Abstract
Intravascular catheter-related infections are still a major problem in health care and are associated with significant morbidity, mortality, and additional cost. The formation of microbial biofilm on catheters makes these infections particularly complicated, as microbial cells that detach from the biofilm can lead to infection, and because these microorganisms are highly resistant to many antimicrobial agents; thus, catheter removal is often required to successfully treat infection. To reduce the risks of catheter-related infections, many strategies have been applied, such as improvements in aseptic insertion and post-insertion care practices, implantation techniques, and antibiotic coated or impregnated materials. However, despite significant advances in using these methods, it has not been possible to completely eradicate biofilm infections. Currently, nanotechnology approaches seem to be among the most promising for preventing biofilm formation and resultant catheter-related bloodstream infection (especially with multi-resistant bacterial strains). In this review, current knowledge about catheter technology and design, the mechanisms of catheter-related bloodstream infection, and the insertion and care practices performed by medical staff, are discussed, along with novel, achievable approaches to infection prevention, based on nanotechnology.
Keywords: catheter related infections, biofilm, nanotechnology
Introduction
Intravascular catheter- (IVC-) related infections lead to high morbidity and mortality for patients, and increase costs of health care.1 Infections develop when microorganisms adhere to catheter surfaces and produce extracellular substances that facilitate adhesion (and provide a structural matrix) for forming biofilms.2 Following biofilm development, microbial cells from the biofilm maturate and can periodically disperse into the bloodstream, causing serious infections.2 Biofilms are resistant to host defence mechanisms and antibiotic agents, making the treatment of catheter-related infections more challenging. In order to prevent catheter-related infections, a large number of strategies and approaches have been developed, including strict hygienic procedures during catheter insertion and use; total or partial (tunneling) implantation of long-term catheters; surface modification of catheter biomaterials with antimicrobial coatings or impregnation; and antibiotic or antimicrobial locking solutions.2 However, it remains impossible to achieve a completely anti-adhesive catheter material since catheter surfaces can be rapidly covered by plasma and matrix proteins, on which bacteria display specific adhesions. The challenge of biofilm-related infections remains. Nanotechnologies, especially nanomaterials, are one of the more promising new strategies that aim to prevent biofilm infections in patients with IVCs.
This review will summarize current knowledge about catheter-related bloodstream infections (CRBSIs), as well as the nanotechnologies in use, or in development, to prevent catheter-related infections occurring due to colonization and/or biofilm formation on catheter surfaces. We also discuss the knowledge gained from microbial research in other medical and non-medical applications that may be helpful to understanding the IVC context. In addition, published theories and data regarding microbial colonization and biofilm development, specifically related to IVCs, are reviewed. This review aims to provide baseline information for the future development of new and effective strategies to prevent catheter-related infections.
Catheter technologies and catheter design
Intravenous catheters have evolved considerably, from the early prototypes of goose quill, silver, glass, and steel tubes, to modern day polyurethane- and silicone-based models. The ideal catheter exhibits a high tensile strength, is soft and pliable, inherently chemical-resistant, biocompatible, and meets flow requirements. Common catheter materials include polyurethane, silicone, polyethylene and Teflon®. A comparison of the relevant characteristics of these catheter materials is shown in Table 1. Polyurethane catheters are now often the preferred material. They are highly biocompatible (therefore, well-tolerated by patients), compatible with the majority of drugs, and resistant to many chemicals.3 Nevertheless, the repeated application of alcohol-based cleaning agents potentially can perish the catheter. Polyurethane is thromboresistant, and softens within the body. Therefore, mechanical trauma and irritation within the vein are reduced, compared against harder materials.4 Other advantages of polyurethane are its tensile strength, multiple lumens, and smaller external diameters, which maximize blood flow in a vessel with catheter. Modern polyurethane catheters are now available as semirigid yet flexible materials, often used for short-term catheter access. They are sufficiently stiff for percutaneous insertion over a wire without splitting the sheath, yet soften in the body after insertion. Silicone catheters are also biocompatible and compatible with most drugs, as well as alcohol-based cleaning solutions, although they can be damaged by peroxide and some povidone-iodine solutions. Silicone is soft, pliable, and thromboresistant,4 but it has limited tensile strength, resulting in easy rupture, and it requires a restricted infusion flow pressure.5 Silicone catheters need insertion through a sheath or cannula. Other IVC materials such as polyethylene and Teflon® (tetrafluoroethylene-hexafluoropropylene) are less favorable catheter materials, and have nearly been replaced by polyurethane and silicone, since they are less biocompatible, stiffer, and have poor flexibility.4 Teflon has also been shown to have higher infection rates, compared against the other three catheter materials.6
Table 1.
Comparison of catheter material characteristics
| Polyurethane | Silicone | Polyethylene | Teflon® | |
|---|---|---|---|---|
| Biocompatiblity | Excellent | Excellent | Fair | Fair |
| Stiffness | Softens in body | Soft | Stiff | Stiff |
| Ease of insertion | Difficult | Fair | Easy | Easy |
| Ease of modifying | Fair | Easy | Difficult | Difficult |
| Tensile strength | Excellent | Fair | Excellent | Excellent |
| Flexibility | Fair | Excellent | Poor | Poor |
| Coefficient of friction | Excellent | Fair | Good | Excellent |
| Infection rate | Low | Fair | High | High |
There are numerous types of IVC, with varying designs, for different functional requirements and durations of use. Short-term central venous catheters are usually made of polyurethane.7 They are relatively short tapered, open-ended mutilumen catheters, used for 3–10 days. Long-term central venous catheters may be used for months or years, and have a catheter portion “tunneled” or “cuffed” under the skin. Alternatively, totally implanted “ports” may be implanted into a central vein, and periodically accessed using a needle, for long-term therapy. Small, peripheral venous or arterial catheters are single lumen, and are also commonly made of polyurethane. Midline catheters are single or dual lumen, predominantly composed of silicone, and are used for 2–4 weeks.8 Peripherally-inserted central catheters (PICCs) can be made of polyurethane or silicone, with single to triple lumen types available. They are recommended for mid-term therapy: from 6 weeks to 1 year.9 PICCs can be valved or non-valved. Non-valved PICCs are open-ended and require regular positive flushing after use, to prevent blood backflow causing occlusion.10 Valved PICCs contain a pressure-sensitive valve at the side of the distal end, which allows both fluid infusion and blood aspiration;10 therefore, heparinized flushes are not required. Hemodialysis catheters are composed of silicone or polyurethane and can be cuffed, or non-cuffed, depending on the duration of usage. These catheters have large bores and relatively stiff constructions, to avoid wall collapse under negative pressure from pumped systems.11 The lumen has one end-hole and multiple side holes, to improve flows and mixing of blood. Two functionally separate catheters can be inserted in parallel, their tips lying slightly separated, in the same vein.11 Alternatively, a combined dual or triple lumen catheter can be used, which has a staggered design: one lumen positioned 3–5 cm above the other, to prevent recirculation of treated blood. To prevent thrombotic events occurring within this type of catheter, heparin locks are essential. Late catheter malfunction is often the consequence of fibrin deposition around the catheter tip – an unpredictable process, but one that occurs more frequently in some patients and less frequently in others.11
Catheter-related bloodstream infection
The use of IVCs is essential for the successful management of critically and chronically ill patients.12 However, CRB-SIs have become a leading cause of health care-associated bloodstream infections, and are associated with substantial morbidity and mortality.13 More than 250,000 CRBSIs occur annually in the USA, with an attributable mortality ranging from 12%–25% in critically ill patients, and with an added cost ranging from US$4,000–$56,000.14,15
CRBSI is defined as 1) fever and other clinical manifestations of bloodstream infection in a patient with an IVC; 2) the presence of positive simultaneous quantitative blood cultures from the IVC and the peripheral vein, yielding the same organism; 3) no apparent source for the bloodstream infection, other than the catheter; and 4) positive semi-quantitative catheter tip cultures with ≥15 colony forming units (CFUs) of the same microorganisms as isolated from the blood cultures. Alternatively, CRBSI can be diagnosed through simultaneous quantitative blood cultures, whereby the number of colonies isolated from the blood drawn through the IVC is at least three times greater (ratio: ≥3:1) than the number of colonies isolated from blood drawn via a peripheral vein, or from one of two different lumen-drawn blood cultures from multi-lumen catheters (“possible CRBSI”).1 The differential between time-to-positivity of peripherally-drawn blood cultures of ≥2 hours growth, and simultaneously drawn peripheral venous blood cultures, is also diagnostic of CRBSI.1
CRBSI most frequently develops in seriously ill patients admitted to hematology-oncology and intensive care units (ICUs) of acute care hospitals. The most commonly reported causative pathogens for CRBSI are coagulase-negative staphylococci, Staphylococcus aureus, enterococci, and Candida spp. (Table 2).16,17 According to the US Centers for Disease Control and Prevention’s database, approximately 19% of CRBSI cases involve gram-negative bacilli.18 Antimicrobial resistance is a concern with all pathogens responsible for CRBSIs. It has been demonstrated that methicillin-resistant S. aureus (MRSA) accounts for more than 50% of all S. aureus isolated in ICUs – although the incidence of CRBSI caused by skin organisms (particularly MRSA) in ICUs has decreased in recent years, due to coordinated efforts in many countries to improve sterile insertion procedures to prevent insertion-related contamination of central venous catheters.16,17 By contrast, with regard to gram-negative rods, the incidence of antimicrobial resistance to third-generation cephalosporins among Klebsiella pneumonia and Escherichia coli is increasing significantly, along with imepenem and ceftazidine reistance among Pseudomonas aeruginosa.18
Table 2.
