Significance
p73 is a tumor suppressor protein that also plays a central role in brain development. But how p73 modulates neurite outgrowth and neurite arborization is not clear. Neurotrophins are small molecules, which send signals between cells. Neurotrophins can induce cells to either apoptosis or survival. We show that TAp73 is a transcriptional activator of the p75 neurotrophin receptor (p75NTR). TAp73 knockout mice have reduced levels of p75NTR and show peripheral nerve defect, including reduced myelin thickness and thermal sensitivity. p75NTR is expressed most strongly in the peripheral nerve, where it is important for nerve development. Relatively limited information exists on p75NTR gene regulation; by linking the p53/p73 gene family to p75NTR transcription, we introduce possibly controversial mechanisms of regulation.
Keywords: sciatic nerve, NGF, p53 family, CGRP
Abstract
Total and N-terminal isoform selective p73 knockout mice show a variety of central nervous system defects. Here we show that TAp73 is a transcriptional activator of p75 neurotrophin receptor (p75NTR) and that p75NTR mRNA and protein levels are strongly reduced in the central and peripheral nervous systems of p73 knockout mice. In parallel, primary cortical neurons from p73 knockout mice showed a reduction in neurite outgrowth and in nerve growth factor-mediated neuronal differentiation, together with reduced miniature excitatory postsynaptic current frequencies and behavioral defects. p73 null mice also have impairments in the peripheral nervous system with reduced thermal sensitivity, axon number, and myelin thickness. At least some of these morphological and functional impairments in p73 null cells can be rescued by p75NTR re-expression. Together, these data demonstrate that loss of p75NTR contributes to the neurological phenotype of p73 knockout mice.
p73 is a transcription factor belonging to the p53 family, whose members share similarities in sequence and function (1–4). p73 can be transcribed from two different promoters: transcription from the upstream P1 promoter generates TAp73 isoforms containing a transactivation (TA) domain, and transcription from the P2 promoter generates truncated proteins that lack the TA domain and are known as ΔNp73. The C-terminal region of p73 is expressed, at least at the mRNA level, as a number of alternatively spliced TAp73 and ΔNp73 C-terminal isoforms (α, β, γ, ζ, δ, ε, η), although it remains unclear whether all of these are also represented at the protein level (5, 6).
The p75 neurotrophin receptor (p75NTR) is a member of the death receptor family, which can also be expressed in a variety of isoforms and functions as a low-affinity receptor for neurotrophins (7, 8). It is abundantly expressed on neurons and glia in the developing nervous system (as well as on some other cell types such as B lymphocytes and natural killer cells), although it is much less abundant in adult brain. The effects of ligand binding to p75NTR depend on p75NTR’s interaction with other coreceptors. Thus, interaction with tropomyosin-related kinase A (TrkA) results in high-affinity binding of neurotrophins such as nerve growth factor (NGF) and cell survival. In contrast, neurotrophin interaction with the p75NTR/sortilin complex leads to apoptosis, whereas p75NTR can also form part of the Nogo receptor complex, which is activated by myelin proteins to inhibit axonal growth (9). Deletion of p75NTR results in a profound loss of sensory but not motor neurons in dorsal root ganglia, with an increase in basal forebrain cholinergic neurons, confirming that p75NTR can mediate both survival and apoptotic effects. The defect in sensory innervation arises because the peripheral axons are stunted and show reduced arborization (10, 11). In the central nervous system, p75NTR-deficient mice also show significantly reduced hippocampal neurogenesis, with a smaller granular cell layer and dentate gyrus, and increased cell death of newly divided cells. These morphological alterations in the hippocampus are associated with impaired performance in a number of behavioral tests (12, 13).
In this study, we show that p75NTR is a direct target of TAp73 and mediates many of the neurological abnormalities associated with loss of p73.
Results
p75NTR Expression Is Reduced in the Brains of TAp73 Null Mice, Resulting in Behavioral and Electrophysiological Abnormalities.
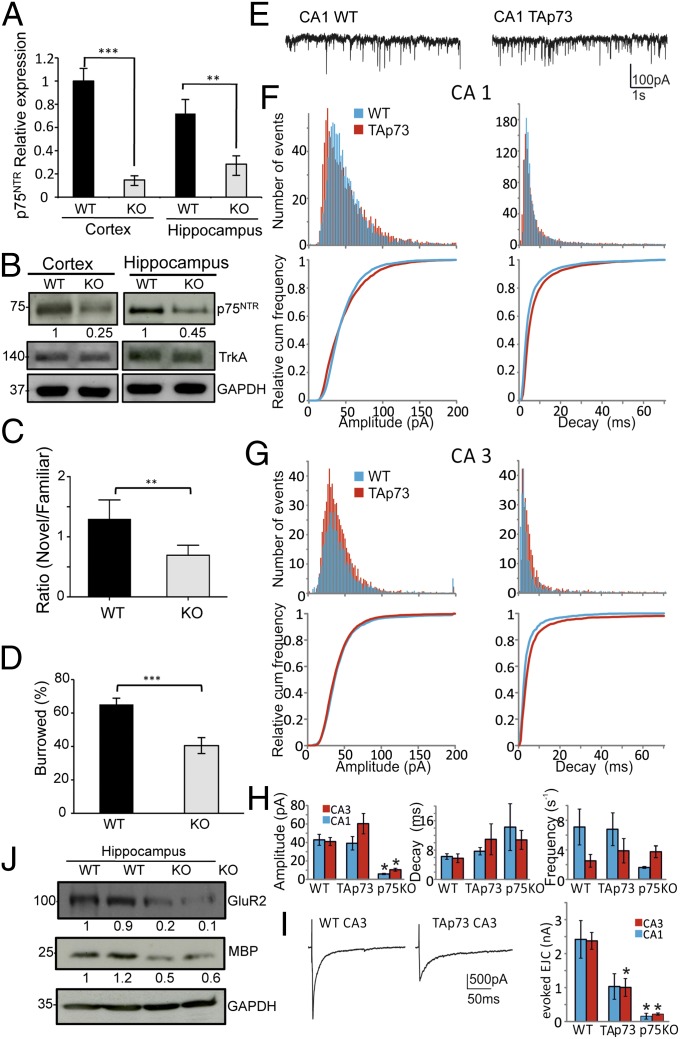
Microarray analysis suggested that p73 induces expression of p75NTR. To assess whether expression of p75NTR was altered in the brains of TAp73 null mice, we compared p75NTR expression in the cortex and hippocampus of 2-mo-old wild-type (WT) and knockout (KO) animals. There was a marked reduction in p75NTR mRNA and protein expression in both cortex and hippocampus (Fig. 1 A and B and Fig. S1A); moreover, immunohistochemistry of the motor cortex also showed a profound reduction in the numbers of p75NTR-positive cells in the KO mice (Fig. S1B), although TrkA expression was unchanged at the protein level in the KO animals (Fig. 1B).
