Abstract
It is recognised that some mutated cancer genes contribute to the development of many cancer types whilst others are cancer-type specific. Amongst genes that affect multiple cancer classes, mutations are usually similar in the different cancer types. Here, however, we observed exquisite tumour-type specificity of different histone 3.3 driver mutations. In 73/77 (95%) cases of chondroblastoma we found K36M mutations predominantly in H3F3B, which is one of two genes encoding histone 3.3. By contrast, 92% (49/53) of giant cell tumours of bone harboured histone 3.3 variants exclusively in H3F3A, which were G34W or, in one case, G34L. The mutations were restricted to the stromal cell population and not detected in osteoclasts or their precursors. In the context of previously reported H3F3A K27M and G34R/V mutations of childhood brain tumours, a remarkable picture of tumour-type specificity of histone 3.3 mutations emerges, indicating distinct functions of histone 3.3 residues, mutations and genes.
Chondroblastoma is a cartilaginous tumour presenting in childhood to early adulthood which arises in active growth plates, most commonly of long bones1. Although locally destructive and prone to recurrence, it is considered a benign tumour which rarely metastasises1. The biology of chondroblastoma is not understood, including its relation to other tumours sited in the epiphysis, such as giant cell tumour of bone.
Using massively parallel sequencing, we sequenced the complete genomes of six chondroblastomas and normal DNAs from the same individuals (Supplementary Table 1). Overall, we identified a relatively low somatic mutation burden, comprising between 542 – 668 substitutions (0.2 / megabase) and 15-25 indels (0.006 / megabase) per genome (Table 1; Supplementary Table 2). The predominant substitution class was C to T, which showed enrichment at CpG dinucleotides. Copy number and rearrangement analysis showed that the tumours were overall diploid with a paucity of structural changes (Table 1; Supplementary Figure 1).
Table 1. Genome wide somatic mutation burden of six chondroblastoma cases.
For each tumour genome the absolute numbers of subs and indels are shown, along with histone 3.3 mutations.
| Case | PD7516a | PD7517a | PD7518a | PD7520a | PD7522a | PD7523a |
|---|---|---|---|---|---|---|
| Substitutions | 547 | 542 | 653 | 531 | 668 | 585 |
| Indels | 15 | 17 | 23 | 21 | 15 | 25 |
|
Histone 3.3
mutation |
H3F3B K36M |
H3F3B K36M |
H3F3A K36M |
H3F3B K36M |
H3F3B K36M |
H3F3B K36M |
A striking observation was that all six chondroblastomas harboured recurrent somatic mutations in one of two genes, H3F3A or H3F3B, encoding the replication-independent histone 3.3. H3F3A and H3F3B reside on chromosomes 1 and 17, respectively. They have different exonic and intronic DNA sequences, but both encode histone 3.3 proteins of identical amino acid sequence2,3. Cancer driver mutations have previously been identified in H3F3A, but not in H3F3B. All six chondroblastomas carried K36M histone 3.3 mutations, five were in H3F3B and one in H3F3A. No other putative driver variants were identified. We extended the investigation of H3F3A and H3F3B to an additional 71 chondroblastomas and, in total, found histone 3.3 mutations in 73/77 (95%). All 73 variants were K36M, with 68 presenting in H3F3B and five in H3F3A (Figure 1; Table 2).