Incidence rates of most commonly isolated pathogens from MNBSIs, and associated crude mortality rates, for patients in ICU and non-ICU wards 17
| Pathogen | CRBSI per 10,000 admissions | Percentage of CRBSI
|
Crude mortality (%)
|
||||
|---|---|---|---|---|---|---|---|
| Total (n=20,978) | ICU (n=10,515) | Non-ICU ward (n=10,442) | Total | ICU | Non-ICU ward | ||
| Coagulase-negative | 15.8 | 31.3 | 35.9 | 26.6 | 20.7 | 25.7 | 13.8 |
| Staphylococcus | |||||||
| S. aureus | 10.3 | 20.2 | 16.8 | 23.7 | 25.4 | 34.4 | 18.9 |
| Enterococcus spp. | 4.8 | 9.4 | 9.8 | 9.0 | 33.9 | 43.0 | 24.0 |
| Candida spp. | 4.6 | 9.0 | 10.1 | 7.9 | 39.2 | 47.1 | 29.0 |
| Escherichia coli | 2.8 | 5.6 | 3.7 | 7.6 | 22.4 | 33.9 | 16.9 |
| Klebsiella spp. | 2.4 | 4.8 | 4.0 | 5.5 | 27.6 | 37.4 | 20.3 |
| Pseudomonas aeruginosa | 2.1 | 4.3 | 4.7 | 3.8 | 38.7 | 47.9 | 27.6 |
| Enterobacter spp. | 1.9 | 3.9 | 4.7 | 3.1 | 26.7 | 32.5 | 18.0 |
| Serratia spp. | 0.9 | 1.7 | 2.1 | 1.3 | 27.4 | 33.9 | 17.1 |
| Acinetobacter baumannii | 0.6 | 1.3 | 1.6 | 0.9 | 34.0 | 43.4 | 16.3 |
Abbreviations: MNBSI, monomicrobial nosocomial blood stream infection; ICU, intensive care unit; CRBSI, catheter-related bloodstream infection.
It is believed that there are four pathways for bacteria to enter into the sterile bloodstream and cause catheter-related infections.16 The first is extraluminal contamination of the catheter with skin organisms, occurring during insertion, or migration of such organisms down the catheter tract while it is in place. This is the most common infection route for short-term catheters. The second route is intraluminal, involving contamination of catheter hubs and connectors through contact with the hands of hospital staff who use the catheter to install medicine or take blood. The third route is direct contamination of the catheter by bacteria circulating in the bloodstream; for example, following translocation of gastrointestinal flora through the intestinal wall. The fourth pathway is through contaminated infusate, which may occur at the manufacturing stage (intrinsic contamination), or during manipulation by health care staff when preparing or administering fluids (extrinsic contamination).
If contamination occurs, the initial microbial attachment of bacteria onto the inner and outer surfaces of IVCs is almost inevitably followed by biofilm development and maturation, which is followed by dispersion of microbial cells from the biofilm into the bloodstream, causing CRBSI. Microorganisms embedded in biofilms typically present phenotypic and genotypic characteristics different to those grown planktonically.19 They are able to obtain and concentrate a number of different nutrients from the environment.19 They are resistant to a number of antimicrobial agents, not only because the antimicrobials cannot penetrate into all the biofilm layers, but also because the organisms grow slowly and may then be resistant to immune defence mechanisms.20 The biofilm mode can also facilitate dissemination of organisms. Furthermore, microorganisms may exhibit different virulence phenotypes when growing within a biofilm; these phenotypes may not have been detected from IVCs in the past, because traditional hospital diagnostic tests, such as the semiquantitative roll-plate technique, used to culture catheter tip segments, involve growth of organisms on rich nutrient media, under planktonic conditions.21 Rather than being amorphous aggregates, biofilms are complex, structured communities in which physiological conditions, such as nutrient and oxygen availability, vary at different depths.22 Therefore, the microorganisms at different depths are phenotypically, morphologically, and functionally different. Once biofilm infection occurs, the host will establish an immune response to antigens released from the biofilm. However, not only may the host’s immune system fail to eradicate the biofilm, it may also result in damage to surrounding tissues.
The pathogenesis of fibrin sheath formation from biofilms is still not understood. After biofilm formation, fibrin and many other molecules, such as laminin, collagen, and even muscle cells, convert the biofilm to a mature sheath.23 Metallic cations, such as magnesium, calcium, and iron, may stabilize the biofilm and facilitate biofilm development and bacterial growth.24 Catheter thrombosis on the fibrin sheath may be facilitated by platelet activation, decreased levels of protein C and antithrombin III, hyperfibrinogenemia, and homocysteine elevation. It has been demonstrated that the presence of catheter-related thrombosis increases the risk of CRBSI.25,26 Many patients require long-term IVCs, and attempts have been made to treat CRBSI without removing the IVC, with variable results. However, as understanding of biofilm structure and function develops, it is clear why often the only solution to an infected IVC is catheter removal, which brings increased hospital costs, additional painful invasive procedures for patients, and interruption to medical therapy.
There are numerous recommended preventive strategies for clinicians to avoid CRBSIs, and these are supported by varying levels of evidence for their effectiveness. Strategies include issues of education, training, and staffing among health care providers who insert and maintain IVCs; selection of catheters and sites; hand hygiene and aseptic technique; maximal sterile barrier precautions on insertion; pre-insertion skin preparation; catheter site dressing regimens; patient cleansing (bathing); catheter securement devices; antimicrobial/antiseptic impregnated catheters (for >5 day catheters, if other education-based interventions have not been effective); antibiotic/antimicrobial ointments for dialysis catheter sites; antimicrobial lock solutions for patients with repeated CRBSI and long-term IVCs; at least weekly replacement of IV infusion tubing; disinfection of needleless connectors prior to use; and replacement of connectors at least every 72 hours.16
The majority of recommended CRBSI prevention strategies target improved clinical practices among hospital staff, rather than technological approaches. The most successful development in recent years has been the implementation of central venous catheter “care bundles”, which are quality campaigns targeting CRBSI in ICUs. Such initiatives comprise simultaneous implementation, and focus on consistent use of, five best practice procedures, in the context of key stakeholder championship, with ongoing audit and feedback of infection rates to staff. Numerous national and international campaigns have been launched, stemming from the successful Keystone ICU Project in Michigan, USA.27 The bundles focus predominantly on the insertion procedure, including hand hygiene and maximal sterile barrier precautions by the inserter; decontamination of the skin site pre-insertion (with 2% chlorhexidine gluconate in 70% alcohol); avoidance of the femoral insertion site, if possible; and removal of unnecessary IVCs. It is widely agreed that such campaigns have greatly reduced, but not eliminated, CRBSIs, particularly those stemming from extra-luminal contamination, occurring during the insertion procedure.16 There is a resultant need to now focus on maintaining those successful infection-prevention practices, and extending them outside of the ICU setting, while adding improvements in post-insertion care, to focus on intraluminal colonization-related infections.28
Nanotechnology
The foundation material of the IVC can also be coated or impregnated with antibiotic or antiseptic agents, to reduce the risk of CRBSIs. Despite significantly higher initial purchase costs, such catheters have been associated with an overall decrease in hospital costs associated with treating CRBSIs. Comparing infection rates of non-impregnated and impregnated catheters, results indicate that impregnation could reduce catheter-related infection rates in various settings and countries (Table 3). However, concerns exist about the potential for development of antimicrobial/antibiotic resistance. Currently, antiseptic/antibiotic catheters are only recommended for short-term use if the CRBSI rate does not decrease, despite adherence to basic prevention measures.29 While antiseptic/antibiotic-impregnated intravascular catheters have been shown to decrease the rate of CRBSI in patients with short-term catheters, the benefit in patients with long-term catheters remains unclear. There is additional concern about the potential to generate multidrug-resistant organisms. In all, none of these strategies seems able to totally prevent CRBSIs; nanotechnology might bring new hope.