Fig. 1.
TAp73 knockout mice show reduced p75NTR and impairment in behavioral and electrophysiological tests. p75NTR mRNA and protein levels were analyzed in 2-mo-old WT and TAp73KO mice. (A) RT-PCR of p75NTR in cortex and hippocampus. (B) Protein levels of p75NTR and TrkA in cortex and hippocampus. (C and D) NOR and burrowing test were performed in WT and TAp73KO male animals aged 1–2 mo. TAp73KO mice show significantly reduced NOR (**P < 0.005) and burrowing (***P < 0.0007). (E–I) Spontaneous miniature events in hippocampal acute slice CA1 or CA3 pyramidal neurons are unaffected in 20-d-old TAp73KO mice, but evoked synaptic transmission in CA3 neurons is reduced. (E) Raw traces showing spontaneous miniature excitatory postsynaptic current amplitudes from neurons in WT and TAp73KO hippocampal neurons. (F) CA1. (G) CA3 histograms (Upper) for amplitude (Left) and decay (Right) distributions in pyramidal neurons from WT (blue) and TAp73KO (red) mice. (Lower) Relative cumulative distributions for amplitude (Left) and decay (Right) distributions in pyramidal neurons form WT (blue) and TAp73KO (red) mice. (H) Mean values for miniature amplitudes, decay, and frequencies for WT, TAp73KO, and p75NTRKO. CA1 (blue) and CA3 (red) hippocampal neurons. (I) (Left) Raw traces of EPSC from WT and TAp73KO CA3 pyramidal neurons. (Right) Mean values for evoked mEPSC from CA1 and CA3 pyramidal neurons from WT, TAp73KO, and p75NTRKO mice. Data denote mean ± SEM; n = 4–6 neurons; *P < 0.05. (J) Protein levels of GluR2, MBP, and GAPDH in hippocampus from WT and TAp73KO mice.
To assess whether the reduced p75NTR expression in the brain of TAp73KO mice was associated with any behavioral and electrophysiological abnormalities, we performed the novel object recognition test (NOR), together with burrowing and open field tests. The NOR was performed in mice from 1 to 2 mo old. Fig. 1C shows that WT mice exhibited a stronger preference for the novel object than the TAp73KO mice. Hippocampal dysfunction has previously been associated with reduction in burrowing and open field tests (14). One- to 2-mo-old TAp73KO animals also exhibited a reduction in burrowing (Fig. 1D and Fig. S1C) and in both speed and rearing time in open field tests (Fig. S1 D–F), and the degree of these impairments progressed with age (Fig. S1 C–F).
To determine if these behavioral deficits were associated with electrophysiological abnormalities, we performed recordings from the hippocampal CA1 or CA3 regions in acute slices from WT and TAp73KO mice (Fig. 1 E–I). p73 deletion had no effect on miniature excitatory postsynaptic current (mEPSC) amplitudes, decay kinetics, or event frequencies recorded from CA1 or CA3 pyramidal neurons (Fig. 1 E–H), but suppressed evoked EPSC amplitudes in CA3 neurons, suggesting a compromised evoked transmitter release at the presynaptic terminal (Fig. 1I). Moreover, p75NTRKO mice also show a strong reduction in mEPSC amplitudes and evoked EPSC amplitudes recorded from CA1 or CA3 pyramidal neurons (Fig. 1I).
Because the total number of synapses was not affected, with no change in the pre- and postsynaptic proteins (Fig. S2A), although the evoked EPSC amplitudes were reduced, we speculated that this electrophysiological impairment could be due to a defect in the AMPA receptor subunit and/or myelin basic protein (MBP), which could have effects on action potential propagation and thereby explain the deficits in evoked transmitter release. AMPA receptor subunit 2 (GluR2) and MBP are known to be p75NTR targets (15–17), and GluR2 and MBP at RNA and protein levels are indeed reduced in hippocampus and cortical neurons from TAp73KO mice (Fig. 1J and Fig. S2 B–E). Cortical neurons from p75NTRKO mice also show a strong reduction in GluR2 (Fig. S2F).
p73 Regulates Neuronal Differentiation After Stimulation with NGF via p75NTR.
