Abstract
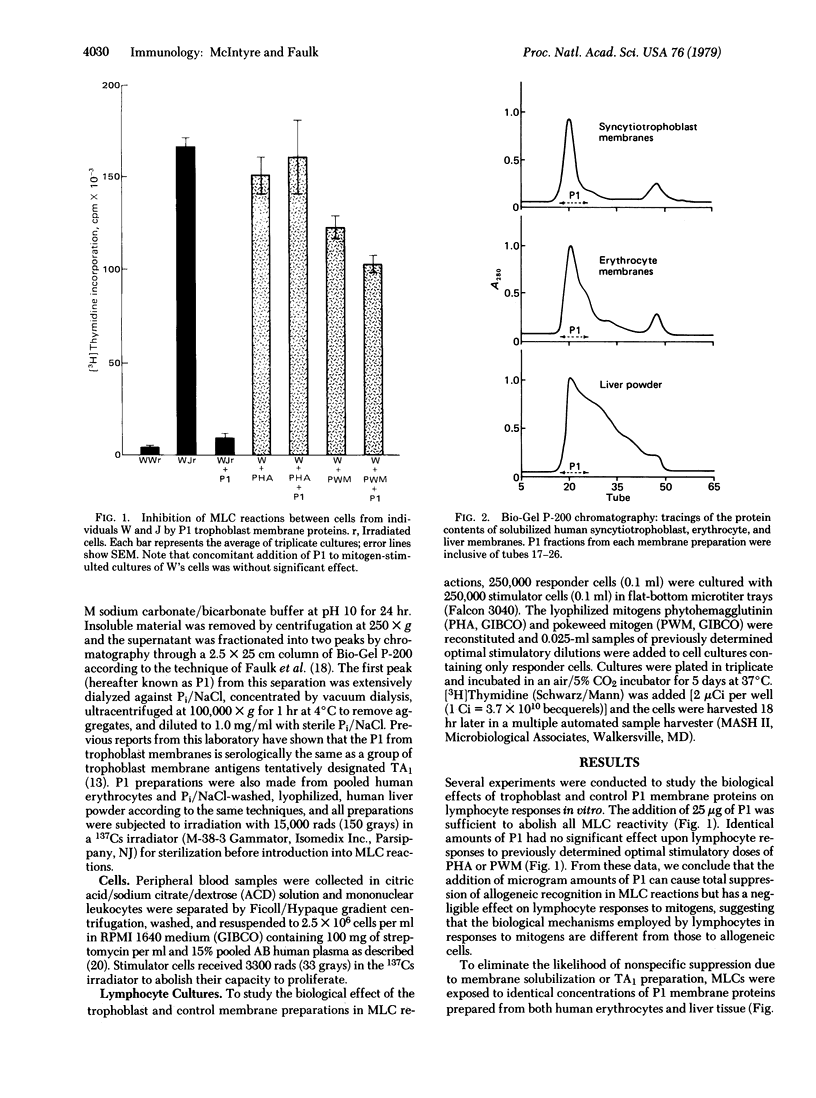
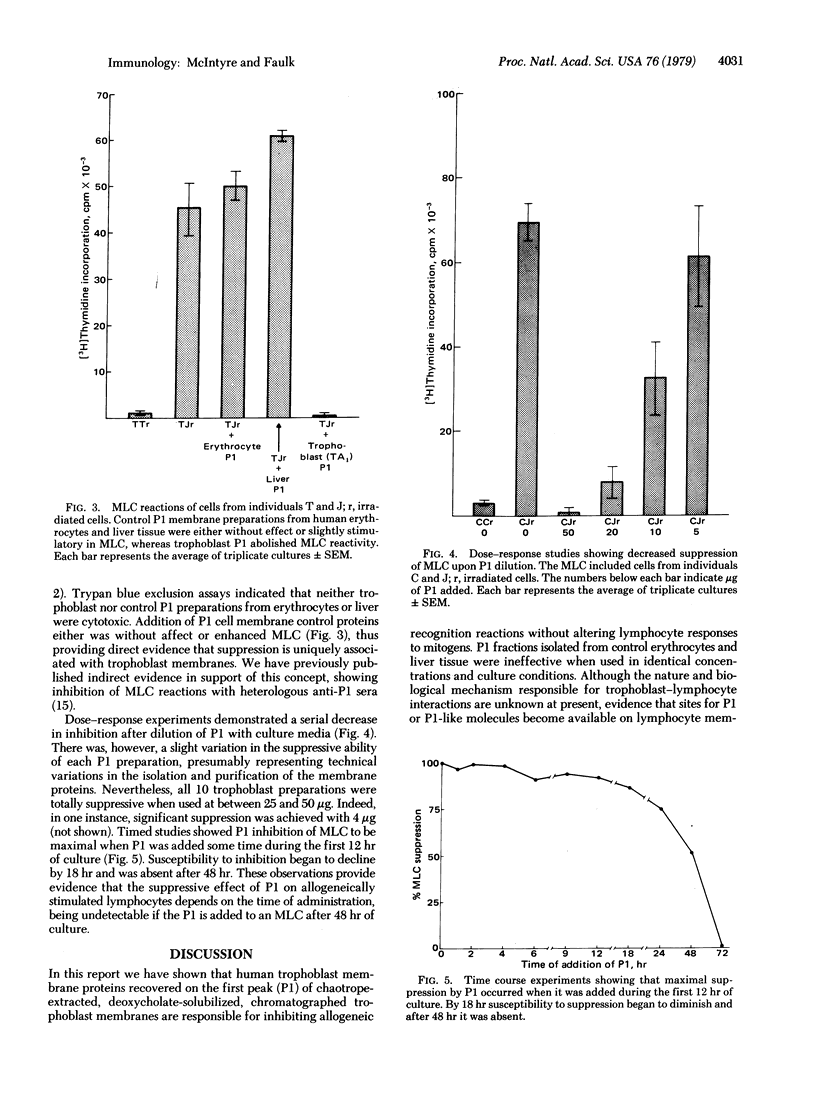
Human syncytiotrophoblast cell membranes prepared by differential ultracentrifugation were extracted with 3 M KCl, solubilized in 1% deoxycholate, and chromatographically separated into two peaks by passage through a column of Bio-Gel P-200. Previous reports from this laboratory have shown that the first peak (PI) is serologically the same as a group of trophoblast membrane antigens tentatively designated as TA1. Microgram amounts of P1 protein were found to completely inhibit the mixed lymphocyte culture (MLC) reaction but had no suppressive effect on lymphocyte responses to the lectins phytohemagglutinin or pokeweed mitogen. Control P1 membrane fractions identically prepared from human erythrocytes and liver powder had no inhibitory effects on either MLC reactions or lymphocyte responses to mitogens. Dose response experiments with P1 from 10 different placentae showed total inhibition of MLC by all preparations when used between 25 and 50 microgram/0.2-ml MLC mixture, but some P1 fractions inhibited significantly at much lower concentrations. Timed experiments revealed that MLC suppression was maximal when P1 was added within 12 hr after culture initiation and that no effect could be found with addition after 48 hr. We have previously shown that TA1 is a lymphocyte product of allogeneic responses, and the present results indicate that P1 proteins are themselves involved in the biology of lymphocyte responses to allogeneic cells. Pregnancy is one of the few natural circumstances in which a mixing of allogeneic cells occurs in vivo, and the presence of P1 proteins at the operational interface in the host-parasite relationship of human pregnancy suggests that this trophoblast membrane constituent is involved in the modulation of maternal allogeneic responses.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Faulk W. P., Galbraith G. M. Trophoblast transferrin and transferrin receptors in the host--parasite relationship of human pregnancy. Proc R Soc Lond B Biol Sci. 1979 Mar 26;204(1154):83–97. doi: 10.1098/rspb.1979.0014. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., Jeannet M., Creighton W. D., Carbonara A. Immunological studies of the human placenta. Characterization of immunoglobulins on trophoblastic basement membranes. J Clin Invest. 1974 Nov;54(5):1011–1019. doi: 10.1172/JCI107844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk W. P., Sanderson A. R., Temple A. Distribution of MHC antigens in human placental chorionic villi. Transplant Proc. 1977 Jun;9(2):1379–1384. [PubMed] [Google Scholar]
- Faulk W. P., Temple A. Distribution of beta2 microglobulin and HLA in chorionic villi of human placentae. Nature. 1976 Aug 26;262(5571):799–802. doi: 10.1038/262799a0. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., Temple A., Lovins R. E., Smith N. Antigens of human trophoblasts: a working hypothesis for their role in normal and abnormal pregnancies. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1947–1951. doi: 10.1073/pnas.75.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk W. P., van Loghem E., Stickler G. B. Maternal antibody to fetal light chain (Inv) antigens. Am J Med. 1974 Mar;56(3):393–397. doi: 10.1016/0002-9343(74)90621-4. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Barnstable C. J., Bodmer W. F., Snary D., Crumpton M. J. Expression of HLA system antigens on placenta. Transplantation. 1976 Dec;22(6):595–603. doi: 10.1097/00007890-197612000-00009. [DOI] [PubMed] [Google Scholar]
- Hoyes A. D. Structure and function of the amnion. Obstet Gynecol Annu. 1975;4:1–38. [PubMed] [Google Scholar]
- Jeannet M., Werner C., Ramirez E., Vassalli P., Faulk W. P. Anti-HLA, anti-human "Ia-like" and MLC blocking activity of human placental IgG. Transplant Proc. 1977 Jun;9(2):1417–1422. [PubMed] [Google Scholar]
- Kahn C. R., Baird K., Filier J. S., Jarrett D. B. Effects of autoantibodies to the insulin receptor on isolated adipocytes. Studies of insulin binding and insulin action. J Clin Invest. 1977 Nov;60(5):1094–1106. doi: 10.1172/JCI108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Gorden P., Flier J. S., Kahn C. R., Freychet P. Anti-insulin receptor antibodies inhibit insulin binding and stimulate glucose metabolism in skeletal muscle. Diabetologia. 1978 May;14(5):311–317. doi: 10.1007/BF01223022. [DOI] [PubMed] [Google Scholar]