Table 3.
Rates of catheter-related colonization and bloodstream infection associated with non-impregnated and antiseptic/antibiotic-impregnated intravascular catheters
| Catheter type | Study | Country | Setting | Number | Catheter colonization | Catheter-related bloodstream infection |
|---|---|---|---|---|---|---|
| Non-impregnated | Hanna et al37 | USA | Single center | 174 | n/a | 14 (8.0%)a |
| Ostendorf et al34 | Germany | Single center | 94 | 31 (33%) | 7 (7%)b | |
| Jaeger et al102 | Germany | Single center | 55 | 9 (16.4%) | 8 (14.5%)c | |
| Sheng et al103 | Taiwan | Single center | 122 | 25 (20.5%) | 2 (1.6%)d | |
| Lorente et al104 | Spain | Single center | 287 | n/a | 12 (4.18%)e | |
| Impregnated with | ||||||
| Silver sulfadiazine/chlorhexidine | Walder et al33 | Switzerland | Multiple centers | 1,544 | n/a | 65 (4.2%) |
| Ostendorf et al34 | Germany | Single center | 90 | 11 (12%) | 3 (3%)b | |
| Jaeger et al102 | Germany | Single center | 51 | 5 (9.8%) | 1 (1.96%)c | |
| Darouiche et al36 | USA | Multiple centers | 382 | 87 (22.8%) | 13 (3.4%)f | |
| Minocycline/rifampin | Darouiche et al36 | USA | Multiple centers | 356 | 28 (7.9%) | 1 (0.3%)f |
| Hanna et al37 | USA | Single center | 182 | n/a | 3 (1.65%)a | |
| Sheng et al103,d | Taiwan | Single center | 113 | 9 (7.1%) | 1 (0.9%)d | |
| Lorente et al104 | Spain | Single center | 238 | n/a | 0e | |
Notes:
Odds ratio for CRBSI (catheter-related bloodstream infection): 0.1 for minocycline/rifampin, compared against non-impregnated catheters; P<0.001
odds ratio for colonization: 0.36 silver sulfadiazine/chlorhexidine-impregnated, compared against non-impregnated catheters; P=0.01. No significant differences for CRBSI
odds ratio for colonization: 0.46 silver sulfadiazine/chlorhexidine-impregnated, compared against non-impregnated catheters; P=0.035. Odds ratio for CRBSI: 0.12 silver sulfadiazine/chlorhexidine-impregnated, compared against non-impregnated catheters, P=0.02
odds ratio for colonization: 0.34 silver sulfadiazine/chlorhexidine-impregnated, compared against non-impregnated catheters; P=0.006. No significant differences for CRBSI
odds ratio for CRBSI: 0.13 for minocycline/rifampin, compared against non-impregnated catheters; P<0.05
odds ratio for colonization: 0.31 for minocycline/rifampin, compared against silver-sulfadiazine/chlorhexidine impregnated catheters; P<0.001. Odds ratio for CRBSI: 0.08 for minocycline/rifampin, compared against silver-sulfadiazine/chlorhexidine impregnated catheters; P<0.0001.
Abbreviation: n/a, not available.
The use of antimicrobial agents was extended to IVC dressing. In one randomized multicenter assessor-blind trial, 1,636 patients with catheter dressings, with or without chlorhexidine-impregnated sponges as part of the standard, transparent, semipermeable polyurethane IVC dressing, were evaluated.30 A total of 3,778 catheters were enrolled (28,931 catheter-days). The chlorhexidine-impregnated sponge dressings decreased rates of major catheter-related infections (0.6 per thousand catheter-days versus [vs] 1.4 per thousand catheter-days; hazard ratio [HR], 0.39; 95% confidence interval [CI], 0.17–0.93 per thousand catheter-days; P=0.03) and CRBSIs (0.40 per thousand catheter-days vs 1.3 per thousand catheter-days; HR, 0.24;CI, 0.09–0.65 per thousand catheter-days; P<0.001).30 A randomized controlled study of a chlorhexidine-impregnated sponge dressing in 74 children showed that it could reduce the rates of catheter colonization (HR, 0.61; CI, 0.3716–1.023; P=0.0446), but there was no statistical difference in CRBSIs when compared with no antimicrobial dressings.31 One possible reason is that the study was underpowered to detect these differences.
Catheters whose outer surfaces are impregnated with chlorhexidine and silver sulfadiazine have been confirmed to reduce the risks of CRBSIs.32 They remained effective when the median duration of insertion time was less than 7 days (median, 6 days; interquartile range [IQR] 5.2–7.5 days) compared with control catheters (median 12 days; IQR 7.8–20 days).33 Second-generation catheters are manufactured with chlorhexidine coating on the internal surfaces, extending into the extension set and hubs, while the external luminal surface is coated with chlorhexidine and silver sulfadiazine.34,35 Although it has been shown that second-generation catheters can reduce catheter colonization, a significant decrease of the CRBSI rate was not detected.34,35 Catheters impregnated with minocycline and rifampin on external and internal surfaces were associated with lower risks of CRBSI, compared against catheters with external coating of chlorhexidine and silver sulfadiazine.36 Silicone catheters impregnated in both the external and internal surfaces with a combination of minocycline and rifampin can decrease the rate of CRBSIs, compared against controls, even with an average dwell time of 60 days.37 No correlation has been shown between the usage of minocycline- and rifampin-impregnated catheters and the development of antimicrobial resistance, or the selection of resistant flora.
Thrombus proteins can also increase bacterial attachment on IVCs, and have been associated with CRBSIs. It has been shown that the formation of a fibrin sheath around the catheter greatly increases catheter colonization.38 Heparin-coated catheters were reported to be able to decrease thrombosis, and the risks of CRBSIs.39 However, the potential benefits of heparin, or heparin-coated catheters, must be balanced against the small, but important, risk of heparin-induced thrombocytopenia. Because heparin solutions contain preservatives with antimicrobial activity, it is unknown whether a decrease in the CRBSI rate is due to decreased thrombus formation, or due to the preservative.
Many metal ions have antimicrobial activity. Among these, silver has the highest level of toxicity for microorganisms and the lowest toxicity for animal cells.40 Silver nanoparticles are clusters of silver atoms that exhibit strong bactericidal activity, against both gram-positive and gram-negative bacteria.41 Silver ions can inhibit replication of bacteria (through binding to the microbial DNA) and/or switch off important enzymes, leading to microbial death (Figure 1).42 It has also been suggested that silver nanoparticles could prevent biofilm formation, since they have an affinity for proteinaceous compounds, where they combine with the sulfhydryl group, inducing protein denaturation and corresponding enzyme inactivation.43 As yet, silver nanoparticles have not been shown to cause microbial resistance, in contrast to increasing microbial resistance towards many traditional antimicrobial agents, and the consequent development of resistant strains. A possible explanation is that silver nanoparticles do not only exert their antibacterial effects at a particular site, but at several locations, such as the bacterial wall, during proteosynthesis, and in DNA.41 Silver nanoparticle-coatings could exert their antimicrobial properties in vivo, by slowly releasing silver ions.44 Silver nanoparticles enable a constant local supply of silver ions at the coating–tissue interface, and also allow improved contact with the microorganisms.45 Therefore, the prevention of microbial adhesion and biofilm formation is more prolonged than with other antimicrobial approaches. Thus, IVC coating with silver nanoparticles could protect both outer and inner surfaces of catheters through continuous release of silver ions, to provide antimicrobial activity.
Figure 1.
Mechanisms of silver ions against bacterium.