We examined the role of the p73/p75NTR axis in neuronal differentiation in two models: cortical neurons treated with NGF and retinoid-induced differentiation of neuroblastoma cell lines. The role of p75NTR in the regulation of neuronal growth after NGF stimulation has been well documented (18, 19). Because neurons in culture express p75NTR and not TrkA (Fig. S3A), NGF signaling is due to p75NTR and not to TrkA.
Cortical neurons.
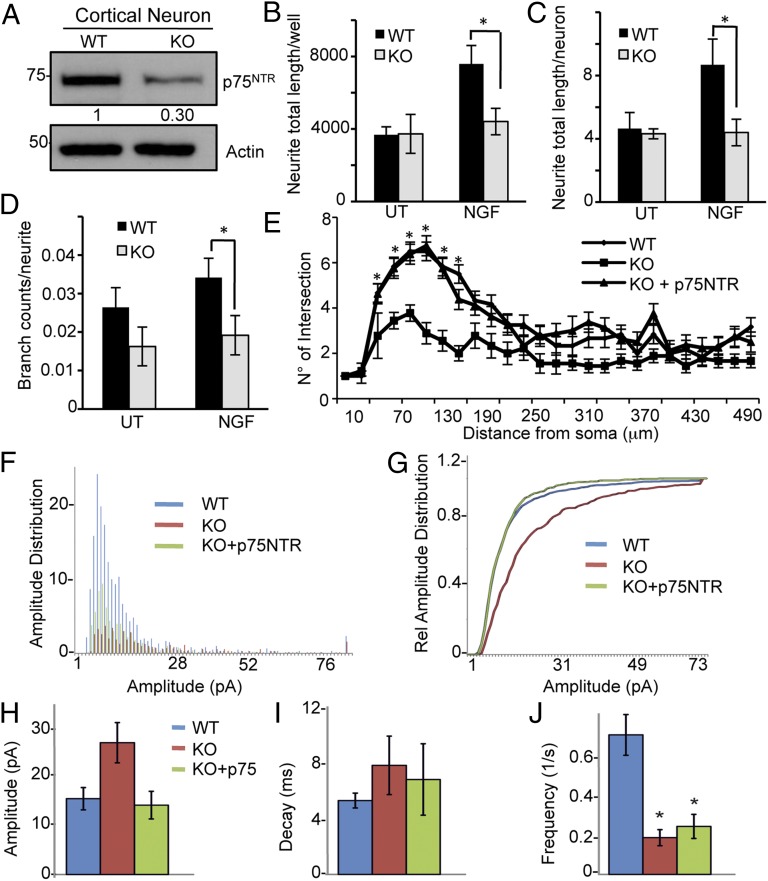
NGF is a potent neurotrophin that regulates axon growth and branching in neurons via p75NTR. Cortical neurons from p73KO, TAp73KO, or p75NTRKO mice do not show any signs of apoptosis with or without NGF in vitro (Fig. S3 B–E). p75NTR expression was reduced in cortical neurons from embryo day (E) 17.5 p73KO mice (Fig. 2A). To investigate whether cortical neurons isolated from WT or p73KO mice have different responses to NGF treatment, we grew low-density cultures of dissociated cortical neurons in the presence of NGF. Cellomic analysis showed a reduction in total neurite length and branch points after NGF treatment in neurons from the p73KO animals (Fig. 2 B–D), and Sholl analysis confirmed that the complexity of neurite arborization was reduced in the p73KO mice with increasing distances from the soma (Fig. 2E). Transfection of p73KO cortical neurons with p75NTR shows the expected increase in p75NTR mRNA and protein levels (Fig. S3 F and G) and rescues these morphological abnormalities in neurite complexity (Fig. 2E).
Fig. 2.
Cortical neurons from TAp73KO mice have reduced p75NTR and neuronal complexity after NGF treatment. (A) Western blot of p75NTR in cortical neurons from E17 WT and p73KO mice. (B–D) Cortical neurons from WT and p73KO mice were plated and, after 36 h of treatment with or without 100 ng/mL NGF, the cells were fixed and stained with β-III tubulin and Hoechst 33342. Images were captured and analyzed using Cellomics Kinetic Scan for neurite total length expressed both per well (B) and per neuron (C) and branch counts per neurite (D). UT, untreated. (E) Sholl analysis of WT, p73KO, or p73KO+p75NTR cortical neurons at day in vitro (DIV) 3 after transfection with scramble-GFP or p75NTR-GFP in the presence of 100 ng/mL NGF for 36 h. n = 10 cells; *P < 0.05. Data are means ± SEM. (F–J) Recordings from cortical neuronal at DIV 4 from WT are shown in blue, p73KO cultures in red, and p73KO+p75NTR in green. p73 null neurons showed increased amplitudes but reduced frequencies of spontaneous miniature events. (F) mEPSC amplitude histograms for WT, p73KO, and p73KO+p75NTR cultures. (G) Relative cumulative frequency plots for amplitude distributions. Mean values for miniature amplitudes (H), decay (I), and frequency (J) from WT, p73KO, and p73KO+p75NTR cultures. Data denote mean ± SEM; n = 8–9 neurons; *P < 0.05.
Next, we investigated if the differences in neuronal morphology following NGF treatment had functional consequences. Cortical cultures from WT and p73KO mice were supplemented with 20 ng/mL NGF for 4 d, and the cultures were examined electrophysiologically (Fig. 2 F–J). Spontaneous miniature excitatory postsynaptic currents were recorded, and neurons from KO animals showed enhanced amplitudes (Fig. 2 F–H) and strongly reduced frequencies (Fig. 2J), suggesting enhanced quantal release/receptor availability but a reduction in the number of functional synapses and network connectivity. We partially rescued this phenotype with re-expression of p75NTR (Fig. 2 F–J).
