Abstract
Human β defensin-3 (hBD-3) is an antimicrobial peptide with diverse functionality. We investigated the capacity of hBD-3 and, for comparison, Pam3CSK4 and LL-37 to induce co-stimulatory molecules and chemokine expression in monocytes. These stimuli differentially induced CD80 and CD86 on the surface of monocytes and each stimulant induced a variety of chemokines including monocyte chemoattractant protein 1 (MCP-1), Gro-α, macrophage-derived chemokine (MDC) and macrophage inflammatory protein 1β (MIP1β), while only hBD-3 and Pam3CSK4 significantly induced the angiogenesis factor, vascular endothelial growth factor (VEGF). Human BD-3 induced similar chemokines in monocyte-derived macrophages and additionally induced expression of Regulated upon activation normal T-cell expressed and presumably secreted (RANTES) in these cells. Comparison of monocytes from HIV+ and HIV– donors indicated that monocytes from HIV+ donors were more likely to spontaneously express certain chemokines (MIP-1α, MIP-1β and MCP-1) and less able to increase expression of other molecules in response to hBD-3 (MDC, Gro-α and VEGF). Chemokine receptor expression (CCR5, CCR2 and CXCR2) was relatively normal in monocytes from HIV+ donors compared with cells from HIV– donors with the exception of diminished expression of the receptor for MDC, CCR4, which was reduced in the patrolling monocyte subset (CD14+ CD16++) of HIV+ donors. These observations implicate chemokine induction by hBD-3 as a potentially important mechanism for orchestrating cell migration into inflamed tissues. Alterations in chemokine production or their receptors in monocytes of HIV-infected persons could influence cell migration and modify the effects of hBD-3 at sites of inflammation.
Keywords: chemokine, defensin, HIV, macrophage, monocyte
Introduction
Human β defensin-3 (hBD-3) is an inducible antimicrobial peptide that is produced by epithelial cells. This molecule mediates the killing of microbes,1 chemotaxis of CCR2+ cells such as monocytes2 and activation of antigen-presenting cells (monocytes and myeloid dendritic cells3,4). These diverse functions indicate that hBD-3 could play an important role in both innate and adaptive defences. Increased expression of hBD-3 is observed in inflammatory microenvironments including psoriasis and oral carcinoma.1,5 Because monocytes are chemoattracted by hBD-35,6 and can potentially migrate into inflamed tissues,7 it is important to consider the functional effects of hBD-3 on these cells. Our previous studies identified Toll-like receptor 1/2 (TLR1/2) -dependent signalling as a mechanism by which hBD-3 could cause activation of these cells.3 Human BD-3-mediated activation of monocytes induced expression of co-stimulatory molecules (CD80 and CD86) as well as expression of various cytokines including interleukin-6 (IL-6), IL-1β and IL-8.3,8
Other antimicrobial molecules have chemotactic properties and may also activate monocytes. For example, the cathelicidin-derived peptide, LL-37, can enhance IL-1β release from lipopolysaccharide-primed monocytes via a P2X7-dependent mechanism and can also induce the production of monocyte chemoattractant protein-1 (MCP-1) chemokine from these cells.9 LL-37 is also reported to influence monocyte maturation, potentially resulting in cells with more pro-inflammatory characteristics.10
To further assess the effects of antimicrobial peptides on monocytic cells, we examined the induction of co-stimulatory molecules, CD80 and CD86, as well as an array of chemokines by hBD-3, LL-37 and a well-defined Toll-like receptor 1/2 (TLR1/2) agonist, PAM3CSK4. In addition, we asked if chemokine induction by hBD-3 might be diminished in cells from HIV+ donors because we have previously found evidence for decreased induction of CD80 in cells from HIV+ donors compared with cells from healthy controls.11 Our results suggest that hBD-3 activation of monocytic cells could play an important role in orchestrating inflammatory microenvironments by inducing chemokine expression and this activity may be modified in HIV disease.
Materials and methods
Donors and cells
Cells were obtained from healthy adult volunteers and HIV+ donors with IRB-approved protocols and informed consent. For chemokine production studies, the HIV+ donors consisted of three viraemic and six aviraemic subjects. Purified monocytes were prepared with EasySep monocyte isolation kits (STEMCELL Technologies) and achieved > 85% purity. Monocyte-derived macrophages were generated by incubating cells with 100 ng/ml macrophage colony-stimulating factor (M-CSF) for 7 days. Cells were incubated in complete medium consisting of RPMI 10% fetal calf serum plus l-glutamine.