In addition to use in catheters, nanotechnology offers promise in reducing post-insertion intraluminal IVC colonization related to staff handling of IVC needleless connectors. Needleless connectors are used to cap off IVCs temporarily not in use, to connect infusion administration sets to the catheter, and to provide an access point for administration of medicines and withdrawal of blood specimens. Recently, connector devices have increasingly attracted the attention of commercial manufacturers and researchers alike.46 Many such devices have recently entered the market, with a variety of internal engineering. Some needleless connectors incorporate a valve, to prevent backflow of blood and intravenous fluids into the connector, which aims to prevent catheter occlusion or thrombosis.47,48 However, many studies suggest that some valved needleless connectors actually increase the risk of CRBSI. Jarvis et al compared split septum needleless connectors against mechanical valve-type needleless connectors, and demonstrated that mechanical valve needleless connectors have higher CRBSI rates, despite similar bloodstream infection surveillance, definitions, and prevention strategies.49 One investigation found CRBSIs increased after a switch from a negative fluid displacement to a positive displacement mechanical valve.50 However, in another observational study, a switch from a negative displacement mechanical valve to a different, luer-activated positive displacement mechanical valve led to a significant decrease in CRBSIs.51 Definitive reasons for these sometimes conflicting results with different types of needleless connectors are still unknown. Regardless of their make, frequent handling, and accessing of catheter hubs by staff, needleless connectors and injection ports have great potential to put patients at risk of primary bloodstream infections, since they facilitate entry of bacteria into the connector and fluid path.52 Research from Donlan et al showed a high incidence of biofilm formation on the interior surface of valved connectors that had been used clinically.53
As a consequence, increasing numbers of studies are being added to the literature on reducing CRBSIs, related to use of needleless connectors. It has been shown that external disinfection of the devices with chlorhexidine/alcohol, rather than alcohol alone, can reduce IVC colonization.47,54 In addition, the time spent on applying the disinfectant is important. Results from one study suggest that wiping the connector with 70% isopropyl alcohol for 3–5 seconds did not adequately disinfect the septal surface.55 Many studies have also shown that conventional disinfection may not be able to prevent entry of microorganisms, if significant contamination of the membranous septum is present prior to the injection or infusion of fluids.56,57
A novel silver nanoparticle-coated connector has been introduced recently. Designed as a single use, disposable valved connector, it is constructed of polycarbonate. However, with the exception of the silicone membranous septum, the entire surface of the connector, including the entirety of the internal fluid path and the external casing, has a silver nano-particle coating.46 Silver nanoparticles are stably imbedded in the polycarbonate matrix, and release minute quantities of bactericidal ionic silver from the surface, into the fluid path.46 Simulation studies have shown that the total amount of ionic silver eluted into the fluid pathway (with continuous infusion), and infused into the patient, is far less than the level of silver exposure considered to pose a risk to human health.58,59 Most of the silver absorbed is excreted in feces and urine. Therefore, silver nanoparticle coating might be safely applied, to prevent contamination and the formation of biofilm on the internal surface IVCs. This has great potential to reduce the risk of CRBSI, but has not yet been studied in clinical trials. In 2010, Maki examined the efficiency of a silver nanoparticle-coated connector, compared against non-medicated connectors, in reducing fluid path colonization, by filling these connectors with six bacteria: Staphylococcus epidermidis, methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, Enterobacter cloacae, Pseudomonas aeruginosa, amd Candida albicans.46 After 24 hours and 72 hours of incubation, the remaining viable microorganisms were quantified, and compared with concentrations in control connectors. The biofilm formation of Enterobacter cloacae on silver nanoparticle-coated connectors was also examined, concluding that more than 99% of bacteria were killed and biofilm formation was also completely suppressed.46 However, human bodies are much more complex than the models used in these studies, and large clinical trials are required to assess the clinical efficacy and safety of silver nanoparticle-coated connectors for preventing CRBSIs.
Another advance could be in the use of liposomes, which are artificially prepared vesicles, made of a lipid biolayer, that can be used as drug carriers, especially against colonizing microorganisms. Liposomes can target the matrix (or biofilm) by specific attachment, allowing drugs to be released in the vicinity of the microorganisms (although, in the case of microbial cell adhesion to human cells, there is a need for further knowledge regarding the ability of this system to prevent microbial adhesion but not affect adhered native cells).60 Therefore, this nanotechnology is a promising research area, but it requires more research to fully understand the mechanism behind the antimicrobial activity. However, several non-clinical studies have been performed on the interaction between liposomes and bacterial biofilms. Halwani et al showed that liposomes were very effective in eradicating antibiotic-resistant P. aeruginosa isolates growing in a planktonic or biofilm community.61 DiTizio et al developed a liposomal hydrogel system that significantly reduced bacterial adhesion to silicone catheters. The system consists of a polyethylene glycol–gelatin hydrogel, in which liposomes containing the antibiotic ciprofloxacin are sequestered.62 Liposomal antimicrobial lock therapy can potentially be considered as a possible alternative to catheter removal.63 This technique opens new perspectives for the development of novel antimicrobial catheters.64
Polymer drug delivery systems are based on nanocarriers that are formed by mixing polymeric chemical compounds with drugs, to form large and complex molecules that carry the drug across physiological barriers.65 Polymeric microspheres, polymer micelles, and hydrogel-type materials have been shown to be effective nanocarriers, for enhancing drug targeting specificity, lowering systemic drug toxicity, improving treatment absorption rates, and providing protection for the pharmaceuticals against biochemical degradation.66 In addition, this system has the possibility to add a pore-forming polymer, which can increase the amount of drug able to be loaded into the carrier.67 It has been shown that polymeric matrices can be mixed with different antimicrobial agents to prevent bacterial colonization and biofilm formation on medical devices.65 This system possesses features that are potentially amenable to the manufacture of antimicrobial medical devices, including IVCs.68 However, in vivo studies are yet to be performed, to test the efficacy of this antibiotic delivery carrier system in reducing bacterial colonization and biofilm formation on catheters.
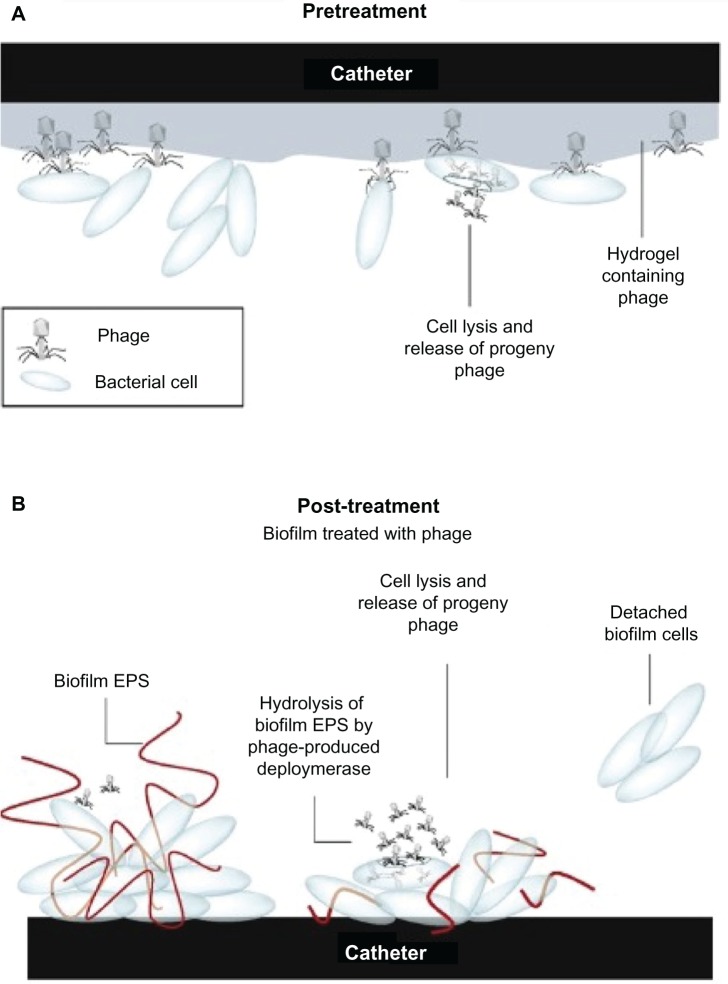
The use of bacteriophages to control CRBSIs caused by biofilms has advantages over treatment with other conventional antimicrobial agents, since phages have very strong bactericidal activity and can replicate at the site of infection.69 It was reported that a progeny phage could treat a biofilm formation, due to its ability to propagate radially throughout a biofilm phage, to infect adjacent cells, and degrade the biofilm matrix (Figure 2).70 In addition, it has been demonstrated that some phages are able to produce enzymes that hydrolyze and degrade the extracellular polymeric matrix of biofilms (Figure 2).70 A phage active against S. epidermidis, incorporated into a hydrogel coating on a catheter, significantly reduced biofilm formation on catheter surfaces.71 Recently, Fu et al studied the effect (in vitro) of pre-treating hydrogel-coated catheters with P. aeruginosa phages, and observed a significant reduction of biofilm formation.72 This shows that the combination of two nanotechnological approaches can further reduce IVC biofilm formation72. However, prior to the use of a phage in humans, there are other aspects to be considered, such as, bacterial resistance to phage, phage inactivation by the human immune system, endotoxins in impure phage, and virulence genes encoded by the phage that can be incorporated into the host bacterial genome.70 The application of phage mixtures, or engineered phages, might provide better solutions for these problems.