Neuroblastoma cell lines.
To explore further the role of the p73/p75NTR axis in neuronal differentiation, we used the neuroblastoma cell line, SH-SY5Y, treated with retinoic acid (RA). Basal levels of p73 and p75NTR in SH-SY5Y cells were extremely low but both increased following treatment with 10 μM RA for 48 h (Fig. S4 A and B) in parallel with neurite outgrowth. Silencing of both total or TAp73 reduced the RA-induced increase in p75NTR expression at both mRNA (Fig. S4 C and D) and protein levels after 48 h (Fig. S4 A and B). Importantly, silencing of p73 and p75NTR significantly reduced neurite outgrowth following RA treatment (Fig. S4E), although p73 and p75NTR silencing did not result in increased apoptosis (Fig. S4 F and G).
TAp73 Knockout Mice Show Defects in Heat Sensitivity with Decreased Innervation of Sensory Nerve Fibers.
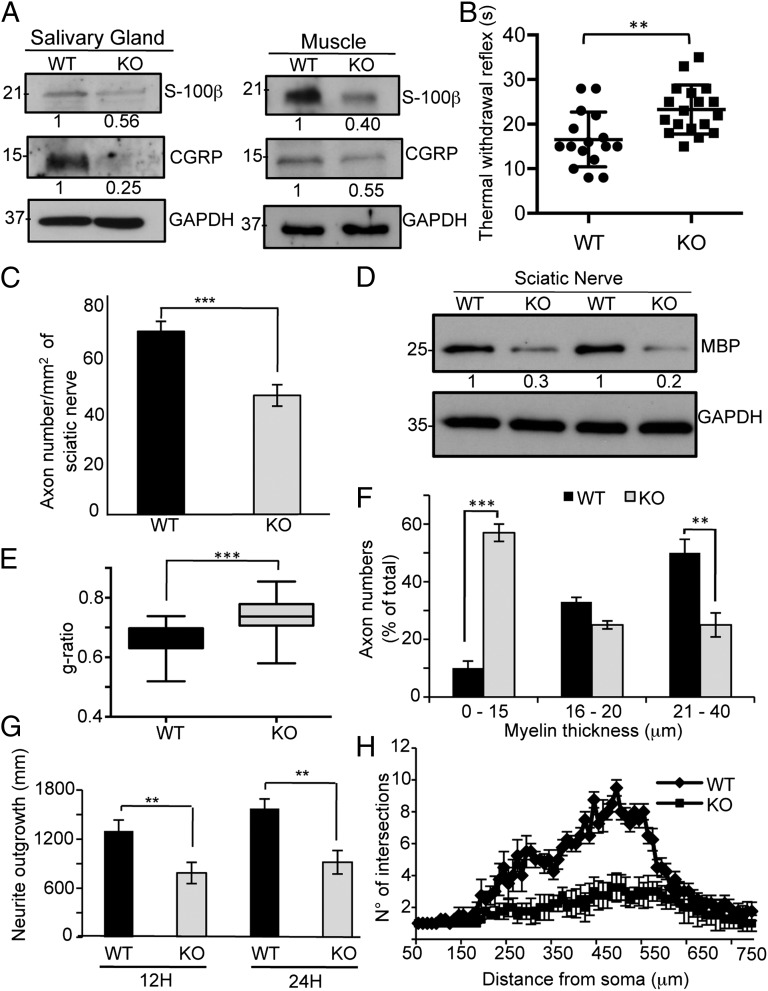
We next addressed, in addition to the reported central nervous system (CNS) defects, whether TAp73KO mice also had deficiencies in the peripheral nervous system (PNS). p75NTRKO mice showed a marked defect in sensory innervation of the footpad skin with reduced levels of calcitonin gene-related peptide (CGRP) (20, 21). Also, the sciatic nerves of p75NTRKO mice had a thinner myelin sheath (18). Therefore, to assess whether the selective TAp73KO mice have similar PNS defects, we performed Western blots for CGRP and S-100β in salivary gland and muscle. Fig. 3A shows a reduced level of these proteins in the TAp73-deficient animals.
Fig. 3.
TAp73KO mice have reduced sensory nerve innervations. (A) Western blot of S-100β and CGRP from salivary gland and muscle from 2-mo-old WT and TAp73KO mice. (B) Heat sensitivity tests were performed in WT and TAp73KO animals aged 2–4 mo. TAp73KO mice show a longer latency to paw withdrawal following thermal stress (**P < 0.0028). (C) Axon number assessed from electron microscopy images of transverse sections from 2-mo-old WT and p73KO mice. The images cover 48 × 37 μm. At least 100 randomly selected axons were analyzed per animal. Three animals were used per genotype. (D) Western blot of MBP in sciatic nerve of 20-d-old WT and p73KO mice. (E) g-Ratio analysis (axon diameter/fiber diameter) in 2-mo-old WT and p73KO mice derived from electron microscopy images of transverse sections of the sciatic nerve. (F) Myelin thickness analyzed by electron microscopy from sciatic nerves of 2-mo-old WT and p73KO mice. (G and H) DRG explants after 12 h of growth were analyzed for neurite outgrowth (G) and complexity of arborization using Sholl analysis (H). DRG from the knockout mice show reduced neurite complexity in response to NGF treatment (**P < 0,001).
