.1006/abio.2000.4487. [DOI] [PubMed] [Google Scholar]
- Malien-Aubert C, Dangles O, Amiot MJ. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. protective effects by intra- and inter-molecular copigmentation. Journal of Agricultural and Food Chemistry. 2001;49(1):170–176. doi: 10.1021/jf000791o. [DOI] [PubMed] [Google Scholar]
- Mallery SR, Budendorf DE, Larsen MP, Pei P, Tong M, Holpuch AS, et al. Effects of human oral mucosal tissue, saliva, and oral microflora on intraoral metabolism and bioactivation of black raspberry anthocyanins. Cancer Prevention Research. 2011;4(8):1209–1221. doi: 10.1158/1940-6207.CAPR-11-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallery SR, Stoner GD, Larsen PE, Fields HW, Rodrigo KA, Schwartz SJ, et al. Formulation and in-vitro and in-vivo evaluation of a mucoadhesive gel containing freeze dried black raspberries: Implications for oral cancer chemoprevention. Pharmaceutical Research. 2007;24(4):728–737. doi: 10.1007/s11095-006-9192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallery SR, Zwick JC, Pei P, Tong M, Larsen PE, Shumway BS, et al. Topical application of a bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein in human premalignant oral lesions. Cancer Research. 2008;68(12):4945–4957. doi: 10.1158/0008-5472.CAN-08-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markakis P. Anthocyanins and their stability in foods. CRC Critical Reviews in Food Technology. 1974;4:437–456. [Google Scholar]
- McDougall GJ, Dobson P, Smith P, Blake A, Stewart D. Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. Journal of Agricultural and Food Chemistry. 2005;53(15):5896–5904. doi: 10.1021/jf050131p. [DOI] [PubMed] [Google Scholar]
- McGhie TK, Walton MC. The bioavailability and absorption of anthocyanins: Towards a better understanding. Molecular Nutrition & Food Research. 2007;51(6):702–713. doi: 10.1002/mnfr.200700092. [DOI] [PubMed] [Google Scholar]
- Moon DO, Choi YH, Moon SK, Kim WJ, Kim GY. Butein suppresses the expression of nuclear factor-kappa B-mediated matrix metalloproteinase-9 and vascular endothelial growth factor in prostate cancer cells. Toxicology in Vitro. 2010;24(7):1927–1934. doi: 10.1016/j.tiv.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Oomen AG, Rompelberg CJ, Bruil MA, Dobbe CJ, Pereboom DP, Sips AJ. Development of an in vitro digestion model for estimating the bioaccessibility of soil contaminants. Archives of Environmental Contamination and Toxicology. 2003;44(3):281–287. doi: 10.1007/s00244-002-1278-0. [DOI] [PubMed] [Google Scholar]
- Prior RL, Wu X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radical Research. 2006;40(10):1014–1028. doi: 10.1080/10715760600758522. [DOI] [PubMed] [Google Scholar]
- Proctor GB, Pramanik R, Carpenter G, Rees GD. Salivary proteins interact with dietary constituents to modulate tooth staining. Journal of Dental Research. 2005;84(1):73–78. doi: 10.1177/154405910508400113. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Ichiyanagi T, Komiyama T, Hatano Y, Konishi T. Superoxide radical- and peroxynitrite-scavenging activity of anthocyanins; structure-activity relationship and their synergism. Free Radical Research. 2006;40(9):993–1002. doi: 10.1080/10715760600815322. [DOI] [PubMed] [Google Scholar]
- Rayment SA, Liu B, Offner GD, Oppenheim FG, Troxler RF. Immunoquantification of human salivary mucins MG1 and MG2 in stimulated whole saliva: Factors influencing mucin levels. Journal of Dental Research. 2000;79(10):1765–1772. doi: 10.1177/00220345000790100601. [DOI] [PubMed] [Google Scholar]