Figure 2.
Mechanisms of hydrogel catheter coated with phage on (A) prevention of bacterial attachment on its surfaces, and (B) treatment of existing biofilm on catheter surfaces.
Note: Reprinted from Trends Microbiol, 17, Donlan RM, Preventing biofilms of clinically relevant organisms using bacteriophage, 66–72, Copyright (2009), with permission from Elsevier.70
Abbreviation: EPS, extracellular polymeric substance.
The bioelectric effect is an approach that uses electrical current to prevent biofilm formation and to enhance the activity of antimicrobials against established biofilms.73 The activity of antimicrobial agents against biofilm microorganisms is enhanced through a relatively weak and continuous electrical current.73 However, there are few published in vivo studies on using electrical current to prevent medical device-related infection. Del Pozo et al introduced a new concept – the electricidal effect – by demonstrating dose- and time-dependent killing of S. epidermidis biofilms after prolonged exposure to low-intensity direct electrical current.74 The electricidal effect was also tested in vivo, in a rabbit model of S. epidermidis chronic foreign body osteomyelitis, which confirmed the bactericidal activity of low amperage electrical current against bacterial biofilms.75 These results highlight the possibility of applying this therapy to different medical devices, including IVCs.
Safety and tolerability
The fundamental safety of health care technology is established by the manufacturer, and certified by the relevant national regulatory body for medical drugs and devices (eg, Australian Therapeutic Goods Administration, US Food and Drug Administration, UK Medicines and Healthcare products Regulatory Agency). Efficacy in the clinical setting is tested in trials, as described earlier in this paper. However, the safety of any device in the clinical setting also relies on adherence to the manufacturer’s guidelines, and competency of the end user: the clinician. Sadly, a plethora of research has identified a common, large variability in clinical practice and outcomes, when it comes to IVC insertion and aftercare.76–80
Improved patient outcomes for IVC insertion have been associated with dedicated IVC teams, and clinicians with improved skills and increased competencies, as a consequence of effective training and procedural volume.81–84 In addition, research has demonstrated that clinicians with minimal experience of inserting central venous catheters have a higher risk of complications arising.85 Improving and standardizing IVC practices, using multi-modal interventions, has demonstrated a significant reduction in infection rates (2.7 per thousand catheter-days reduced to 0 per thousand catheter-days, at 3 months post-intervention; P≤0.002) (7.7 per thousand catheter-days reduced to 1.4 per thousand catheter-days, at 18 months post-intervention; P≤0.002).27 Furthermore, the results of a prepost study of a post-insertion bundle of evidence-based cares, such as appropriate dressings, suggested a reduction in CRBSI from 5.7 per thousand catheter-days to 1.1 per thousand catheter-days.28 Another study, on the impact of the introduction of evidence based guidelines on peripheral venous catheter practice, was also associated with a reduction in infection rates, as well as with other improvements, including: completed insertion records (76% vs 58%; P<0.01), correct and sterile fixation localized (92% vs 80%; P<0.05), and catheter complications (4% vs 15%; P<0.01).86
The significance of these studies is that they demonstrate the positive impact of evidence-based “best practice” principles and systems, in guiding the insertion and care of IVCs. However, ensuring clinician understanding, acceptance, and adherence to recommended practice is challenging beyond the trial research setting. It involves a careful and strategic combination of staff education and the implementation of systems that promote best practice. Staff need to be educated not only about the clinical problem and related risks, but also about research methodology and levels of evidence. However, in acknowledging the difficulty of achieving adequate compliance levels in practice, and understanding the fallibility of humans, we need to minimize risk and harm through forced decision making systems, or use of technology and equipment that negate clinician choice, and error (eg, central venous catheter insertion trolleys, or pre-filled flush syringes) and protect the patient (eg, impregnated catheters and nanotechnology).
Some clinicians, and even academics, believe that increased use of guidelines or standardized systems reduces and devalues clinical judgment. But, accompanying the freedom of clinical judgments is the risk of inappropriate judgment and error.87,88 Clinical judgment is not adequately accounted for in a systems analysis approach that may oversimplify health professionals’ choices, and so, within clinical practice. a tradeoff exists between forced choice and free-thinking design.89
Patient-focused perspectives
The financial cost of treating CRBSI is well documented. The US Institute for Healthcare Improvement has quantified the extra cost associated with each CRBSI episode at US$25,000–$55,000, which included an extension of the admission by 7 days, on average. The CRBSI-associated mortality rate ranges between 12%–25%.90 However, the “cost” to patients is less well documented. What is the impact of extended hospital stay (sometimes in isolation), the discomfort associated with local and systemic infection and related treatment, including ongoing surveillance and laboratory and radiological examinations?
There is a paucity of research describing the impact of CRBSI on the individual patient. Studies that have explored patients’ experiences of being nursed in single room isolation, due to infection while in hospital, have identified adverse effects including boredom, lowered or disturbed mood, and feelings of stigma, along with anxiety about passing the infections to relatives and carers.91–94 An integrated review of nineteen studies that explored patients’ experiences of infection and/or isolation identified some key common themes. These included a perceived lack of consistent information about the infection, poor understanding of the infection by health care staff, associated fear and stigma, and isolation.95
One small, qualitative study explored the patients’ experiences of CRBSI.96 In this study patients (n=18) were somewhat confident about asking staff about health care-associated infection and related infection control issues. But, on the whole, patients were reluctant to question or challenge staff, because they did not want to alienate themselves. Most patients stated that they received little or no information about their infection, until later in their admission, if at all. Patients believed that low staffing levels, the use of temporary (bank) nursing staff, and poor cleanliness were causes of infection. While some patients were resigned to the potential risk of acquiring an infection in hospital, others dreaded it. There was no discussion about how each patient experienced the infection itself, its treatment, and sequelae.
There are few studies also to have examined patient experience of intravenous (IV) therapy or devices. An older paper discussed the potential stress associated with IV therapy, and suggested a number of potential causes, using case studies as references.97 The author identified three principal sources of anxiety about IV therapy: fear of pain, fear of needles, and fear of confinement. Having identified these factors, the author proposed a range of strategies and approaches for minimizing the stress. These included projection of confidence and competence by the clinician inserting or caring for the IV device, and the use of diversion tactics, for some patients, or detailed explanations of the procedure, equipment, and implications of therapy, for others.
Other research about the patient experience of IV therapy and devices comes from the community setting.98–101 In a US study conducted using interviews, patients spoke positively about the independence and autonomy that home-based IV therapy afforded them, compared against regular in-hospital treatment. They valued the expertise and support of clinicians associated with the service. But patients also reflected on the inflexibility of some clinicians and/or guiding protocols, and (sometimes) the use of “blaming or accusatory language” by staff, such as “poor access” or “bad veins”.100 An Australian study of home-based IV therapy patients echoed similar feelings of independence and liberation in patients who had formerly been required to attend hospital for IV therapy – of experiencing feelings of “getting their life back”. When it is well managed and well supported, infectious outcomes of home-based IV therapy and patient self-management of IVs have been shown to be equivalent to, if not better than, clinician-controlled care. The incidence of catheter-related infections was significantly reduced in the patient education group (2.55 per thousand catheter-days) compared against a control group, with standard care (5.91 per thousand catheter-days) (P<0.01).101
The significance of these studies’ results is that they demonstrate patients’ acceptance of, and willingness to embrace, health care technology. Future, trial-based research should incorporate patient evaluation and satisfaction in study protocols.
Conclusion
Catheter-related infections remain a major problem in health care, being associated with significant morbidity, mortality, and additional medical cost. Microbial biofilm formation makes these infections more complicated, as microbial cells detached from the biofilm can lead to acute infection, and these microorganisms are highly resistant to a large number of antimicrobial agents. New nanotechnologies are being developed in order to overcome problems associated with bacterial or fungal biofilm formation. The nanotechnology approach seems to be one of the most promising research fields for preventing biofilm formation and catheter-related infection, especially against multiresistant strains. However, these will never completely eradicate CRBSI, without additional and ongoing efforts to ensure health professionals consistently adhere to infection prevention measures. In addition, the patient’s experience and acceptance of CRBSI avoidance strategies, including nanotechnologies, must be considered, to maximize the success of such prevention measures.
Acknowledgments
Li Zhang’s work has been supported by a National Health and Medical Research Council training clinical research fellowship (Australian Government grant number 597491).