For studies of chemokine receptor expression, freshly isolated peripheral blood mononuclear cells (PBMC) were stained with anti-CD14 Peridinin chlorophyll protein (PerCP; BD Biosciences, San Jose, CA), anti-CD16 allophycocyanin-chychrom 7 (APC-Cy7; Biolegend, San Diego, CA), anti-CCR5 APC (BD Pharmingen), anti-CCR2 PerCP Cy5.5 (Biolegend), anti-CXCR2 FITC (Biolegend) and anti-CCR4 phycoerythrin-Cy7 (BD Pharmingen, Franklin Lakes, NJ). Cells were incubated for 10 min at room temperature, washed in PBS/BSA buffer, fixed in 1% paraformaldehyde and analysed by flow cytometry. Subjects for these studies included 27 HIV+ donors and 18 healthy control donors. The HIV+ donors had a median CD4 cell count of 589 cells/μl and a median plasma HIV RNA of 33 copies/ml. All but three HIV+ donors were receiving anti-retroviral therapy at the time of the study and all but four of the HIV+ donors had a viral load below 500 copies/ml. The age of the HIV+ donors (median = 47 years) and HIV– donors (median = 38 years) was not significantly different.
Activation of monocytes and monocyte-derived macrophages
Purified monocytes were incubated overnight in medium alone or medium plus hBD-3 (20 μg/ml; Peptide International, Louisville, KY), LL-37 (20 μg/ml; AnaSpec, Fremont, CA) or Pam3CSK4 (500 ng/ml; Invivogen, San Diego, CA). Cells were harvested the next day for flow cytometric analyses. Supernatants were collected and stored at −80°C until analysed by infrared array.
Monocyte-derived macrophages were washed at the end of 7 days and replenished with fresh medium. Cells were then either stimulated with hBD-3 or incubated in medium alone overnight. Culture supernatants were harvested and stored at −80°C until analysed by infrared chemokine array. Cells were harvested with ice-cold PBS and gently scraped. The recovered cells were analysed by flow cytometry.
Flow cytometric analyses
Monocytes were stained with antibodies reactive to CD14, CD80 and CD86. Propidium iodide (PI) was used to assess viability. Propidium iodide (10 μg/ml) was added to cells 10 min before analysis. Cells were examined on an LSRII flow cytometer.
Chemokine arrays
Searchlight IR custom Array kits were used for multiplex infrared analyses (Aushon Biosystems, Billerica, MA). Briefly, chemokine capture antibodies were spotted to the bottom of 96-well plates. Fifty microlitres of supernatants or standards were added to 96-well plates and non-bound proteins were washed away after 3 hr incubation at room temperature. Secondary biotinylated detecting antibodies were added and incubated 30 min at room temperature. Plates were washed and streptavidin-DyLightTM 800 Fluor was added for 30 min at room temperature. Plates were rotated for the duration of incubations. After another wash, plates were centrifuged and scanned with an Odyssey infrared imager and analysed with Searchlight Array software.
Statistical analyses
Non-parametric paired tests were used to assess differences between chemokine concentrations in supernatants from cells that were stimulated compared with cells incubated in medium alone. Mann–Whitney U-tests were used to compare results with cells from HIV+ and HIV− donors. Analyses were performed with spss software (IBM, Armonk, NY).
Results
Differential induction of co-stimulatory molecules by hBD-3, Pam3CSK4 and LL-37
To assess monocyte responses to hBD-3, LL-37 or Pam3CSK4, we incubated purified monocytes with these various stimuli in overnight cell cultures and subsequently examined induction of co-stimulatory molecule surface expression by flow cytometric analysis. Human BD-3, and to a modest extent Pam3CSK4, induced CD80 expression in monocytes whereas LL-37 did not affect the expression of this co-stimulatory molecule (Fig. 1a). All three stimuli induced CD86 expression, although hBD-3 provided the most pronounced effects (Fig. 1b). As the intensity of CD86 expression among CD86+ cells appeared to be different depending on the stimuli, we further assessed MFI of CD86+ cells in each experimental condition (medium or medium plus various stimulants). Both hBD-3 and LL-37 tended to increase the intensity of CD86 expression above the levels observed in unstimulated monocytes, whereas Pam3CSK4 did not (Fig. 1b). Hence, co-stimulatory molecule expression is differentially modulated by hBD-3, LL-37 and Pam3CSK4 in human monocytes.
Figure 1.