In addition, we performed in footpad from WT and p73KO a staining with PGP9.5 and CGRP (Fig. S5 A and B). Reduced innervations were found in KO mice. We then tested whether this reduced innervation had functional significance by comparing the heat sensitivity of 2- to 4-mo-old WT and TAp73KO mice. The knockout mice showed a significantly longer time to footpad withdrawal in response to 52 °C, which was independent of age (Fig. 3B).
p75NTR plays an important role in the development of the PNS (11, 19), and it is strongly expressed in the sciatic nerve (22). Therefore, we analyzed electron microscopy cross-sections of sciatic nerves from WT and p73KO mice. WT and p73KO animals did not show any significant difference in the diameter of the sciatic nerve (Fig. S6A), but p73KO mice had a reduction in axon number and in MBP protein levels (Fig. 3 C and D and Fig. S6A). The g-ratio is used as a functional and structural index of optimal axonal myelination (23–25), and an increased g-ratio is associated with demyelination. Fig. 3E shows a high g-ratio in p73KO mice, which was associated with an increased proportion of more thickly myelinated axons in WT compared with KO mice (Fig. 3F). TAp73KO mice also display a reduction in myelin thickness, axon number, and MBP level (Fig. S6 A–D).
We also cultured dorsal root ganglion (DRG) explants from WT and KO mice (Fig. S7A) and evaluated p75NTR RNA and protein levels; p73KO mice showed a 50% reduction in p75NTR mRNA (Fig. S7B). These molecular changes were associated with short axons (Fig. S7A) and reduction both in neurite outgrowth (Fig. 3G) and in the complexity of neurite arborization assessed by Sholl analysis (Fig. 3H); the short axons may contribute to the defect in nociceptive sensory input (Fig. 3B). These molecular and morphological changes were not due to increased DRG apoptosis (Fig. S7C).
Next, we used the ST88-14 human Schwann cell line and performed a scratch assay after silencing p73. p73 silencing reduced p73 and p75NTR mRNA and protein levels (Fig. S8 A–C) and reduced cell cumulative distance and velocity (Fig. S8 D–F; Movies S1–S3). Importantly, transfection of the p73-silenced cells with p75NTR restored p75NTR levels (Fig. S8 B and C), with a corresponding increase in mobility to levels comparable to those in the scrambled transfected cells (Fig. S8 D–F).
p75NTR Is a Direct Transcriptional Target of TAp73.
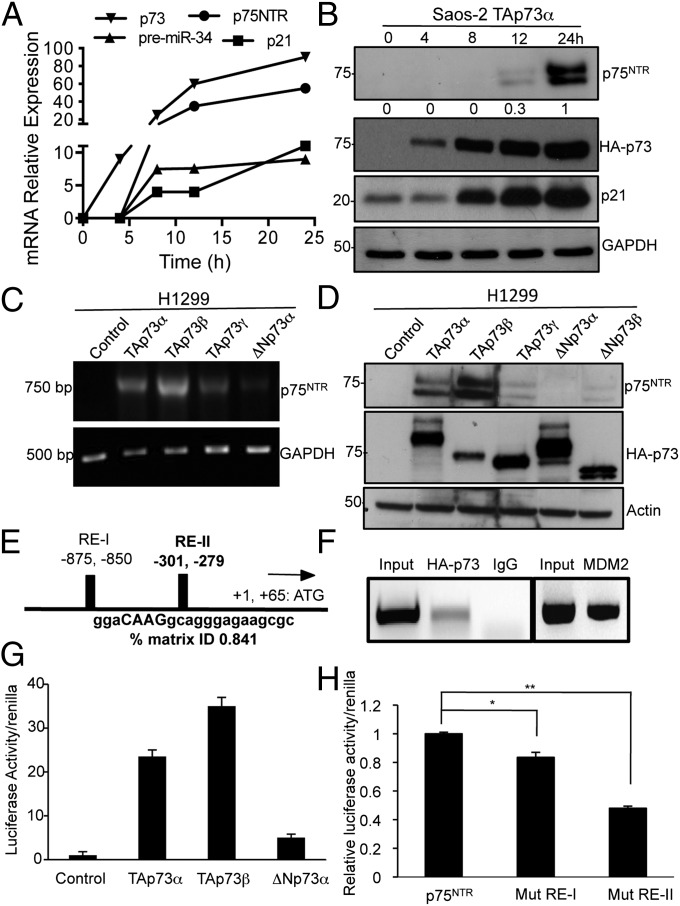
To identify whether p75NTR is a direct transcriptional target of TAp73, we first used a tet-on inducible Saos-2 cell line overexpressing HA-TAp73α or HA-TAp73β. TAp73α mRNA expression was detected 4 h after addition of doxycycline, and p75NTR mRNA expression was detectable after 8 h (Fig. 4A). Expression of the known p73 targets, p21 and premiR 34, also increased on p73 induction with similar time kinetics (Fig. 4A). In parallel, p75NTR protein was also expressed after 12 h of p73 induction and increased further after 24 h (Fig. 4B). Increased p21 protein expression was also detected again with similar kinetics (Fig. 4B). Similar results were obtained with TAp73β (Fig. S9 A and B).
Fig. 4.