Samantha Keogh and Claire M Rickard have received research funding from BD Medical that is unrelated to this work.
Footnotes
Disclosure
The authors declare no conflicts of interest related to this article.
References
- 1.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L, Gowardman J, Rickard CM. Impact of microbial attachment on intravascular catheter-related infections. Int J Antimicrob Agents. 2011;38(1):9–15. doi: 10.1016/j.ijantimicag.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Pizzoferrato A, Arciola CR, Cenni E, Ciapetti G, Sassi S. In vitro biocompatibility of a polyurethane catheter after deposition of fluorinated film. Biomaterials. 1995;16(5):361–367. doi: 10.1016/0142-9612(95)93853-6. [DOI] [PubMed] [Google Scholar]
- 4.Stenqvist O, Curelaru I, Linder LE, Gustavsson B. Stiffness of central venous catheters. Acta Anaesthesiol Scand. 1983;27(2):153–157. doi: 10.1111/j.1399-6576.1983.tb01926.x. [DOI] [PubMed] [Google Scholar]
- 5.Cohen AB, Dagli M, Stavropoulos SW, Jr, et al. Silicone and polyurethane tunneled infusion catheters: a comparison of durability and breakage rates. J Vasc Interv Radiol. 2011;22(5):638–641. doi: 10.1016/j.jvir.2011.01.433. [DOI] [PubMed] [Google Scholar]
- 6.Gaukroger PB, Roberts JG, Manners TA. Infusion thrombophlebitis: a prospective comparison of 645 Vialon and Teflon cannulae in anaesthetic and postoperative use. Anaesth Intensive Care. 1988;16(3):265–271. doi: 10.1177/0310057X8801600305. [DOI] [PubMed] [Google Scholar]
- 7.Ezingeard E, Coudrot M, Guyomarc’h S, et al. Evaluation of colonization of peripheral venous catheters inserted by prehospital emergency service teams (SMUR) in France. J Hosp Infect. 2009;72(2):169–175. doi: 10.1016/j.jhin.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Giuliani J, Andreetta L, Mattioli M, et al. Intravenous midline catheter usage: which clinical impact in homecare patients? J Palliat Med. 2013;16(6):598. doi: 10.1089/jpm.2012.0615. [DOI] [PubMed] [Google Scholar]
- 9.Bourgeois FC, Lamagna P, Chiang VW. Peripherally inserted central catheters. Pediatr Emerg Care. 2011;27(6):556–561. doi: 10.1097/PEC.0b013e31821dc9b6. quiz 562–553. [DOI] [PubMed] [Google Scholar]
- 10.Hoffer EK, Borsa J, Santulli P, Bloch R, Fontaine AB. Prospective randomized comparison of valved versus nonvalved peripherally inserted central vein catheters. AJR Am J Roentgenol. 1999;173(5):1393–1398. doi: 10.2214/ajr.173.5.10541127. [DOI] [PubMed] [Google Scholar]
- 11.McDowell DE, Moss AH, Vasilakis C, Bell R, Pillai L. Percutaneously placed dual-lumen silicone catheters for long-term hemodialysis. Am Surg. 1993;59(9):569–573. [PubMed] [Google Scholar]
- 12.Raad II, Hanna HA. Intravascular catheter-related infections – New horizons and recent advances. Arch Intern Med. 2002;162(8):871–878. doi: 10.1001/archinte.162.8.871. [DOI] [PubMed] [Google Scholar]
- 13.Miller SE, Maragakis LL. Central line-associated bloodstream infection prevention. Curr Opin Infect Dis. 2012;25(4):412–422. doi: 10.1097/QCO.0b013e328355e4da. [DOI] [PubMed] [Google Scholar]
- 14.Walz JM, Memtsoudis SG, Heard SO. Prevention of central venous catheter bloodstream infections. J Intensive Care Med. 2010;25(3):131–138. doi: 10.1177/0885066609358952. [DOI] [PubMed] [Google Scholar]
- 15.Trautner BW, Darouiche RO. Catheter-associated infections – Pathogenesis affects prevention. Arch Intern Med. 2004;164(8):842–850. doi: 10.1001/archinte.164.8.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis. 2011;52(9):e162–e193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 18.Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41(6):848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 19.Beveridge TJ, Makin SA, Kadurugamuwa JL, Li ZS. Interactions between biofilms and the environment. FEMS Microbiol Rev. 1997;20(3–4):291–303. doi: 10.1111/j.1574-6976.1997.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 20.Donlan RM. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7(2):277–281. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- 22.Hu ZQ, Hidalgo G, Houston PL, et al. Determination of spatial distributions of zinc and active biomass in microbial biofilms by two-photon laser scanning microscopy. Appl Environ Microbiol. 2005;71(7):4014–4021. doi: 10.1128/AEM.71.7.4014-4021.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costerton JW. Introduction to biofilm. Int J Antimicrob Agents. 1999;11(3–4):217–221. doi: 10.1016/s0924-8579(99)00018-7. [DOI] [PubMed] [Google Scholar]
- 24.Raad II, Fang X, Keutgen XM, Jiang Y, Sherertz R, Hachem R. The role of chelators in preventing biofilm formation and catheter-related bloodstream infections. Curr Opin Infect Dis. 2008;21(4):385–392. doi: 10.1097/QCO.0b013e32830634d8. [DOI] [PubMed] [Google Scholar]
- 25.Raad II, Luna M, Khalil SA, Costerton JW, Lam C, Bodey GP. The relationship between the thrombotic and infectious complications of central venous catheters. JAMA. 1994;271(13):1014–1016. [PubMed] [Google Scholar]
- 26.van Rooden CJ, Schippers EF, Barge RM, et al. Infectious complications of central venous catheters increase the risk of catheter-related thrombosis in hematology patients: a prospective study. J Clin Oncol. 2005;23(12):2655–2660. doi: 10.1200/JCO.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 28.Guerin K, Wagner J, Rains K, Bessesen M. Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control. 2010;38(6):430–433. doi: 10.1016/j.ajic.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Mermel LA, Allon M, Bouza E, et al. Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timsit JF, Schwebel C, Bouadma L, et al. Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA. 2009;301(12):1231–1241. doi: 10.1001/jama.2009.376. [DOI] [PubMed] [Google Scholar]
- 31.Levy I, Katz J, Solter E, et al. Chlorhexidine-impregnated dressing for prevention of colonization of central venous catheters in infants and children: a randomized controlled study. Pediatr Infect Dis J. 2005;24(8):676–679. doi: 10.1097/01.inf.0000172934.98865.14. [DOI] [PubMed] [Google Scholar]
- 32.Veenstra DL, Saint S, Saha S, Lumley T, Sullivan SD. Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection: a meta-analysis. JAMA. 1999;281(3):261–267. doi: 10.1001/jama.281.3.261. [DOI] [PubMed] [Google Scholar]
- 33.Walder B, Pittet D, Tramer MR. Prevention of bloodstream infections with central venous catheters treated with anti-infective agents depends on catheter type and insertion time: evidence from a meta-analysis. Infect Control Hosp Epidemiol. 2002;23(12):748–756. doi: 10.1086/502005. [DOI] [PubMed] [Google Scholar]
- 34.Ostendorf T, Meinhold A, Harter C, et al. Chlorhexidine and silver-sulfadiazine coated central venous catheters in haematological patients – a double-blind, randomised, prospective, controlled trial. Support Care Cancer. 2005;13(12):993–1000. doi: 10.1007/s00520-005-0812-9. [DOI] [PubMed] [Google Scholar]
- 35.Rupp ME, Lisco SJ, Lipsett PA, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med. 2005;143(8):570–580. doi: 10.7326/0003-4819-143-8-200510180-00007. [DOI] [PubMed] [Google Scholar]
- 36.Darouiche RO, Raad II, Heard SO, et al. A comparison of two antimicrobial-impregnated central venous catheters. Catheter Study Group. N Engl J Med. 1999;340(1):1–8. doi: 10.1056/NEJM199901073400101. [DOI] [PubMed] [Google Scholar]
- 37.Hanna H, Benjamin R, Chatzinikolaou I, et al. Long-term silicone central venous catheters impregnated with minocycline and rifampin decrease rates of catheter-related bloodstream infection in cancer patients: a prospective randomized clinical trial. J Clin Oncol. 2004;22(15):3163–3171. doi: 10.1200/JCO.2004.04.124. [DOI] [PubMed] [Google Scholar]
- 38.Mehall JR, Saltzman DA, Jackson RJ, Smith SD. Fibrin sheath enhances central venous catheter infection. Crit Care Med. 2002;30(4):908–912. doi: 10.1097/00003246-200204000-00033. [DOI] [PubMed] [Google Scholar]