- Levine P. Biological and clinical significance of differences between RBC membrane (Rh) and non-membrane (ABH, MN, P) antigenic sites: illegitimate ABO, M-N (T), P (Tja) antigens in malignancy. Rev Fr Transfus Immunohematol. 1976 Mar;19(1):213–229. doi: 10.1016/s0338-4535(76)80100-6. [DOI] [PubMed] [Google Scholar]
- McIntyre J. A., Faulk W. P. Antigens of human trophoblast. Effects of heterologous anti-trophoblast sera on lymphocyte responses in vitro. J Exp Med. 1979 Apr 1;149(4):824–836. doi: 10.1084/jem.149.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre J. A., Faulk W. P. Suppression of mixed lymphocyte cultures by antibodies against human trophoblast membrane antigens. Transplant Proc. 1978 Dec;10(4):919–922. [PubMed] [Google Scholar]
- McIntyre J. A., Turner R. E., 3rd, LeRoy E. C. Human leukocyte antigens (HLA). South Med J. 1978 Oct;71(10):1269–1279. doi: 10.1097/00007611-197810000-00021. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Kitzmiller J. L., Carpenter C. B., Garovoy M. R., David J. R. Maternal-fetal relation. Absence of an immunologic blocking factor from the serum of women with chronic abortions. N Engl J Med. 1976 Nov 25;295(22):1209–1213. doi: 10.1056/NEJM197611252952201. [DOI] [PubMed] [Google Scholar]
- Smith N. C., Brush M. G., Luckett S. Preparation of human placental villous surface membrane. Nature. 1974 Nov 22;252(5481):302–303. doi: 10.1038/252302b0. [DOI] [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J., Hockley D. J. Host antigens in schistosomiasis. Proc R Soc Lond B Biol Sci. 1969 Feb 25;171(1025):483–494. doi: 10.1098/rspb.1969.0007. [DOI] [PubMed] [Google Scholar]
- Taylor P. V., Hancock K. W. Antigenicity of trophoblast and possible antigen-masking effects during pregnancy. Immunology. 1975 May;28(5):973–982. [PMC free article] [PubMed] [Google Scholar]