TAp73 isoforms induce p75NTR mRNA and protein expression. (A and B) Saos-2-tet-on cells expressing HA-TAp73α were treated with 2 μg/μL doxycyclin for 0, 4, 8, 12, and 24 h. (A) Expression of p75NTR, premiR-34, p21, and TAp73 was analyzed by RT-PCR. (B) Western blots were probed with antibodies to p75NTR, HA-p73, p21, and GAPDH. (C and D) H1299 cells were transfected with plasmids expressing HA-TAp73α, -β, and -γ and HA-ΔNp73α and -β for 72 h. (C) The levels of p75NTR and GAPDH were analyzed by PCR. (D) Western blots were probed with antibodies to p75NTR, HA-p73, and actin. (E) Bioinformatics analysis of human p75NTR promoters using the MatInspector program by Genomatix has been analyzed for putative p53 REs. Two REs in humans were found; the first (RE-I; 5′) is located at −875/−850 bp from the ATG and the second (RE-II; 3′) at −301/−279 bp from the ATG. (F) ChIP assay was performed using nuclear extracts from the Saos-2 cell line overexpressing HA-TAp73β. Protein-chromatin complexes were immunoprecipitated with HA-TAp73 or nonimmune IgG. RT-PCR was performed with primers designed against the p75NTR promoter region containing the predicted and validated p53 RE. MDM2 was used as a positive control. (G) Luciferase assay on the p75NTR promoter readings was normalized against Renilla activity. Both TAp73α and -β, but not ΔNp73α, activate the p75NTR promoter. (H) Mutation of RE-I and -II reduce TAp73 activity on the p75NTR promoter. Luciferase assays on the p75NTR, Mut RE-I, and Mut RE-II promoters were normalized against Renilla activity.
We also transiently transfected H1299 cells with the α, β, and γ isoforms of HA-TAp73 and HA-ΔNp73. The results showed that only TAp73 isoforms, but not ΔNp73, induced p75NTR at both mRNA and protein levels (Fig. 4 C and D). To further confirm role of the TA isoforms, we analyzed p75NTR RNA and protein levels in cortex and hippocampus of 20-d-old ΔNp73KO mice and found these to be increased rather than reduced (Fig. S9 C and D).
We next performed an in silico analysis to identify putative p73-binding sites within the p75NTR human promoters. This showed the presence of two p53 consensus responsive elements (RE), with the first (RE-I) being located at −875/−850 bp and the second (RE-II) at −301/−279 bp from the ATG in the human sequence (Fig. 4E and Fig. S10A). The RE-II has a similarity of 0.841 and is the more conserved. In the mouse p75NTR promoter, RE-I is located at −1063/−1030 and RE-II at −578/−559 (Fig. 4E and Fig. S10A). The p75NTR promoter sequence is highly conserved throughout evolution, and we found putative p53 REs in the p75NTR promoters of a wide range of species (Fig. S10B).
To confirm these in silico data, we performed a chromatin immunoprecipitation (ChIP) assay using either a HA-TAp73 antibody or nonimmune IgG as control; RT-PCR was performed with primers designed against the p75NTR promoter region containing the predicted p53 RE-II (Fig. 4F). Next, we performed a luciferase assay, cotransfecting the p75NTR promoter, containing the p53 REs, upstream of a luciferase reporter, together with expression plasmids for TAp73α, TAp73β, and ΔNp73α. TAp73α and -β, but not ΔNp73, significantly increased the luciferase activity (Fig. 4G). Mutation of RE-I and, particularly, of RE-II significantly reduced promoter activity (Fig. 4H and Fig. S10C). Together, these data show that TAp73 is a direct transactivator of p75NTR.
Discussion
In this paper, we show that TAp73 is a direct transcriptional activator of the neurotrophin receptor, p75NTR, and that p75NTR expression is correspondingly reduced in the brains and cortical neurons of p73 null mice. However, expression of TrkA is unchanged, suggesting that TAp73 acts on p75NTR alone. p73 null mice have defects in cortical neuronal morphology and function and behavioral abnormalities. The rescue of the morphological phenotype by expression of p75NTR in neurons from p73 null mice suggests that p75NTR is an important mediator of the neuronal effects of p73. Furthermore, reconstitution of p73-deficient Schwann cells by p75NTR also restores their migration in response to NGF, suggesting that the p73/p75NTR axis is important in at least some glial cells as well as in neurons.p75NTR is a member of the tumor necrosis factor receptor family that transduces signals from pro- and mature neurotrophins, including NGF. p75NTR has multiple functions within the nervous system, ranging from neurite outgrowth and survival to apoptosis, and this diversity of function is a reflection of the variety of its cognate ligands, as well as its ability to interact with other receptors such as Trk and sortilin (26, 27). Indeed, the activation of TrkA by NGF is not detectable in p75NTRKO hippocampal neurons (28). In contrast, there is evidence that neurotrophins such as NGF induce neuronal apoptosis through p75NTR when TrkA is absent (29). p75NTR is widely expressed on neurons and glia during embryonic and early postnatal life, although expression decreases markedly in adulthood. For this reason, we have used primary cortical neuronal cultures from embryonic p73 null mice. p75NTR is particularly highly expressed on Schwann cells during the development and regeneration of peripheral nerves, and myelin formation in cocultures of neurons from dorsal root ganglia and Schwann cells requires p75NTR (18).