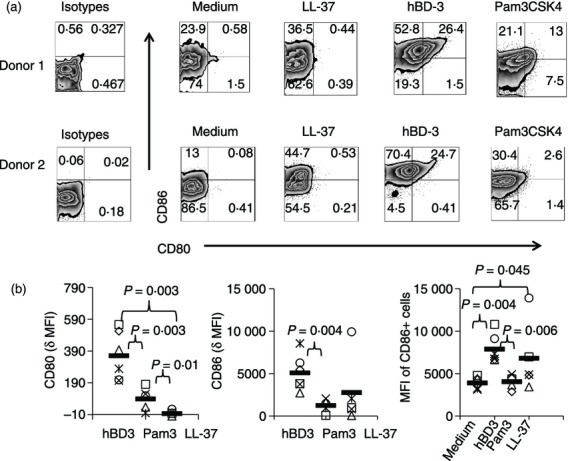
Differential induction of co-stimulatory molecules in monocytes activated by human β-defensin-3 (hBD-3), Pam3CSk4 or LL-37. Purified monocytes were incubated overnight in medium alone or in medium supplemented with hBD-3 (20 μg/ml), Pam3CSK4 (500 ng/ml) or LL-37 (20 μg/ml). CD80 and CD86 expression was measured among CD14+ cells by flow cytometry. Representative histograms are shown (a). Cumulative data from five or six donors are shown for each condition (b). Delta CD80 (or CD86) mean fluorescence intensity (MFI) was calculated by subtracting the MFI of CD80 (or CD86) on monocytes in medium alone from the CD80 (or CD86) MFI in monocytes incubated with each stimulus. MFI of CD86+ cells refers to the MFI observed on cells gated only among the CD14+ CD86+ events. Horizontal bars represent the mean of the data.
Importantly, we have previously tested hBD-3 from this source for contaminants that might explain its activity. The chemically synthesized hBD-3 reagent failed to activate a variety of TLR-expressing cell lines including TLR4+ cells lines, the levels of endotoxin by Limulus amoebocyte lysate assay were below the limits of detection and the activity of the reagent was completely inhibited by boiling.3 Therefore, the greater activity of hBD-3 relative to the other stimulants is not readily explained by contamination of the reagent.
Induction of chemokines by hBD-3, LL-37 or Pam3CSK4 in monocytes
Inflammatory responses are shaped by activation of antigen-presenting cells and by expression of chemokines that draw different cell types into tissues. We considered the possibility that hBD-3, LL-37 and Pam3CSK4 might differentially induce chemokines from human monocytes. Purified monocytes were stimulated overnight with hBD-3, Pam3CSK4 or LL-37 at concentrations that optimally induced co-stimulatory molecule expression on the surface of monocytes. Cell culture supernatants were collected for infrared cytokine arrays. Pam3CSK4 and hBD-3 induced a variety of chemokines from human monocytes including Gro-α, macrophage-derived chemokine (MDC), MCP-1, macrophage inflammatory protein 1α and 1β (MIP-1α and MIP-1β) as well as the angiogenic factor, vascular endothelial growth factor (VEGF) (Fig. 2). LL-37 had similar activity, although the responses appeared less pronounced in general and not statistically significant for VEGF induction. In contrast to the induction of chemokines described above, we did not find evidence for significant induction of a variety of other chemokines or cytokines including Regulated upon activation normal T-cell expressed and presumably secreted (RANTES), myeloid progenitor inhibitory factor-1 or monokine induced by interferon-γ (MIG) or IL-15 by any of the stimuli tested (not shown). Overall, these data suggest that a similar pattern of chemokine induction can be induced from monocytes by these various stimulants, although LL-37 seems to provide the least robust stimulus at the concentrations tested.
Figure 2.
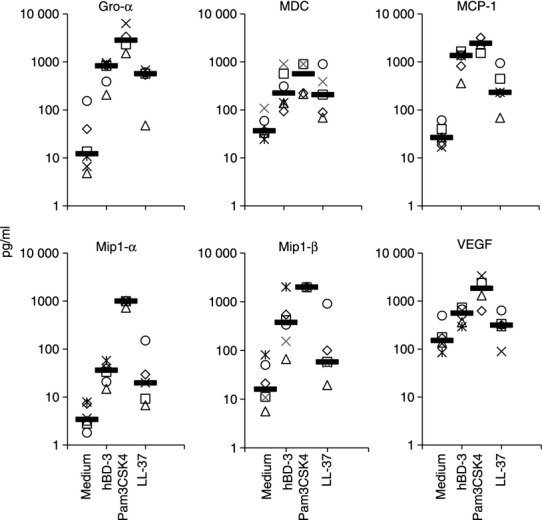
Induction of chemokines and angiogenesis factors in monocytes stimulated with human β-defensin-3 (hBD-3), Pam3CSK4 or LL-37. Purified monocytes were incubated overnight in medium alone (n = 6), hBD-3 (n = 6), Pam3CSK4 (n = 4) or LL-37 (n = 5) as described in Fig. 1. Cell culture supernatants were stored until analysed in batch by infrared chemokine arrays. Statistically significant differences (P < 0·05) were observed between stimulated cells and unstimulated cells in each condition except for vascular endothelial growth factor (VEGF) induction by LL-37. The lower limits of detection for each of the assays was 2·5 pg/ml (Gro-α), 2·1 pg/ml (macrophage-derived chemokine; MDC), 2·5 pg/ml (monocyte chemoattractant protein 1; MCP-1), 2·5 pg/ml (macrophage inflammatory protein 1α; MIP-1α), 3 pg/ml (MIP-1β) and 21 pg/ml (VEGF). Horizontal bars represent the median of the data.