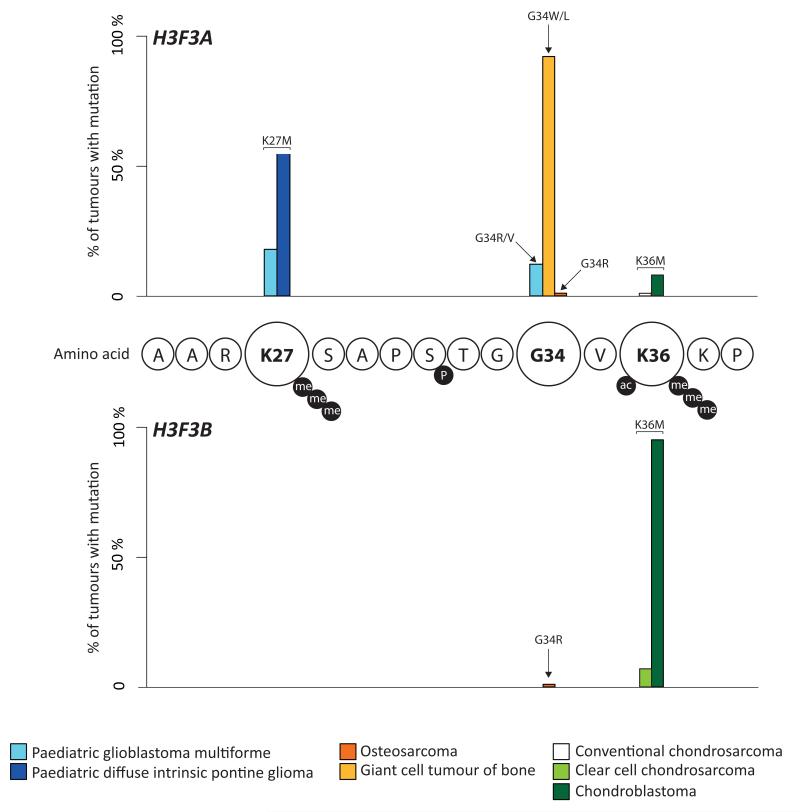
Figure 1. Prevalence and distribution of histone 3.3 mutations in different tumour types.
The percentage of cases in each series that harbour a specific histone 3.3 mutation is indicated: paediatric glioblastoma multiforme4 (light blue); paediatric diffuse intrinsic pontine glioma6 (dark blue); osteosarcoma (dark orange); giant cell tumour of bone (light orange); clear cell chondrosarcoma (light green); conventional chondrosarcoma (white); chondroblastoma (dark green). Circles represent amino acid residues which are identical for H3F3A and H3F3B. Black circles represent post-translational modifications that occur at residues: me = methylation; ac = acetylation; P = phosphorylation.
Table 2. Histone 3.3 mutations in bone and cartilage tumours.
Prevalence of histone 3.3 mutations identified in eight different tumour types are shown. K36M and G34W mutations are mutually exclusive in chondroblastoma and giant cell tumour of bone (p<1×10−15). Amino acid changes are specified above the bars.
| Tumour type | Number screened |
Mutated | H3F3A | H3F3B | |||||
|---|---|---|---|---|---|---|---|---|---|
| G34L | G34R | G34V | G34 W |
K36M | G34R | K36M | |||
| Chondroblastoma | 77 | 73 (95%) | 5 | 68 | |||||
| Giant cell tumour of bone |
53 | 49 (92%) | 1 | 48 | |||||
| Clear cell chondrosarcoma |
15 | 1 (7%) | 1 | ||||||
| Osteosarcoma | 103 | 2 (2%) | 1 | 1 | |||||
| Conventional chondrosarcoma |
75 | 1 (1%) | 1 | ||||||
| Chondromyxoid fibroma |
43 | 0 | |||||||
| Chordoma | 25 | 0 | |||||||
| Chondroma | 7 | 0 | |||||||
We screened seven additional types of bone and cartilage tumours for the presence of H3F3A or H3F3B mutations (Table 2). In giant cell tumour of bone we found mutations in 49/53 (92%) cases. All variants were in H3F3A encoding changes at glycine 34; 48 were G34W and one was G34L (Figure 1; Table 2).