Defects in the CNS, including cortical hypoplasia, hydrocephalus, and hippocampal dysgenesis are features of p73 null mice (30), and hippocampal dysgenesis, with almost total loss of the lower blade of the dentate gyrus, is the dominant neurological phenotype of selective TAp73 null mice (30). p75NTR-deficient mice also show significantly reduced hippocampal neurogenesis, with a smaller granular cell layer and dentate gyrus and increased cell death of newly born cells; therefore, the p75NTR-deficient animals largely phenocopy the CNS abnormalities of the TAp73 selective knockout mice (12, 13). Cultures of cortical neurons from total and TAp73-deficeint mice show reduced neurite arborization, which is at least partially dependent on miR-34a (31, 32). However, the role of p75NTR in neurite morphology remains controversial. Hypomorphic expression of p75NTR has been reported to reduce the number of DRG neurons (33), and suppression of p75NTR expression in PC12 cells reduces neurite outgrowth in response to NGF (34). However, p75NTR null mice show an increase in the size of forebrain cholinergic neurons together with an increase in cholinergic hippocampal target innervation (35). In our experiments, we found that p75NTR partially reverted the reduced neurite complexity in NGF-treated cultured cortical neurons from p73 null mice, suggesting that, at least in this model, p75NTR signaling is required for optimal arborization, in addition to the effects mediated through miR-34a. The reduced activity in the open field test in the p73KO mice could also be secondary to reduced p75NTR signaling, because higher levels of NGF in the amygdala have been reported to be associated with reduced anxiety and a corresponding increase in exploratory behavior (36), and the morphological alterations in the hippocampus of p75NTR-deficient mice has also been reported to be associated with impaired performance in a number of behavioral tests.
Deletion of p75NTR also results in a profound loss of sensory but not motor neurons in dorsal root ganglia, which is due to stunting and reduced arborization of peripheral axons (10, 11). We also observed reduced neurite outgrowth and arborization in peripheral nerves from p73KO mice, associated with reduced p75NTR expression. In addition, peripheral nerves from p73KO mice showed a reduction in Schwann cell number, with reduced myelination and nociceptive defects. Moreover, p73-deficient Schwann cells show impaired migration, which is restored by p75NTR expression.
Together, these data demonstrate that the neurological abnormalities associated with p73 deficiency are mediated by loss of transcriptional activation of p75NTR as well as that of miR-34a. β-Amyloid binds p75NTR and mediates β-amyloid–induced neuronal apoptosis in Alzheimer’s disease (AD) hippocampus (37). Hippocampal p75NTR expression is also increased in AD (38). Because we have shown that p73 expression is increased in AD hippocampus (32), the p73/p75NTR axis may play an important role in the pathology of neurodegenerative disease.
Materials and Methods
All results are expressed as mean values ± SD or ± SEM of at least three independent experiments. Unpaired Student t test was used to generate statistical analysis. P values < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Berna Sayan, Maria Guerra, Tim Smith, Jenny Edwards, David Read, Lucia Pinon, Alison Smart, Aimee Craighead, and Colin Molloy at the Medical Research Council Toxicology Unit; Dr. Alessandro Terrinoni at the Istituto Dermopatico dell’Immacolata–Istituto di Ricovero e Cura a Carattere Scientifico (IDI-IRCCS); Dr. Michael Coleman at the Babraham Institute; Dr. Jim Norman at the Beatson Institute; and Moses Chao. This work has been supported by the Medical Research Council (Leicester, UK); grants from “Alleanza contro il Cancro” (ACC12-ACC6); Ministero Istruzione Università Ricerca/Progetti di Ricerca di Interesse Nazionale (RBIP06LCA9_0023); Associazione Italiana per la Ricerca sul Cancro (2008-2010_33-08); 5xmille (#9979); Telethon Grant GGPO9133; Ministero della Salute (Ricerca Oncologica 26/07); and IDI-IRCCS Grant RF06 (RF06 c.73, RF07 c.57, RF08 c.15, RF07 c.57) (to G.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221172110/-/DCSupplemental.
References
- 1.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469(7330):323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dötsch V, Bernassola F, Coutandin D, Candi E, Melino G. p63 and p73, the ancestors of p53. Cold Spring Harb Perspect Biol. 2010;2(9):a004887. doi: 10.1101/cshperspect.a004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Prives C. Are interactions with p63 and p73 involved in mutant p53 gain of oncogenic function? Oncogene. 2007;26(15):2220–2225. doi: 10.1038/sj.onc.1210311. [DOI] [PubMed] [Google Scholar]
- 4.Kaghad M, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90(4):809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 5.Collavin L, Lunardi A, Del Sal G. p53-family proteins and their regulators: Hubs and spokes in tumor suppression. Cell Death Differ. 2010;17(6):901–911. doi: 10.1038/cdd.2010.35. [DOI] [PubMed] [Google Scholar]
- 6.Belyi VA, Levine AJ. One billion years of p53/p63/p73 evolution. Proc Natl Acad Sci USA. 2009;106(42):17609–17610. doi: 10.1073/pnas.0910634106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller FD, Kaplan DR. On Trk for retrograde signaling. Neuron. 2001;32(5):767–770. doi: 10.1016/s0896-6273(01)00529-3. [DOI] [PubMed] [Google Scholar]
- 8.Miller FD, Kaplan DR. Neurotrophin signalling pathways regulating neuronal apoptosis. Cell Mol Life Sci. 2001;58(8):1045–1053. doi: 10.1007/PL00000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cragnolini AB, Friedman WJ. The function of p75NTR in glia. Trends Neurosci. 2008;31(2):99–104. doi: 10.1016/j.tins.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24(3):585–593. doi: 10.1016/s0896-6273(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 11.Bentley CA, Lee KF. p75 is important for axon growth and Schwann cell migration during development. J Neurosci. 2000;20(20):7706–7715. doi: 10.1523/JNEUROSCI.20-20-07706.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catts VS, Al-Menhali N, Burne TH, Colditz MJ, Coulson EJ. The p75 neurotrophin receptor regulates hippocampal neurogenesis and related behaviours. Eur J Neurosci. 2008;28(5):883–892. doi: 10.1111/j.1460-9568.2008.06390.x. [DOI] [PubMed] [Google Scholar]