To confirm that the chemokines induced by hBD-3 were monocyte-derived, PBMC or CD14-depleted PBMC from two different donors were tested for chemokine production after stimulation with hBD-3. With the exception of VEGF, we found evidence of induction of each of these molecules in PBMC treated with hBD-3. Among the other molecules tested, depletion of CD14+ cells resulted in loss of hBD-3-induced chemokine induction in all cases except for MIP1α (see Supplementary material, Fig. S1). Overall, these data are supportive of a primary role of monocytes as a source for these chemokines in hBD-3-stimulated cell cultures.
Human BD-3 induces chemokines in monocyte-derived macrophages
Expression of hBD-3 can be especially increased in inflamed tissues. Therefore, it was important to ascertain if cells that better resemble tissue macrophages might also respond to hBD-3 stimulation. To generate macrophages, purified CD14+ cells (purified by negative selection) were incubated with M-CSF for 7 days as previously described.2 The cells became large and granular by flow cytometric analyses and produced high spontaneous levels of MDC (Fig. 3), consistent with their maturation into macrophages.12,13 After 7 days, culture supernatant from monocyte-derived macrophages was replaced with fresh media or fresh media plus hBD-3 and cells were then incubated overnight. Chemokines were detected in supernatants from these cells by infrared array. In three of four experiments, hBD-3 induced Gro-α, MIP1α, MCP-1, whereas in four of four experiments we found evidence of MIP-1β and RANTES induction (Fig. 3). Unlike monocytes, there was no evidence of induction of MDC (Fig. 3) or VEGF (not shown) in these cells. It is possible in the case of MDC that increased spontaneous production of this chemokine may have limited the capacity for further induction. These data suggest that the induction of chemokines by hBD-3 is also likely to occur in more mature, monocyte-derived macrophages.
Figure 3.
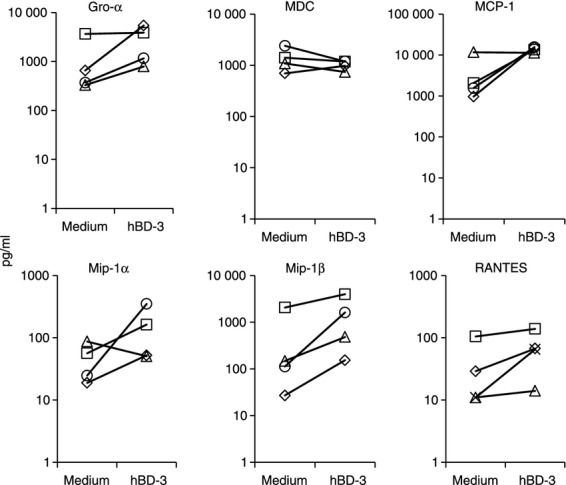
Induction of chemokines in monocyte-derived macrophages by human β-defensin-3 (hBD-3). Purified monocytes were incubated with macrophage colony-stimulating factor for 7 days. Cells were washed and fresh medium was added. Some cells were stimulated overnight with hBD-3 or left unstimulated. Cell culture supernatants were assessed by infrared array technology. Cells from four different donors were tested.
Altered expression of chemokines in cells from HIV+ donors
We have recently demonstrated that monocytes from HIV+ donors respond less well to hBD-3 stimulation as determined by the induction of CD80 surface expression. This defect was observed in cells from viraemic as well as treated, aviraemic donors suggesting that viraemia was not a critical determinant. To investigate the possibility that chemokine induction might also be altered in HIV infection, we compared hBD-3 induction of chemokines in cells from nine HIV+ donors with that in monocytes from six control donors. The HIV+ donors included three viraemic donors (plasma HIV RNA levels of 28 124, 157 792 and 166 206 copies/ml) and six aviraemic donors (< 48 copies/ml). The median CD4 cell count of our HIV+ donors was 370 cells/µl, ranging from 140 to 871 cells/μl. Interestingly, several chemokines including MCP-1, MIP-1α and MIP-1β were produced at heightened levels spontaneously in purified monocytes from HIV+ donors that were incubated overnight in medium alone (Fig. 4). After hBD-3 stimulation, induction of VEGF, Gro-α and MDC were all diminished in cells from HIV+ donors and a similar trend was noticed for MIP-1β (Fig. 4). We have recently shown that hBD-3 can cause membrane damage in monocytes from healthy donors at the concentration used in these studies and that this could result in cell death in a minority of monocytes.14 Comparison of propidium iodide staining in monocytes cultured in medium alone or in medium supplemented with hBD-3 did not demonstrate appreciable differences in PI bright cells when comparing cells from HIV+ and control donors, suggesting that cell death as a result of hBD-3 exposure was not responsible for the differences in chemokine induction by cells from HIV+ and HIV− donors (%PI bright monocytes in cell cultures from HIV+ donors versus % bright from control donors after hBD-3 treatment). These data are consistent with our previous observations suggesting that monocytes from HIV+ donors are generally less readily activated by hBD-3 stimulation than cells from healthy controls11 and provide evidence that monocytes from HIV+ donors may be more likely to spontaneously release certain chemokines into their environment than cells from healthy controls.