In other bone and cartilage tumours, we found a low prevalence of histone 3.3 mutations (Table 2). Two osteosarcomas (2%; 2/103) harboured G34R variants, one in H3F3A and one in H3F3B. One (1%; 1/75) conventional chondrosarcoma had an H3F3A K36M mutation. We identified one clear cell chondrosarcoma (7%; 1/15) with an H3F3B K36M mutation. No histone 3.3 variants were found in chondromyxoid fibroma (n=43), chordoma (n=25) or soft tissue/synovial chondroma (n=7). To search for mutations of other histone 3 genes that are closely related, but not identical, to H3F3A and H3F3B, we analysed whole exome or genome sequence of 49/75 chondrosarcoma and of all 103 osteosarcoma cases included in this study. One osteosarcoma harboured a somatic variant of HIST1H3H of unknown significance (G12R). Unlike in some childhood brain tumours, we did not identify H3F3A or HIST1H3H K27M mutations, nor did we observe an association of histone 3.3 variants with TP53 mutations4-9.
Giant cell tumour of bone, and to a lesser extent chondroblastoma, contains cells of the osteoclast lineage in addition to stromal cells. In giant cell tumour of bone there has been some dispute as to which cell type is neoplastic10. Consequently we separated the stromal compartment (CD51-/CD61-/CD14-) from osteoclasts (CD51+/CD61+) and osteoclast precursors (CD14+) in both giant cell tumour of bone and chondroblastoma harbouring histone 3.3 mutations. The presence of mutations demonstrates that the neoplastic component of these tumours is represented by stromal cells and not cells of the osteoclast lineage (Table 3). It is possible that histone 3.3 mutations directly lead to osteoclast recruitment, for example through alteration of expression of essential osteoclast signals such as RANK ligand or colony stimulating factor 1.
Table 3. Mutant allele fraction in different compartments of chondroblastoma and giant cell tumour of bone.
| Mutant allele fraction in stromal component CD14−/CD51−/CD61− cells |
Mutant allele fraction in mononuclear precursors CD14+/CD51+/CD61+ cells |
|
|---|---|---|
|
Case S00036864 Chondroblastoma (H3F3B K36M) |
10%* | 0 |
|
Case S000297791 Giant cell tumour of bone (H3F3A G34W) |
40% | 0 |
The mutant allele fraction in the stromal component of this tumour was low as it contained a large fraction of reactive fibroblasts.
Taken together, our findings reveal histone 3.3 mutations in five classes of bone and cartilage neoplasms with an exceptionally high prevalence in chondroblastoma and giant cell tumours of bone and a low frequency in osteosarcoma, conventional chondrosarcoma and clear cell chondrosarcoma. Recurrent somatic histone 3.3 mutations have previously been described in paediatric brain tumours4-9, including up to 60% of diffuse intrinsic pontine glioma8. Otherwise, histone 3.3 mutations appear to be exceedingly rare. Amongst 1.14 million coding mutations from 885,000 tumours recorded in the Catalogue of Somatic Mutations in Cancer (COSMIC) 11, there is one mutation at a histone 3.3 hotspot, a K36R variant found in a urinary tract tumour. Therefore, histone 3.3 driver mutations appear to be markedly enriched in glial tumours and a subset of primary skeletal neoplasms.
We observed quite remarkable specificity of histone 3.3 mutations for different histogenetic tumour types, both with respect to which codon is mutated and what amino acid is newly substituted (Figure 1). Histone 3.3 K36M mutations were confined to a subset of cartilage tumours, namely chondroblastoma and chondrosarcoma, whilst glycine 34 was mutated in tumours of osteoblast lineage, osteosarcoma and giant cell tumour of bone. Lysine 27, which is the principal methylation site mutated in paediatric brain tumours, is not affected in bone and cartilaginous tumours. Likewise, lysine 36 which is the principal methylation site mutated in chondroblastoma, is not mutated in bone or brain tumours. At glycine 34, which is mutated in both bone and paediatric brain tumours, mutations to valine or arginine are found in brain tumours while mutations to tryptophan are confined to giant cell tumours of bone. Thus, there is a dramatic association of each tumour type with a specific histone 3.3 mutation. The most likely explanation of this phenomenon is that particular histone 3.3 mutations confer differential growth advantage on specific cell types. Although, in principle, different mutational processes in different neoplasms could account for the tumour-specificity of the histone 3.3 mutations, this appears unlikely as the overall patterns of somatic substitutions are similar in these tumour types.
