Abstract
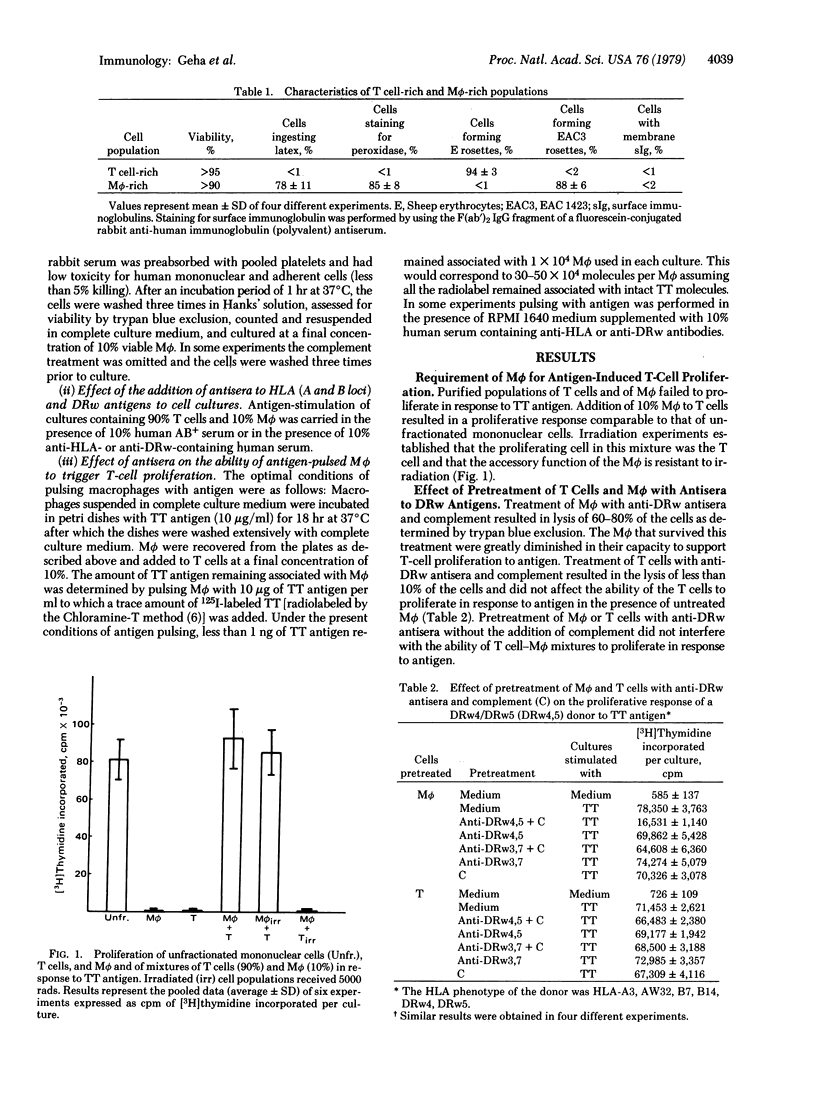
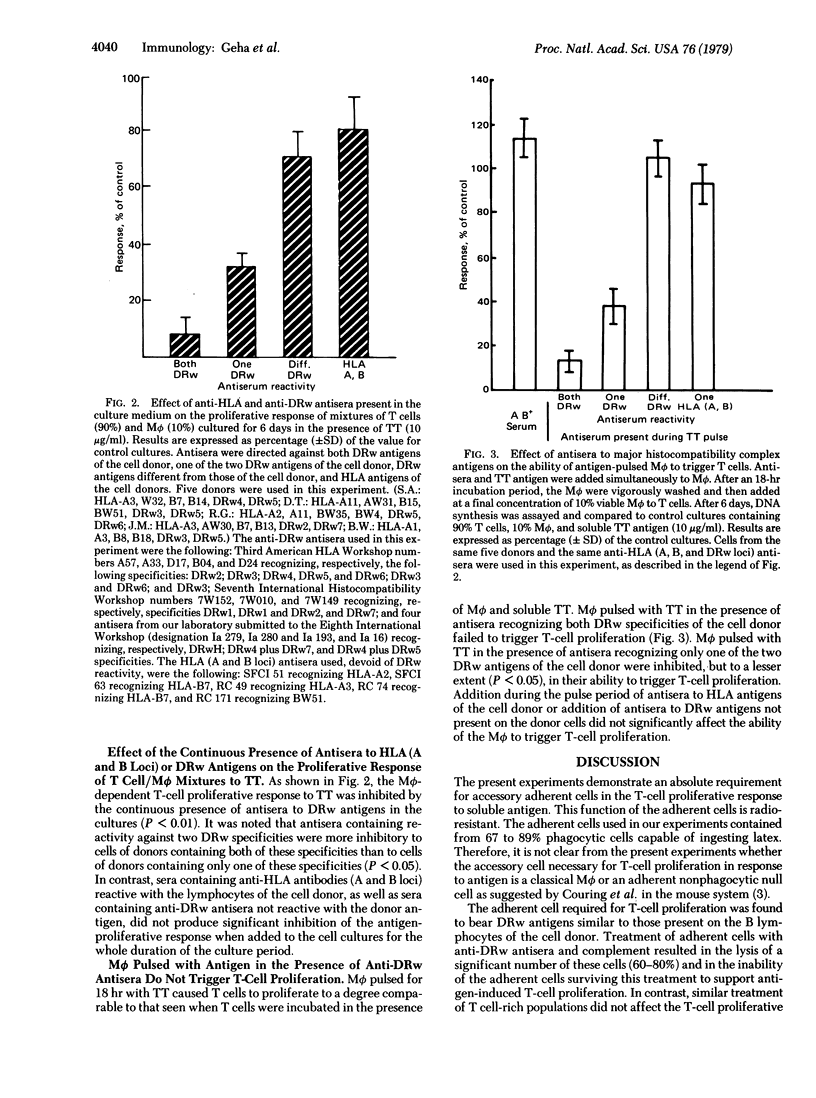
Human T lymphocytes were shown to proliferate in response to tetanus toxoid antigen only in the presence of macrophages. This response was inhibited by anti-DRw but not by anti-HLA (A and B loci) antisera added to the cultures and by pretreatment of macrophages but not of T cells with anti-DRw antisera and complement. Macrophages pulsed for 18 hr with antigen and then washed were capable of triggering T-cell proliferation. Addition of anti-DRw but not anti-HLA (A and B loci) antisera during the pulse period inhibited the macrophages' ability to trigger T-cell proliferation. The data obtained indicate that human T cells recognize and proliferate in response to antigen presented by the macrophages in association with Ia-like antigens.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cowing C., Pincus S. H., Sachs D. H., Dickler H. B. A subpopulation of adherent accessory cells bearing both I-A and I-E or C subregion antigens is required for antigen-specific murine T lymphocyte proliferation. J Immunol. 1978 Nov;121(5):1680–1686. [PubMed] [Google Scholar]