Figure 4.
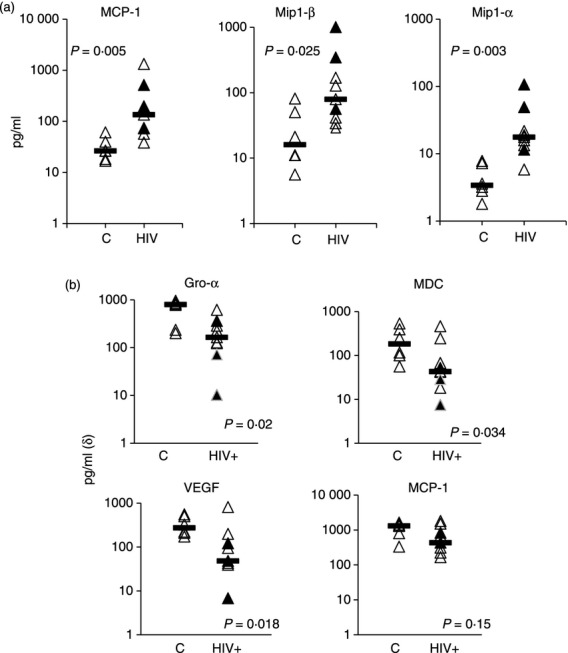
Altered chemokine expression in HIV disease. Purified monocytes from HIV− donors (n = 6) and HIV+ donors (n = 9) were incubated overnight in medium alone or in medium plus human β-defensin-3 (hBD-3; 20 μg/ml). Supernatants were assessed for chemokine concentrations by infrared array. Spontaneous production of certain chemokines were elevated in cells from HIV+ donors (a), while induction of chemokines by hBD-3 above the levels observed in cells incubated in medium alone tended to be diminished in cells from HIV+ donors (b). Horizontal bars represent the median of the data.
Chemokine receptor expression is not significantly altered in cells from HIV+ donors
It is possible that monocytes from HIV+ donors may have modified chemokine receptor expression that compensates for modified chemokine production. Freshly isolated monocytes from 18 healthy donors and 27 HIV+ donors were stained with antibodies reactive against CD14 and CD16 to identify monocyte subsets as CD14++ CD16− (traditional monocytes), CD14++ CD16+ (inflammatory monocytes) and CD14+ CD16++ (patrolling monocytes)15. Each subset was evaluated for expression of CCR2 (MCP-1 receptor), CXCR2 (Gro-α receptor), CCR5 (β chemokine receptor) and CCR4 (MDC receptor). The expression of these receptors was clearly distinguishable between monocyte subsets. CXCR2, CCR2 and CCR4 expression was lower among CD14+ CD16++ patrolling monocytes, whereas, CCR5 expression was markedly increased in this subset compared with the other subsets (Fig. 5). Expression of chemokine receptors was mostly similar when comparing monocytes from HIV+ and HIV− donors with the exception of a significant reduction in CCR4 expression that was observed in CD14+ CD16++ patrolling monocyte subset from HIV+ donors. A trend towards lower CXCR2 expression was noted among CD14++ CD16− traditional monocytes from HIV+ donors, which was not significantly different. The expression of chemokine receptors was not correlated with age, or current or nadir CD4 cell counts within our HIV+ population.
Figure 5.