An association of mutations of specific codons with different cancer types has been reported in other cancer genes. In NRAS, for example, Q61 is more frequently mutated in melanoma than the other two mutation hotspots, G12 and G13, which are predominantly mutated in leukaemias12. Other examples include BRAF and TP53 in which certain mutation hotspots demonstrate a bias for specific tumour types11,13. However, the mutual exclusivity of histone 3.3 mutations between brain tumours, giant cell tumour of bone and chondroblastoma, that we report here, is unprecedented.
To our knowledge, this is also the first description of somatic driver mutations presenting in different genes encoding an identical protein. The reasons for this phenomenon remain uncertain. However, the clear preferences for H3F3A mutations in tumours of osteoblastic lineage and glial cells, and for H3F3B mutations in cartilaginous neoplasms, indicate that the two genes are unlikely to be redundant with respect to function. One possibility is that differential gene expression may account for the selective pattern of mutation in different tumour types. We assessed expression levels of H3F3A and H3F3B in ten cases each of giant cell tumour of bone (H3F3A G34W mutant) and chondroblastoma (H3F3B K36M mutant) and did not find significant differences in gene expression between the tumour types. Our findings do not exclude the possibility that temporal differences underlie the tumour specificity of H3F3A and H3F3B, especially as differential expression patterns of the two genes have been observed during embryonic and post-natal development in normal murine and human tissues14,15.
The accumulation of histone 3.3 mutations in paediatric brain tumours has sparked a number of functional investigations seeking to evaluate the function of key regulatory sites, such as critical lysines which are targets of post-translational methylation. Histone 3.3 lysine to methionine mutations, including K9M, K27M, and K36M, were found to reduce methylation of these residues through inhibition of SET domain-containing enzymes16. Interestingly, G34R and G34V substitutions decreased methylation at K3616. In addition, expression profiling of histone 3.3 K27M mutant cell lines demonstrated genome-wide changes of gene expression which appeared to disrupt multiple cancer pathways17. Amongst these, MYCN upregulation has been identified as a potential downstream target of histone 3.3 mutation in a gene expression study of a mutant H3F3A G34V glioma cell line18. Collectively, these studies begin to provide a mechanistic insight into histone 3.3 mutations and corroborate the genomic evidence that the recurrent somatic mutations of histone 3.3 at regulatory residues K27, G34, and K36 are dominant driver events.
Our study reveals novel histone 3.3 mutations in a variety of bone and cartilage tumours. Given the exceptionally high prevalence of mutually exclusive histone 3.3 mutations in chondroblastoma and giant cell tumour of bone, it seems likely these tumours are defined by distinct histone 3.3 mutations, which may have immediate diagnostic utility, especially in morphologically ambiguous cases. The unprecedented tumour-type specificity of histone 3.3 mutations indicates fundamental differences in the function of histone 3.3 amino acid residues in tumour development that may pertain to normal differentiation of bone and cartilage cells. It is conceivable that chondroblastoma and giant cell tumour of bone derive from a common precursor cell, the differentiation of which is determined by specific histone 3.3 variants. A future challenge will be to investigate the functional repercussions of these mutations in appropriate biological models that control for the tissue specificity of histone 3.3 variants.
Online methods
Patient samples
Informed consent was obtained from all subjects and ethical approval obtained from Cambridgeshire 2 Research Ethics Service (reference 09/H0308/165). Collection and use of patient samples were approved by the appropriate institutional review board (IRB) of each Institution.