- 39.Pierce CM, Wade A, Mok Q. Heparin-bonded central venous lines reduce thrombotic and infective complications in critically ill children. Intensive Care Med. 2000;26(7):967–972. doi: 10.1007/s001340051289. [DOI] [PubMed] [Google Scholar]
- 40.Guggenbichler JP, Boswald M, Lugauer S, Krall T. A new technology of microdispersed silver in polyurethane induces antimicrobial activity in central venous catheters. Infection. 1999;27(Suppl 1):S16–S23. doi: 10.1007/BF02561612. [DOI] [PubMed] [Google Scholar]
- 41.Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 42.Chaiyakunapruk N, Veenstra DL, Lipsky BA, Saint S. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med. 2002;136(11):792–801. doi: 10.7326/0003-4819-136-11-200206040-00007. [DOI] [PubMed] [Google Scholar]
- 43.Gordon O, Vig Slenters T, Brunetto PS, et al. Silver coordination polymers for prevention of implant infection: thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical induction. Antimicrob Agents Chemother. 2010;54(10):4208–4218. doi: 10.1128/AAC.01830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roe D, Karandikar B, Bonn-Savage N, Gibbins B, Roullet JB. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J Antimicrob Chemother. 2008;61(4):869–876. doi: 10.1093/jac/dkn034. [DOI] [PubMed] [Google Scholar]
- 45.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1):76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Maki DG. In vitro studies of a novel antimicrobial luer-activated needleless connector for prevention of catheter-related bloodstream infection. Clin Infect Dis. 2010;50(12):1580–1587. doi: 10.1086/652764. [DOI] [PubMed] [Google Scholar]
- 47.Casey AL, Burnell S, Whinn H, Worthington T, Faroqui MH, Elliott TS. A prospective clinical trial to evaluate the microbial barrier of a needleless connector. J Hosp Infect. 2007;65(3):212–218. doi: 10.1016/j.jhin.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 48.Yebenes JC, Vidaur L, Serra-Prat M, et al. Prevention of catheter-related bloodstream infection in critically ill patients using a disinfectable, needle-free connector: a randomized controlled trial. Am J Infect Control. 2004;32(5):291–295. doi: 10.1016/j.ajic.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Jarvis WR, Murphy C, Hall KK, et al. Health care-associated bloodstream infections associated with negative- or positive-pressure or displacement mechanical valve needleless connectors. Clin Infect Dis. 2009;49(12):1821–1827. doi: 10.1086/648418. [DOI] [PubMed] [Google Scholar]
- 50.Maragakis LL, Bradley KL, Song X, et al. Increased catheter-related bloodstream infection rates after the introduction of a new mechanical valve intravenous access port. Infect Control Hosp Epidemiol. 2006;27(1):67–70. doi: 10.1086/499166. [DOI] [PubMed] [Google Scholar]
- 51.Costello JM, Morrow DF, Graham DA, Potter-Bynoe G, Sandora TJ, Laussen PC. Systematic intervention to reduce central line-associated bloodstream infection rates in a pediatric cardiac intensive care unit. Pediatrics. 2008;121(5):915–923. doi: 10.1542/peds.2007-1577. [DOI] [PubMed] [Google Scholar]
- 52.Do AN, Ray BJ, Banerjee SN, et al. Bloodstream infection associated with needleless device use and the importance of infection-control practices in the home health care setting. J Infect Dis. 1999;179(2):442–448. doi: 10.1086/314592. [DOI] [PubMed] [Google Scholar]
- 53.Donlan RM, Murga R, Bell M, et al. Protocol for detection of biofilms on needleless connectors attached to central venous catheters. J Clin Microbiol. 2001;39(2):750–753. doi: 10.1128/JCM.39.2.750-753.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soothill JS, Bravery K, Ho A, Macqueen S, Collins J, Lock P. A fall in bloodstream infections followed a change to 2% chlorhexidine in 70% isopropanol for catheter connection antisepsis: a pediatric single center before/after study on a hemopoietic stem cell transplant ward. Am J Infect Control. 2009;37(8):626–630. doi: 10.1016/j.ajic.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 55.Menyhay SZ, Maki DG. Preventing central venous catheter-associated bloodstream infections: development of an antiseptic barrier cap for needleless connectors. Am J Infect Control. 2008;36(10):S174. e171–e175. doi: 10.1016/j.ajic.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Segura M, AlvarezLerma F, Tellado JM, et al. A clinical trial on the prevention of catheter-related sepsis using a new hub model. Ann Surg. 1996;223(4):363–369. doi: 10.1097/00000658-199604000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menyhay SZ, Maki DG. Disinfection of needleless catheter connectors and access ports with alcohol may not prevent microbial entry: the promise of a novel antiseptic-barrier cap. Infect Control Hosp Epidemiol. 2006;27(1):23–27. doi: 10.1086/500280. [DOI] [PubMed] [Google Scholar]
- 58.Wan AT, Conyers RA, Coombs CJ, Masterton JP. Determination of silver in blood, urine, and tissues of volunteers and burn patients. Clin Chem. 1991;37(10 Pt 1):1683–1687. [PubMed] [Google Scholar]
- 59.Armitage SA, White MA, Wilson HK. The determination of silver in whole blood and its application to biological monitoring of occupationally exposed groups. Ann Occup Hyg. 1996;40(3):331–338. doi: 10.1016/0003-4878(95)00076-3. [DOI] [PubMed] [Google Scholar]
- 60.Tamilvanan S, Venkateshan N, Ludwig A. The potential of lipid- and polymer-based drug delivery carriers for eradicating biofilm consortia on device-related nosocomial infections. J Control Release. 2008;128(1):2–22. doi: 10.1016/j.jconrel.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 61.Halwani M, Yebio B, Suntres ZE, Alipour M, Azghani AO, Omri A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J Antimicrob Chemother. 2008;62(6):1291–1297. doi: 10.1093/jac/dkn422. [DOI] [PubMed] [Google Scholar]
- 62.DiTizio V, Ferguson GW, Mittelman MW, Khoury AE, Bruce AW, DiCosmo F. A liposomal hydrogel for the prevention of bacterial adhesion to catheters. Biomaterials. 1998;19(20):1877–1884. doi: 10.1016/s0142-9612(98)00096-9. [DOI] [PubMed] [Google Scholar]
- 63.Buckler BS, Sams RN, Goei VL, et al. Treatment of central venous catheter fungal infection using liposomal amphotericin-B lock therapy. Pediatr Infect Dis J. 2008;27(8):762–764. doi: 10.1097/INF.0b013e318170b68b. [DOI] [PubMed] [Google Scholar]
- 64.Finelli A, Burrows LL, DiCosmo FA, et al. Colonization-resistant antimicrobial-coated peritoneal dialysis catheters: evaluation in a newly developed rat model of persistent Pseudomonas aeruginosa peritonitis. Perit Dial Int. 2002;22(1):27–31. [PubMed] [Google Scholar]
- 65.Donelli G, Francolini I, Romoli D, et al. Synergistic activity of dispersin B and cefamandole nafate in inhibition of staphylococcal biofilm growth on polyurethanes. Antimicrob Agents Chemother. 2007;51(8):2733–2740. doi: 10.1128/AAC.01249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanli O, Bicer E, Isiklan N. In vitro release study of diltiazem hydrochloride from poly(vinyl pyrrolidone)/sodium alginate blend microspheres. J Appl Pharm Sci. 2008;107:1973–1980. [Google Scholar]
- 67.Ruggeri V, Francolini I, Donelli G, Piozzi A. Synthesis, characterization, and in vitro activity of antibiotic releasing polyurethanes to prevent bacterial resistance. J Biomed Mater Res A. 2007;81(2):287–298. doi: 10.1002/jbm.a.30984. [DOI] [PubMed] [Google Scholar]
- 68.Martinelli A, D’Ilario L, Francolini I, Piozzi A. Water state effect on drug release from an antibiotic loaded polyurethane matrix containing albumin nanoparticles. Int J Pharm. 2011;407(1–2):197–206. doi: 10.1016/j.ijpharm.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 69.Smith HW, Huggins MB. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J Gen Microbiol. 1982;128(2):307–318. doi: 10.1099/00221287-128-2-307. [DOI] [PubMed] [Google Scholar]