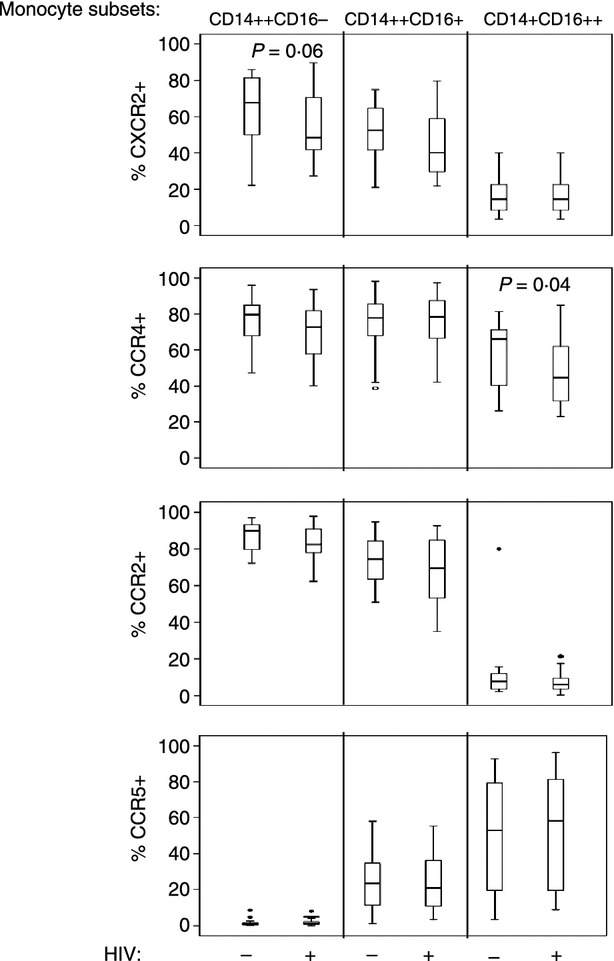
Reduced CCR4 expression in freshly isolated CD14+ CD16++ monocytes from HIV+ donors. Chemokine receptor expression was assessed in freshly isolated peripheral blood mononuclear cells by staining cells with antibodies reactive to CD14, CD16, CCR2, CXCR2, CCR5 and CCR4. The percentage of monocyte subsets in HIV+ and HIV− donors is shown on the y-axis. Cell gating is shown in Supplementary material, Fig. S2.
Discussion
We have previously shown that hBD-3 and Pam3CSK4 differentially induce expression of co-stimulatory molecules in the surface of monocytes such that hBD-3 induces expression of CD86 and CD80, whereas Pam3CSK4 only marginally affects CD86 expression and may even cause down-modulation of this molecule.8 Our results from these studies suggest that Pam3CSK4 can induce CD86 although the density of CD86 expression is not enhanced above background levels. As our previous studies demonstrated a dependence on IL-10 production for diminished CD86 induction by Pam3CSK4, it is possible that differences in the levels of IL-10 produced in these cultures could account for the differences between these studies and our previous observations.8 In addition, we find that LL-37 induces increases in both percentages and density of CD86 expression in monocytes in the absence of CD80 induction. Interestingly, in most samples, CD86 induction is limited to a subset of monocytes after LL-37 stimulation, suggesting that some monocyte subsets may be more responsive to LL-37 than others. Further studies of monocyte subset responses may provide insight into this possibility.
The significance of CD86 induction without CD80 induction by LL-37 is unknown as both of these molecules serve as co-stimulatory ligands for CD28. Nonetheless, CD80 seems to interact better than CD86 with the immunoregulatory receptor CTLA-4,16 and immune activation in the absence of either CD80 or CD86 may have differential consequences for T-cell maturation.17 The differential modulation of these co-stimulatory molecules may therefore have important consequences for directing T-cell maturation.
Induction of chemokines is a key mechanism for shaping inflammatory microenvironments. Here we find evidence that hBD-3 induces the expression of several chemokines and angiogenesis factors (MCP-1, MIP-1α, MIP-1β, MDC, Gro-α and VEGF) in monocytes and macrophages. MCP-1 acts in a similar manner to hBD-3 and can chemoattract monocytes via CCR2.18 Both MIP-1α and MIP-1β are β chemokines that interact with CCR5 to attract memory T cells19,20 and MDC mediates chemotaxis via CCR4, resulting in the potential recruitment of T helper type 2 cells and dendritic cells.21 Gro-α binds CXCR2 and causes the chemotaxis of neutrophils and monocytes.22,23 Similar to VEGF, Gro-α can also play a role in the vascularization of tissues.23,24 These findings provide evidence that hBD-3 orchestrates the influx of diverse pro-inflammatory cell types not just by direct recruitment of CCR2+ cells but also by activating monocytes and macrophages to release additional chemokines. Furthermore, induction of angiogenesis factors by hBD-3 could contribute to tissue repair in some cases and may also exacerbate tumour growth in circumstances where hBD-3 expression may be increased in or near cancerous lesions.5
Monocytes from HIV+ donors display a variety of phenotypic and functional alterations. These cells appear to be activated in HIV disease as indicated by their increased expression of CD69 and HLA-DR25,26 and are also less capable of responding to type I interferon stimulation.26,27 In these studies, we find that monocytes from HIV+ donors more readily produce chemokines (MCP-1, MIP-1α and MIP-1β) spontaneously in the absence of overt stimulation and we find evidence that monocytes are less able to release chemokines or growth factors (VEGF, Gro-α and MDC) after stimulation with hBD-3. Notably, the chemokines that are spontaneously produced at high levels and the chemokines that are less readily induced by hBD-3 in cells from HIV+ donors are not overlapping, suggesting that high background production of chemokines does not account for failure to optimally induce their expression from these cells.