Whole genome sequencing
DNA was extracted from 6 chondroblastomas as well as matched normal tissue derived from the same individuals. Short insert 500bp library construction, flowcell preparation and cluster generation was performed according to the Illumina no-PCR library protocol. 100 base paired-end sequencing was performed on Illumina Hiseq 2000 analysers, as described in the Illumina Genome Analyzer operating manual. Reads were aligned to the reference human genome (NCBI37) by using BWA on default settings19. Reads which were unmapped or PCR-derived duplicates were excluded from the analysis. The average coverage of tumours was 40X and of normal DNA 30X.
Variant detection
The CaVEMan (cancer variants through expectation maximization) algorithm was used to call single nucleotide substitutions20. To call insertions and deletions, we used split-read mapping implemented as a modification of the Pindel algorithm21. To call rearrangements we applied the BRASS (breakpoint via assembly) algorithm, which identifies rearrangements by grouping discordant read pairs that point to the same breakpoint event22. Post-processing filters were applied to the output to improve specificity. Mutations were annotated to Ensembl version 58.
Variant validation
Variants were subjected to validation by manual inspection, and artefacts excluded from the data set. All ambiguous variants (141 substitutions; 24 indels) as well as randomly selected indels (n=25; 12%) and substitutions (n=339; 8%) were experimentally validated. To this end, DNA of the tumours and normal tissue from same individuals was amplified by PCR (primer sequences available on request) targeting the variant location. Resulting PCR amplicons were sequenced on an Illumina MiSeq (read length 150 base pairs) to a median depth of 7400X. The overall specificity for the catalogue of substitutions and indels was thus determined to be at least 95%. All histone 3.3 variants detected in whole genomes were separately validated by capillary sequencing, as described below.
Extension study
185 tumours were screened by capillary sequencing (primers available on request) for the presence of H3F3A and H3F3B variants between codons 29 and 63: 70 chondroblastoma, 43 chondromyxoid fibroma; 51 giant cell tumours of bone; 14 clear cell chondrosarcoma; 7 soft tissue / synovial chondromas. DNA from these 185 tumours was extracted from formalin-fixed paraffin-embedded tissue and from fresh frozen tissue in 22 cases as well, along with normal tissue from the same individuals.
Using DNA from fresh frozen tissue, we used massively parallel sequencing (whole genome, whole exome sequencing or targeted capture of histone 3.3 genes), as previously described20,22, to screen H3F3A and H3F3B for the presence of mutations in 213 tumours: 103 osteosarcoma, 75 chondrosarcoma, 25 chordoma, 2 cases of giant cell tumours of bone, 1 case of clear cell chondrosarcoma, and 1 case of chondroblastoma. Mutations found in histone 3.3 genes or related genes were validated by capillary sequencing or massively parallel sequencing.
TP53 screen
Giant cell tumour of bone cases (47/53) were screened for TP53 mutations. Libraries of targeted 120bp fragments were generated by Fluidigm, according to the manufacturer’s instructions, and sequenced by Ion Torrent One Touch version 2 and Personal Genome Machine with 316 chip, according to the manufacturer’s instructions with minor modifications. Reads were analysed by manual inspection and by samtools mpileup incorporated into custom software.
Detection of copy number variation
Copy number data were derived from whole genome reads using the ASCAT (version 2.2) algorithm23 and validated by SNP6.0 in 5/6 cases. Variant zygosity was derived by determining mutation copy number, as previously described24.