- Rodrigo KA, Rawal Y, Renner RJ, Schwartz SJ, Tian Q, Larsen PE, et al. Suppression of the tumorigenic phenotype in human oral squamous cell carcinoma cells by an ethanol extract derived from freeze-dried black raspberries. Nutrition and Cancer. 2006;54(1):58–68. doi: 10.1207/s15327914nc5401_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Saona LE, Wrolstad RE. Extraction, isolation, and purification of anthocyanins. In: Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, CF, et al., editors. Handbook of food analytical chemistry. Hoboken, New Jersey: John Wiley & Sons, Inc; 2005. pp. F1.1.1–F1.1.11. [Google Scholar]
- Seeram NP, Adams LS, Zhang Y, Lee R, Sand D, Scheuller HS, et al. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. Journal of Agricultural and Food Chemistry. 2006;54(25):9329–9339. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- Shumway BS, Kresty LA, Larsen PE, Zwick JC, Lu B, Fields HW, et al. Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clinical Cancer Research. 2008;14(8):2421–2430. doi: 10.1158/1078-0432.CCR-07-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontology. 2005;2000(38):135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- Steinert RE, Ditscheid B, Netzel M, Jahreis G. Absorption of black currant anthocyanins by monolayers of human intestinal epithelial caco-2 cells mounted in using type chambers. Journal of Agricultural and Food Chemistry. 2008;56(13):4995–5001. doi: 10.1021/jf703670h. [DOI] [PubMed] [Google Scholar]
- Stoner GD, Sardo C, Apseloff G, Mullet D, Wargo W, Pound V, et al. Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. The Journal of Clinical Pharmacology. 2005;45(10):1153–1164. doi: 10.1177/0091270005279636. [DOI] [PubMed] [Google Scholar]
- Tian Q, Giusti MM, Stoner GD, Schwartz SJ. Screening for anthocyanins using high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry with precursor-ion analysis, product-ion analysis, common-neutral-loss analysis, and selected reaction monitoring. Journal of Chromatography A. 2005;1091(1–2):72–82. doi: 10.1016/j.chroma.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Vitaglione P, Donnarumma G, Napolitano A, Galvano F, Gallo A, Scalfi L, et al. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. The Journal of Nutrition. 2007;137(9):2043–2048. doi: 10.1093/jn/137.9.2043. [DOI] [PubMed] [Google Scholar]
- Walle T, Browning AM, Steed LL, Reed SG, Walle UK. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. The Journal of Nutrition. 2005;135(1):48–52. doi: 10.1093/jn/135.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LS, Stoner GD. Anthocyanins and their role in cancer prevention. Cancer Letters. 2008;269(2):281–290. doi: 10.1016/j.canlet.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L, Sissons CH. A comparison of human dental plaque microcosm biofilms grown in an undefined medium and a chemically defined artificial saliva. Archives of Oral Biology. 2001;46(6):477–486. doi: 10.1016/s0003-9969(01)00016-4. [DOI] [PubMed] [Google Scholar]
- Woodward G, Kroon P, Cassidy A, Kay C. Anthocyanin stability and recovery: Implications for the analysis of clinical and experimental samples. Journal of Agricultural and Food Chemistry. 2009;57(12):5271–5278. doi: 10.1021/jf900602b. [DOI] [PubMed] [Google Scholar]
- Wu X, Beecher G, Holden J, Haytowitz D, Gebhardt S, Prior R. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. Journal of Agricultural and Food Chemistry. 2006;54(11):4069. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- Wu X, Gu L, Prior RL, McKay S. Characterization of anthocyanins and proanthocyanidins in some cultivars of ribes, aronia, and sambucus and their antioxidant capacity. Journal of Agricultural and Food Chemistry. 2004;52(26):7846–7856. doi: 10.1021/jf0486850. [DOI] [PubMed] [Google Scholar]
- Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Molecular Nutrition & Food Research. 2007;51(6):675–683. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang SY. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. Journal of Agricultural and Food Chemistry. 2003;51(2):502–509. doi: 10.1021/jf020728u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.