- Evans R. L., Faldetta T. J., Humphreys R. E., Pratt D. M., Yunis E. J., Schlossman S. F. Peripheral human T cells sensitized in mixed leukocyte culture synthesize and express Ia-like antigens. J Exp Med. 1978 Nov 1;148(5):1440–1445. doi: 10.1084/jem.148.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S. M., Chiorazzi N., Wang C. Y., Montrazeri G., Kunkel H. G., Ko H. S., Gottlieb A. B. Ia-bearing T lymphocytes in man. Their identification and role in the generation of allogeneic helper activity. J Exp Med. 1978 Nov 1;148(5):1423–1428. doi: 10.1084/jem.148.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Ruhl H., Shevach E. M. The effect of alloantisera on antigen-induced T cell proliferation. J Immunol. 1975 Dec;115(6):1493–1499. [PubMed] [Google Scholar]
- Seeger R. C., Oppenheim J. J. Synergistic interaction of macrophages and lymphocytes in antigen-induced transformation of lymphocytes. J Exp Med. 1970 Jul 1;132(1):44–65. doi: 10.1084/jem.132.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda S., Schlamowitz M. Studies of 125I trace labeling of immunoglobulin G by chloramine-T. Immunochemistry. 1970 Nov;7(11):885–898. doi: 10.1016/0019-2791(70)90051-0. [DOI] [PubMed] [Google Scholar]