Cell separation experiment
Fresh tissue from 1 case of giant cell tumour of bone (radius) and 1 case of chondroblastoma (femur) was obtained at time of surgery from the Royal National Orthopaedic Hospital, Brockley Hill, Stanmore, UK. The tumours were processed as previously described25 with some modifications. Following collagenase digestion for 2 hours, the cells were cultured for 14 days, and on reaching confluence were passaged twice during that period to enrich for the trypsin-sensitive stromal cell population. The trypsin-resistant large multinucleated osteoclasts, and a smaller population of mononuclear cells persisted in the flasks following the first treatment with trypsin, but new osteoclasts developed when the cells were replated. Following the second passage the cells were incubated with the mouse anti-human monoclonal (IgG1) antibody 23C6 (ab34226; Abcam, Cambridge, UK) directed against CD51 and CD61, the vitronectin receptor (VNR) expressed by osteoclasts26,27, followed by rat anti-mouse IgG1 microbeads (Miltenyi Biotech, Surrey, UK). This was followed by incubation with mouse anti-human monoclonal (IgG2a) CD14 microbeads (Miltenyi Biotech, UK) according to the manufacturers’ recommendations for the purpose of selecting osteoclast precursors. The cells were sorted manually using a Minimax magnetic cell separator (Miltenyi Biotech, UK) according to the manufacturer’s instructions. Each fraction, the CD51/61/CD14-negative, as well as the CD51/CD61/CD14-positive fraction of each tumour, was sorted three times. Using RT-PCR on RNA extracted from paraffin-embedded tissue (RNeasy Minikit, Qiagen, UK) we confirmed that cells from the stromal compartment express genes specific to stromal cells (COL1A1) and do not express markers of osteoclasts and their precursors (CD45, ACP5 and CD14). DNA was extracted from the resulting cell populations and screened for H3F3A and H3F3B mutations by massively parallel sequencing.
Gene expression analyses
Total RNA was isolated from paraffin embedded tissue of 10 cases of giant cell tumour of bone and 10 cases of chondroblastoma and was reverse transcribed using Superscript III First-Strand Synthesis kit (Invitrogen Ltd/Life Technologies Ltd, Paisley, Scotland, UK). Specific gene primers were designed with Primer 3 and real-time PCR was performed with SsoFast EvaGreensupermix from Biorad (Hemel Hempstead, Hertfordshire, UK; primer sequences available upon request). The average of two independent analyses for each target gene and sample was calculated and was normalised to the endogenous reference control gene, GAPDH.
Statistical analysis
The significance of the association between specific histone 3.3 mutations and tumour type was tested using one-sided Fisher exact tests.
Supplementary Material
Acknowledgements
This work was supported by funding the Wellcome Trust (grant reference 077012/Z/05/Z), and Skeletal Cancer Action Trust (SCAT), UK, and Rosetrees Trust UK. The material was obtained from the RNOH Musculoskeletal Research Programme and Biobank, all of which was co-ordinated by Mrs Deidre Brooking and Mrs Ru Grinnell, Biobank staff, RNOH. Support was provided to AMF (UCL) by the National Institute for Health Research, UCLH Biomedical Research Centre, and the CRUK UCL Experimental Cancer Centre. P.J.C. is personally funded through a Wellcome Trust Senior Clinical Research Fellowship (grant reference WT088340MA). O.M. is funded by the Norwegian Research Council (grant reference 218241/H10). P.V.L. is a postdoctoral researcher of the Research Foundation - Flanders (FWO). SB is funded through the Wellcome Trust PhD Programme for Clinicians. We are grateful to the patients for participating in the research and to the clinicians and support staff involved in their care.
Footnotes
Author Information
Informed consent was obtained from all subjects and ethical approval obtained from Cambridgeshire 2 Research Ethics Service (ref 09/H0308/165). Sequencing data have been deposited at the European Genome-Phenome Archive (EGA, http://www.ebi.ac.uk/ega/), which is hosted by the European Bioinformatics Institute (EBI); accession number EGAD00001000646.
Reprints and permissions information is available at www.nature.com/reprints.
The authors declare no competing financial interests.