Our studies also define the expression of chemokine receptors on monocyte subsets in freshly isolated cells from HIV+ donors. CCR5 and CCR2 expression appeared to be relatively unperturbed in cells from HIV+ donors, whereas CXCR2 and CCR4 expression was marginally decreased in certain subsets. The potential reduction in expression of these particular receptors in cells from HIV+ donors together with the diminished induction of their respective ligands after hBD-3 stimulation provides evidence that these chemokine axes may be perturbed in monocytes from HIV+ donors.
Monocyte function may be influenced in HIV disease by exposure to microbial products or to type I interferons;28 however, the mechanism that accounts for diminished responsiveness to hBD-3 stimulation in HIV infection11 remains to be determined. Importantly, our studies of chemokine induction in monocytes from HIV+ donors represent only a small number of subjects and we have only anecdotally examined responses in viraemic and aviraemic subjects. From our previous studies of CD80 induction by hBD-3, viraemia does not seem to play a major role in diminished hBD-3 responsiveness;11 however, this may depend on the functional read-out being investigated. Assessment of monocyte responses to antimicrobial peptide-mediated stimulation and discernment of the mechanism(s) responsible for monocyte dysfunction may provide new insights into immune deficiencies in HIV-infected persons, including those persons receiving anti-retroviral therapy.
Acknowledgments
This work was supported by a National Institutes of Health grant (DE17335), by the Center for AIDS Research at Case Western Reserve University (AI-36219) and by a grant from the James B. Pendleton Charitable Trust.
Disclosures
The authors have no competing interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Peripheral blood mononuclear cells (PBMC) or CD14-depleted PBMC from healthy control donors were incubated overnight in medium alone or medium plus human β-defensin-3 (hBD-3; 20 μg/ml). With the exception of macrophage inflammatory protein 1α (MIP-1α), CD14 depletion diminished chemokine expression in PBMC, providing evidence that hBD-3-induction of chemokines was dependent on CD14+ cells.
Figure S2. Peripheral blood mononuclear cells (PBMC) were incubated with antibodies reactive to CD14, CD16, CCR2, CXCR2, CCR5 and CCR4 for 10 min at room temperature. Cells were washed and analysed on an LSRII flow cytometer. Cellular debris and granulocytes were removed from analyses by forward and side scatter gating. Doublets were removed with FSC-H and FSC-A plots. Cells that were CD14++ CD16−, CD14++ CD16+ and CD14+ CD16++ were identified (a) and evaluated for expression of CCR2, CXCR2, CCR4 and CCR5 as indicated (b). An example from a healthy control donor is shown.
References
- 1.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 2.Jin G, Kawsar HI, Hirsch SA, et al. An antimicrobial peptide regulates tumor-associated macrophage trafficking via the chemokine receptor CCR2, a model for tumorigenesis. PLoS ONE. 2010;5:e10993. doi: 10.1371/journal.pone.0010993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funderburg N, Lederman MM, Feng Z, Drage MG, Jadlowsky J, Harding CV, Weinberg A, Sieg SF. Human β-defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc Natl Acad Sci USA. 2007;104:18631–5. doi: 10.1073/PNAS.0702130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funderburg N, Luciano AA, Jiang W, Rodriguez B, Sieg SF, Lederman MM. Toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis. PLoS ONE. 2008;3:e1915. doi: 10.1371/journal.pone.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawsar HI, Weinberg A, Hirsch SA, Venizelos A, Howell S, Jiang B, Jin G. Overexpression of human β-defensin-3 in oral dysplasia: potential role in macrophage trafficking. Oral Oncol. 2009;45:696–702. doi: 10.1016/j.oraloncology.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Wu Z, Hoover DM, Yang D, Boulegue C, Santamaria F, Oppenheim JJ, Lubkowski J, Lu W. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human β-defensin 3. Proc Natl Acad Sci U S A. 2003;100:8880–5. doi: 10.1073/pnas.1533186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimm MC, Pavli P, Van de Pol E, Doe WF. Evidence for a CD14+ population of monocytes in inflammatory bowel disease mucosa – implications for pathogenesis. Clin Exp Immunol. 1995;100:291–7. doi: 10.1111/j.1365-2249.1995.tb03667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funderburg NT, Jadlowsky JK, Lederman MM, Feng Z, Weinberg A, Sieg SF. The Toll-like receptor 1/2 agonists Pam(3) CSK(4) and human β-defensin-3 differentially induce interleukin-10 and nuclear factor-κB signalling patterns in human monocytes. Immunology. 2011;134:151–60. doi: 10.1111/j.1365-2567.2011.03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1β processing and release. J Immunol. 2004;172:4987–94. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 10.van der Does AM, Beekhuizen H, Ravensbergen B, et al. LL-37 directs macrophage differentiation toward macrophages with a proinflammatory signature. J Immunol. 2010;185:1442–9. doi: 10.4049/jimmunol.1000376. [DOI] [PubMed] [Google Scholar]