- 13.Colditz MJ, et al. p75 neurotrophin receptor regulates basal and fluoxetine-stimulated hippocampal neurogenesis. Exp Brain Res. 2010;200(2):161–167. doi: 10.1007/s00221-009-1947-6. [DOI] [PubMed] [Google Scholar]
- 14.Petit TL, LeBoutillier JC. Zinc deficiency in the postnatal rat: Implications for lead toxicity. Neurotoxicology. 1986;7(1):237–246. [PubMed] [Google Scholar]
- 15.Du Y, Fischer TZ, Clinton-Luke P, Lercher LD, Dreyfus CF. Distinct effects of p75 in mediating actions of neurotrophins on basal forebrain oligodendrocytes. Mol Cell Neurosci. 2006;31(2):366–375. doi: 10.1016/j.mcn.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Woo NH, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8(8):1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 17.Rösch H, Schweigreiter R, Bonhoeffer T, Barde YA, Korte M. The neurotrophin receptor p75NTR modulates long-term depression and regulates the expression of AMPA receptor subunits in the hippocampus. Proc Natl Acad Sci USA. 2005;102(20):7362–7367. doi: 10.1073/pnas.0502460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosgaya JM, Chan JR, Shooter EM. The neurotrophin receptor p75NTR as a positive modulator of myelination. Science. 2002;298(5596):1245–1248. doi: 10.1126/science.1076595. [DOI] [PubMed] [Google Scholar]
- 19.Lee KF, et al. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69(5):737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- 20.Gjerstad MD, Aarsland D, Larsen JP. Development of daytime somnolence over time in Parkinson’s disease. Neurology. 2002;58(10):1544–1546. doi: 10.1212/wnl.58.10.1544. [DOI] [PubMed] [Google Scholar]
- 21.Song XY, Zhou FH, Zhong JH, Wu LL, Zhou XF. Knockout of p75(NTR) impairs re-myelination of injured sciatic nerve in mice. J Neurochem. 2006;96(3):833–842. doi: 10.1111/j.1471-4159.2005.03564.x. [DOI] [PubMed] [Google Scholar]
- 22.Bogenmann E, et al. Generation of mice with a conditional allele for the p75(NTR) neurotrophin receptor gene. Genesis. 2011;49(11):862–869. doi: 10.1002/dvg.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chomiak T, Hu B. What is the optimal value of the g-ratio for myelinated fibers in the rat CNS? A theoretical approach. PLoS ONE. 2009;4(11):e7754. doi: 10.1371/journal.pone.0007754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guy J, Ellis EA, Hope GM, Emerson S. Maintenance of myelinated fibre g ratio in acute experimental allergic encephalomyelitis. Brain. 1991;114(Pt 1A):281–294. [PubMed] [Google Scholar]
- 25.Little GJ, Heath JW. Morphometric analysis of axons myelinated during adult life in the mouse superior cervical ganglion. J Anat. 1994;184(Pt 2):387–398. [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, et al. Multiple roles of the p75 neurotrophin receptor in the nervous system. J Int Med Res. 2009;37(2):281–288. doi: 10.1177/147323000903700201. [DOI] [PubMed] [Google Scholar]
- 27.Hempstead BL. The many faces of p75NTR. Curr Opin Neurobiol. 2002;12(3):260–267. doi: 10.1016/s0959-4388(02)00321-5. [DOI] [PubMed] [Google Scholar]
- 28.Culmsee C, et al. Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75. Neuroscience. 2002;115(4):1089–1108. doi: 10.1016/s0306-4522(02)00539-0. [DOI] [PubMed] [Google Scholar]
- 29.Frade JM, Rodríguez-Tébar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383(6596):166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- 30.Yang A, et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404(6773):99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- 31.Rufini A, et al. p73 in Cancer. Genes Cancer. 2011;2(4):491–502. doi: 10.1177/1947601911408890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agostini M, et al. Neuronal differentiation by TAp73 is mediated by microRNA-34a regulation of synaptic protein targets. Proc Natl Acad Sci USA. 2011;108(52):21093–21098. doi: 10.1073/pnas.1112061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hannila SS, Kawaja MD. Distribution of central sensory axons in transgenic mice overexpressing nerve growth factor and lacking functional p75 neurotrophin receptor expression. Eur J Neurosci. 2003;18(2):312–322. doi: 10.1046/j.1460-9568.2003.02752.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang C, et al. Suppression of p75 neurotrophin receptor surface expression with intrabodies influences Bcl-xL mRNA expression and neurite outgrowth in PC12 cells. PLoS ONE. 2012;7(1):e30684. doi: 10.1371/journal.pone.0030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeo TT, et al. Absence of p75NTR causes increased basal forebrain cholinergic neuron size, choline acetyltransferase activity, and target innervation. J Neurosci. 1997;17(20):7594–7605. doi: 10.1523/JNEUROSCI.17-20-07594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yee BK, Singer P, Chen JF, Feldon J, Boison D. Transgenic overexpression of adenosine kinase in brain leads to multiple learning impairments and altered sensitivity to psychomimetic drugs. Eur J Neurosci. 2007;26(11):3237–3252. doi: 10.1111/j.1460-9568.2007.05897.x. [DOI] [PubMed] [Google Scholar]
- 37.Perini G, et al. Role of p75 neurotrophin receptor in the neurotoxicity by beta-amyloid peptides and synergistic effect of inflammatory cytokines. J Exp Med. 2002;195(7):907–918. doi: 10.1084/jem.20011797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakravarthy B, et al. Hippocampal membrane-associated p75NTR levels are increased in Alzheimer’s disease. J Alzheimers Dis. 2012;30(3):675–684. doi: 10.3233/JAD-2012-120115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.