- 70.Donlan RM. Preventing biofilms of clinically relevant organisms using bacteriophage. Trends Microbiol. 2009;17(2):66–72. doi: 10.1016/j.tim.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 71.Curtin JJ, Donlan RM. Using bacteriophages to reduce formation of catheter-associated biofilms by Staphylococcus epidermidis. Antimicrob Agents Chemother. 2006;50(4):1268–1275. doi: 10.1128/AAC.50.4.1268-1275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu W, Forster T, Mayer O, Curtin JJ, Lehman SM, Donlan RM. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob Agents Chemother. 2010;54(1):397–404. doi: 10.1128/AAC.00669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin P, Lin CW, Mansour R, Gu F. Improving biocompatibility by surface modification techniques on implantable bioelectronics. Biosensors and Bioelectronics. 2013;47:451–460. doi: 10.1016/j.bios.2013.01.071. [DOI] [PubMed] [Google Scholar]
- 74.del Pozo JL, Rouse MS, Mandrekar JN, Steckelberg JM, Patel R. The electricidal effect: reduction of Staphylococcus and pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrob Agents Chemother. 2009;53(1):41–45. doi: 10.1128/AAC.00680-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Del Pozo JL, Rouse MS, Euba G, et al. The electricidal effect is active in an experimental model of Staphylococcus epidermidis chronic foreign body osteomyelitis. Antimicrob Agents Chemother. 2009;53(10):4064–4068. doi: 10.1128/AAC.00432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clemence MA, Walker D, Farr BM. Central venous catheter practices: results of a survey. Am J Infect Control. 1995;23(1):5–12. doi: 10.1016/0196-6553(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 77.Girouard S, Levine G, Goodrich K, et al. Infection control programs at children’s hospitals: a description of structures and processes. Am J Infect Control. 2001;29(3):145–151. doi: 10.1067/mic.2001.115406. [DOI] [PubMed] [Google Scholar]
- 78.Creamer E, McCarthy G, Tighe I, Smyth E. A surevy of 554 peripheral intravenous catheters: infection, duration of cannulation and documentation issues. Brit J Infect Control. 2003;4(4):21–25. [Google Scholar]
- 79.Pujol M, Hornero A, Saballs M, et al. Clinical epidemiology and outcomes of peripheral venous catheter-related bloodstream infections at a university-affiliated hospital. J Hosp Infect. 2007;67(1):22–29. doi: 10.1016/j.jhin.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 80.Rickard CM, Courtney M, Webster J. Central venous catheters: a survey of ICU practices. J Adv Nurs. 2004;48(3):247–256. doi: 10.1111/j.1365-2648.2004.03193.x. [DOI] [PubMed] [Google Scholar]
- 81.Alexandrou E, Murgo M, Calabria E, et al. Nurse-led central venous catheter insertion-procedural characteristics and outcomes of three intensive care based catheter placement services. Int J Nurs Stud. 2012;49(2):162–168. doi: 10.1016/j.ijnurstu.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 82.Alexandrou E, Spencer TR, Frost SA, Parr MJ, Davidson PM, Hillman KM. A review of the nursing role in central venous cannulation: implications for practice policy and research. J Clin Nurs. 2010;19(11–12):1485–1494. doi: 10.1111/j.1365-2702.2009.02910.x. [DOI] [PubMed] [Google Scholar]
- 83.Meier PA, Fredrickson M, Catney M, Nettleman MD. Impact of a dedicated intravenous therapy team on nosocomial bloodstream infection rates. Am J Infect Control. 1998;26(4):388–392. doi: 10.1016/s0196-6553(98)70033-1. [DOI] [PubMed] [Google Scholar]
- 84.Yacopetti N, Alexandrou E, Spencer TR, et al. Central venous catheter insertion by a clinical nurse consultant or anaesthetic medical staff: a single-centre observational study. Crit Care Resusc. 2010;12(2):90–95. [PubMed] [Google Scholar]
- 85.Comfere TB, Brown DR. Central Venous Catheters: COnsiderations regarding placement and clinical use. Contemporary Critical Care. 2007;5(1):1. [Google Scholar]
- 86.Couzigou C, Lamory J, Salmon-Ceron D, Figard J, Vidal-Trecan GM. Short peripheral venous catheters: effect of evidence-based guidelines on insertion, maintenance and outcomes in a university hospital. J Hosp Infect. 2005;59(3):197–204. doi: 10.1016/j.jhin.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 87.Reason J. Human error: models and management. West J Med. 2000;172(6):393–396. doi: 10.1136/ewjm.172.6.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reason J. Human error: models and management. BMJ. 2000;320(7237):768–770. doi: 10.1136/bmj.320.7237.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benner P, Sheets V, Uris P, Malloch K, Schwed K, Jamison D. Individual, practice, and system causes of errors in nursing: a taxonomy. J Nurs Adm. 2002;32(10):509–523. doi: 10.1097/00005110-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 90.Dumont C, Nesselrodt D. Preventing central line-associated bloodstream infections CLABSI. Nursing. 2012;42(6):41–46. doi: 10.1097/01.NURSE.0000414623.31647.f5. quiz 47. [DOI] [PubMed] [Google Scholar]
- 91.Madeo M. Understanding the MRSA experience. Nurs Times. 2001;97(30):36–37. [PubMed] [Google Scholar]
- 92.Rees J, Davies HR, Birchall C, Price J. Psychological effects of source isolation nursing (2): Patient satisfaction. Nurs Stand. 2000;14(29):32–36. doi: 10.7748/ns2000.04.14.29.32.c2805. [DOI] [PubMed] [Google Scholar]
- 93.Newton JT, Constable D, Senior V. Patients’ perceptions of methicillin-resistant Staphylococcus aureus and source isolation: a qualitative analysis of source-isolated patients. J Hosp Infect. 2001;48(4):275–280. doi: 10.1053/jhin.2001.1019. [DOI] [PubMed] [Google Scholar]
- 94.Tarzi S, Kennedy P, Stone S, Evans M. Methicillin-resistant Staphylococcus aureus: psychological impact of hospitalization and isolation in an older adult population. J Hosp Infect. 2001;49(4):250–254. doi: 10.1053/jhin.2001.1098. [DOI] [PubMed] [Google Scholar]
- 95.MacDonald P. Exploring patients’ experiences of MRSA to help reduce HCAIs. Nursing times. 2008;104(9):32–33. [Google Scholar]
- 96.Burnett E, Lee K, Rushmer R, Ellis M, Noble M, Davey P. Healthcare-associated infection and the patient experience: a qualitative study using patient interviews. J Hosp Infect. 2010;74(1):42–47. doi: 10.1016/j.jhin.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 97.Carlquist K. Understanding the psychological needs of the patient on IV therapy: A stress-reducing approach. NITA. 1981;4:368–370. [Google Scholar]
- 98.Bamford KB, Desai M, Aruede MJ, Lawson W, Jacklin A, Franklin BD. Patients’ views and experience of intravenous and oral antimicrobial therapy: room for change. Injury. 2011;42(Suppl 5):S24–S27. doi: 10.1016/S0020-1383(11)70129-2. [DOI] [PubMed] [Google Scholar]
- 99.Breier SJ. Home intravenous therapy down under. J Intraven Nurs. 1999;22(4):187–193. [PubMed] [Google Scholar]
- 100.Hanchett M. Infusion therapy in chronic disease: The patient perspective. JAVA. 2003;8(3):20–22. [Google Scholar]
- 101.Moller T. Individualized supervised patient education – a key role in reinforcement of infection protection and outpatient management of acute leukemia. Copenhagen, DK: University of Copenhagen; 2011. p. 129 p. [Google Scholar]
- 102.Jaeger K, Zenz S, Juttner B, et al. Reduction of catheter-related infections in neutropenic patients: a prospective controlled randomized trial using a chlorhexidine and silver sulfadiazine-impregnated central venous catheter. Ann Hematol. 2005;84(4):258–262. doi: 10.1007/s00277-004-0972-6. [DOI] [PubMed] [Google Scholar]
- 103.Sheng WH, Ko WJ, Wang JT, Chang SC, Hsueh PR, Luh KT. Evaluation of antiseptic-impregnated central venous catheters for prevention of catheter-related infection in intensive care unit patients. Diagn Microbiol Infect Dis. 2000;38(1):1–5. doi: 10.1016/s0732-8893(00)00166-8. [DOI] [PubMed] [Google Scholar]
- 104.Lorente L, Lecuona M, Ramos MJ, Jimenez A, Mora ML, Sierra A. The use of rifampicin-miconazole-impregnated catheters reduces the incidence of femoral and jugular catheter-related bacteremia. Clin Infect Dis. 2008;47(9):1171–1175. doi: 10.1086/592253. [DOI] [PubMed] [Google Scholar]