- 11.Funderburg NT, Sieg SF. Diminished responsiveness to human β-defensin-3 and decreased TLR1 expression on monocytes and mDCs from HIV-1-infected patients. J Leukoc Biol. 2012;92:1103–9. doi: 10.1189/jlb.1111555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray PW. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med. 1997;185:1595–604. doi: 10.1084/jem.185.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodenburg RJ, Brinkhuis RF, Peek R, Westphal JR, Van Den Hoogen FH, van Venrooij WJ, van de Putte LB. Expression of macrophage-derived chemokine (MDC) mRNA in macrophages is enhanced by interleukin-1β, tumor necrosis factor α, and lipopolysaccharide. J Leukoc Biol. 1998;63:606–11. doi: 10.1002/jlb.63.5.606. [DOI] [PubMed] [Google Scholar]
- 14.Lioi AB, Reyes Rodriguez AL, Funderburg NT, Feng Z, Weinberg A, Sieg SF. Membrane damage and repair in primary monocytes exposed to human β-defensin-3. J Leukoc Biol. 2012;92:1083–91. doi: 10.1189/jlb.0112046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–86. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasu C, Wang A, Gorla SR, Kaithamana S, Prabhakar BS, Holterman MJ. CD80 and CD86 C domains play an important role in receptor binding and co-stimulatory properties. Int Immunol. 2003;15:167–75. doi: 10.1093/intimm/dxg017. [DOI] [PubMed] [Google Scholar]
- 17.Perez N, Karumuthil-Melethil S, Li R, Prabhakar BS, Holterman MJ, Vasu C. Preferential costimulation by CD80 results in IL-10-dependent TGF-β1+ -adaptive regulatory T cell generation. J Immunol. 2008;180:6566–76. doi: 10.4049/jimmunol.180.10.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci USA. 1994;91:2752–6. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Combadiere C, Ahuja SK, Tiffany HL, Murphy PM. Cloning and functional expression of CC CKR5, a human monocyte CC chemokine receptor selective for MIP-1α, MIP-1β, and RANTES. J Leukoc Biol. 1996;60:147–52. doi: 10.1002/jlb.60.1.147. [DOI] [PubMed] [Google Scholar]
- 20.Raport CJ, Gosling J, Schweickart VL, Gray PW, Charo IF. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1β, and MIP-1α. J Biol Chem. 1996;271:17161–6. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 21.Imai T, Chantry D, Raport CJ, Wood CL, Nishimura M, Godiska R, Yoshie O, Gray PW. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem. 1998;273:1764–8. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- 22.Geiser T, Dewald B, Ehrengruber MU, Clark-Lewis I, Baggiolini M. The interleukin-8-related chemotactic cytokines GROα, GROβ, and GROγ activate human neutrophil and basophil leukocytes. J Biol Chem. 1993;268:15419–24. [PubMed] [Google Scholar]
- 23.Papadopoulou C, Corrigall V, Taylor PR, Poston RN. The role of the chemokines MCP-1, GRO-α, IL-8 and their receptors in the adhesion of monocytic cells to human atherosclerotic plaques. Cytokine. 2008;43:181–6. doi: 10.1016/j.cyto.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–9. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 25.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang W, Lederman MM, Salkowitz JR, Rodriguez B, Harding CV, Sieg SF. Impaired monocyte maturation in response to CpG oligodeoxynucleotide is related to viral RNA levels in human immunodeficiency virus disease and is at least partially mediated by deficiencies in α/β interferon responsiveness and production. J Virol. 2005;79:4109–19. doi: 10.1128/JVI.79.7.4109-4119.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardy GA, Sieg SF, Rodriguez B, Jiang W, Asaad R, Lederman MM, Harding CV. Desensitization to type I interferon in HIV-1 infection correlates with markers of immune activation and disease progression. Blood. 2009;113:5497–505. doi: 10.1182/blood-2008-11-190231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tilton JC, Johnson AJ, Luskin MR, et al. Diminished production of monocyte proinflammatory cytokines during human immunodeficiency virus viremia is mediated by type I interferons. J Virol. 2006;80:11486–97. doi: 10.1128/JVI.00324-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