References
- 1.Fletcher CDM, et al. Pathology and genetics of tumours of soft tissue and bone. IARC Press; Lyon: 2013. p. 427. [Google Scholar]
- 2.Albig W, et al. The human replacement histone H3.3B gene (H3F3B) Genomics. 1995;30:264–72. doi: 10.1006/geno.1995.9878. [DOI] [PubMed] [Google Scholar]
- 3.Szenker E, Ray-Gallet D, Almouzni G. The double face of the histone variant H3.3. Cell Res. 2011;21:421–34. doi: 10.1038/cr.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gessi M, et al. H3.3 G34R mutations in pediatric primitive neuroectodermal tumors of central nervous system (CNS-PNET) and pediatric glioblastomas: possible diagnostic and therapeutic implications? J Neurooncol. 2013;112:67–72. doi: 10.1007/s11060-012-1040-z. [DOI] [PubMed] [Google Scholar]
- 5.Gielen GH, et al. H3F3A K27M mutation in pediatric CNS tumors: a marker for diffuse high-grade astrocytomas. Am J Clin Pathol. 2013;139:345–9. doi: 10.1309/AJCPABOHBC33FVMO. [DOI] [PubMed] [Google Scholar]
- 6.Khuong-Quang DA, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124:439–47. doi: 10.1007/s00401-012-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartzentruber J, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–31. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 8.Wu G, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44:251–3. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45:602–12. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wulling M, et al. The nature of giant cell tumor of bone. J Cancer Res Clin Oncol. 2001;127:467–74. doi: 10.1007/s004320100234. [DOI] [PubMed] [Google Scholar]
- 11.Bamford S, et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer. 2004;91:355–8. doi: 10.1038/sj.bjc.6601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Medarde A, Santos E. Ras in cancer and developmental diseases. Genes Cancer. 2011;2:344–58. doi: 10.1177/1947601911411084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lasky T, Silbergeld E. P53 mutations associated with breast, colorectal, liver, lung, and ovarian cancers. Environ Health Perspect. 1996;104:1324–31. doi: 10.1289/ehp.961041324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank D, Doenecke D, Albig W. Differential expression of human replacement and cell cycle dependent H3 histone genes. Gene. 2003;312:135–43. doi: 10.1016/s0378-1119(03)00609-7. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Fernandez LA, Lopez-Alanon DM, Castaneda V, Krimer DB, del Mazo J. Developmental expression of H3.3A variant histone mRNA in mouse. Int J Dev Biol. 1997;41:699–703. [PubMed] [Google Scholar]
- 16.Lewis PW, et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340:857–61. doi: 10.1126/science.1232245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan KM, et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013;27:985–90. doi: 10.1101/gad.217778.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjerke L, et al. Histone H3.3 Mutations Drive Pediatric Glioblastoma through Upregulation of MYCN. Cancer Discov. 2013 doi: 10.1158/2159-8290.CD-12-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–95. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarpey PS, et al. Frequent mutation of the major cartilage collagen gene COL2A1 in chondrosarcoma. Nat Genet. 2013 doi: 10.1038/ng.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye K, Schulz MH, Long Q, Apweiler R, Ning Z. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics. 2009;25:2865–71. doi: 10.1093/bioinformatics/btp394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nik-Zainal S, et al. Mutational processes molding the genomes of 21 breast cancers. Cell. 2012;149:979–93. doi: 10.1016/j.cell.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Loo P, et al. Allele-specific copy number analysis of tumors. Proc Natl Acad Sci U S A. 2010;107:16910–5. doi: 10.1073/pnas.1009843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephens PJ, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–4. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor RM, et al. CD14-mononuclear stromal cells support (CD14+) monocyte-osteoclast differentiation in aneurysmal bone cyst. Laboratory Investigation. 2012;92:600–5. doi: 10.1038/labinvest.2012.5. [DOI] [PubMed] [Google Scholar]
- 26.Horton MA, Lewis D, McNulty K, Pringle JA, Chambers TJ. Monoclonal antibodies to osteoclastomas (giant cell bone tumors): definition of osteoclast-specific cellular antigens. Cancer Res. 1985;45:5663–9. [PubMed] [Google Scholar]
- 27.Horton MA, Taylor ML, Arnett TR, Helfrich MH. Arg-Gly-Asp (RGD) peptides and the anti-vitronectin receptor antibody 23C6 inhibit dentine resorption and cell spreading by osteoclasts. Exp Cell Res. 1991;195:368–75. doi: 10.1016/0014-4827(91)90386-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.