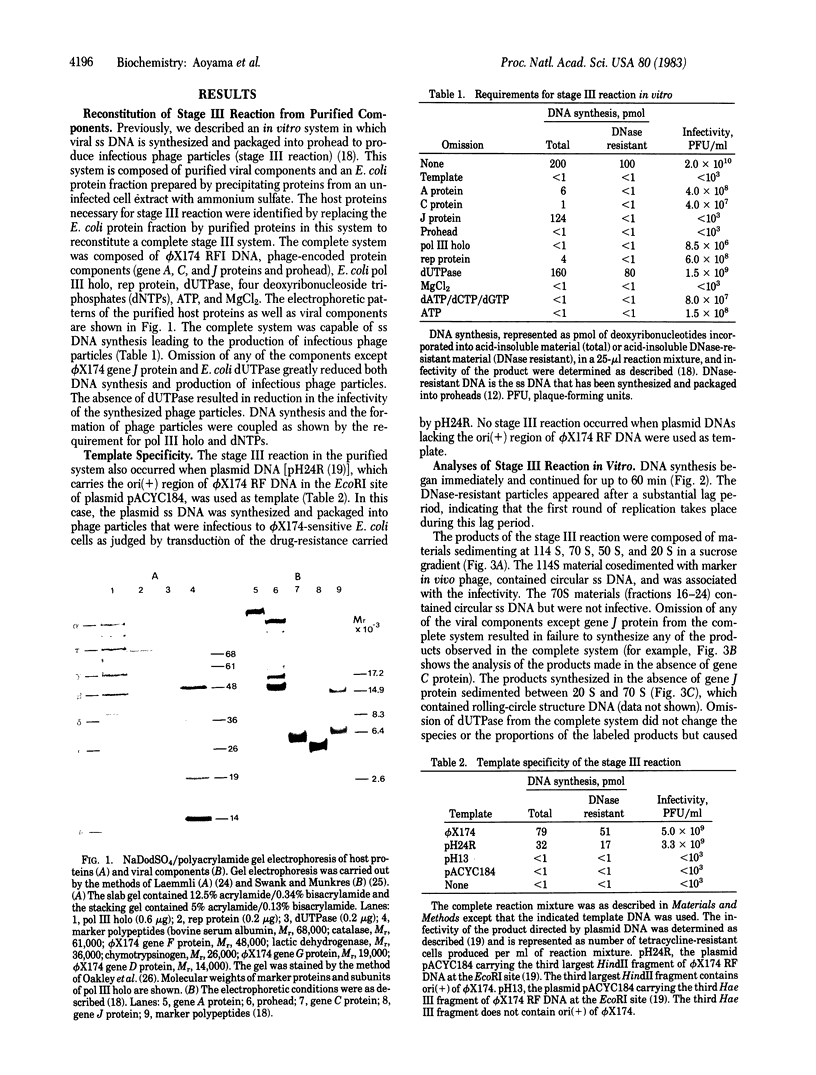
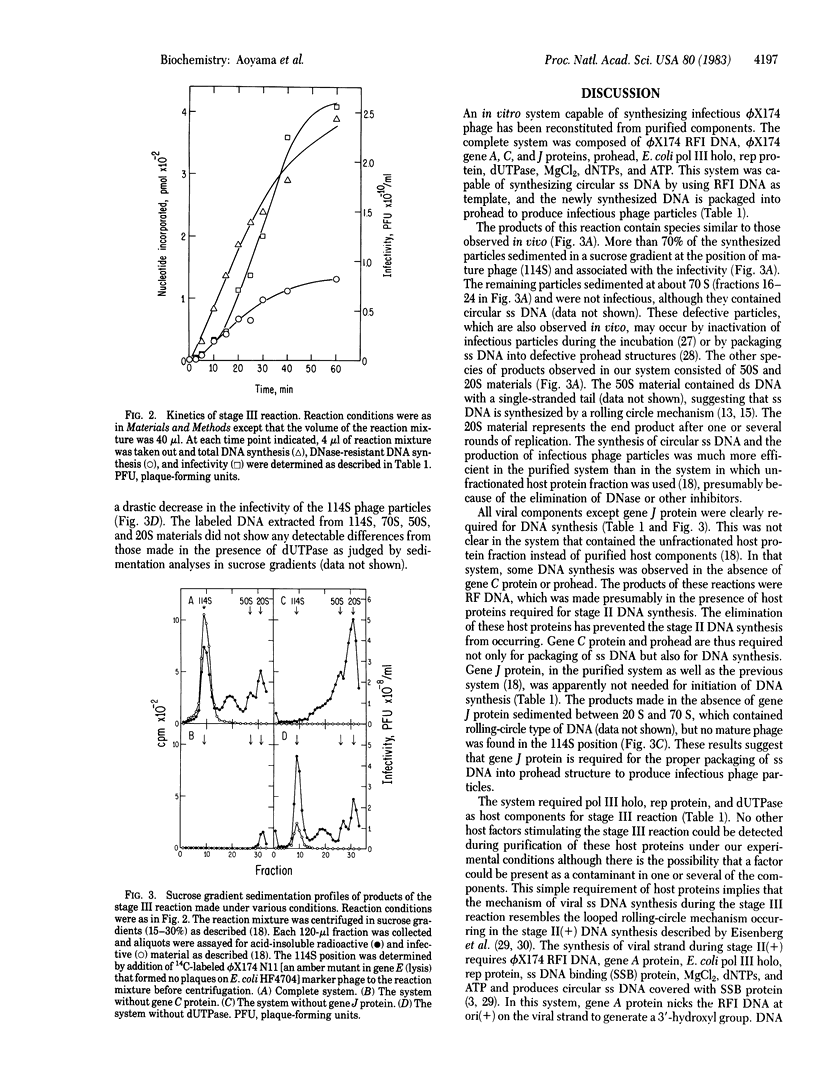
Abstract
An in vitro system capable of synthesizing infectious phi X174 phage particles was reconstituted from purified components. The synthesis required phi X174 supercoiled replicative form DNA, phi X174-encoded proteins A, C, J, and prohead, Escherichia coli DNA polymerase III holoenzyme, rep protein, and deoxyuridinetriphosphatase (dUTPase, dUTP nucleotidohydrolase, EC 3.6.1.23) as well as MgCl2, four deoxyribonucleoside triphosphates, and ATP. Phage production was coupled to the synthesis of viral single-stranded DNA. More than 70% of the synthesized particles sedimented at the position of mature phage in a sucrose gradient and associated with the infectivity. The simple requirement of the host proteins suggests that the mechanism of viral strand synthesis in the phage-synthesizing reaction resembles that of viral strand synthesis during the replication of replicative form DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama A., Hamatake R. K., Hayashi M. Morphogenesis of phi X174: in vitro synthesis of infectious phage from purified viral components. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7285–7289. doi: 10.1073/pnas.78.12.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama A., Hamatake R. K., Mukai R., Hayashi M. Purification of phi X174 gene C protein. J Biol Chem. 1983 May 10;258(9):5798–5803. [PubMed] [Google Scholar]
- Aoyama A., Hayashi M. In vitro packaging of plasmid DNAs into phi X174 bacteriophage capsid. Nature. 1982 Jun 24;297(5868):704–706. doi: 10.1038/297704a0. [DOI] [PubMed] [Google Scholar]
- Arai N., Polder L., Akai K., Kornberg A. Replication of phi X174 DNA with purified enzymes. II. Multiplication of the duplex form by coupling of continuous and discontinuous synthetic pathways. J Biol Chem. 1981 May 25;256(10):5239–5246. [PubMed] [Google Scholar]
- Baas P. D., Jansz H. S. Bacteriophage phiX174 DNA synthesis in a replication-deficient host: determination of the origin of phiX DNA replication. J Mol Biol. 1976 Apr 15;102(3):633–656. doi: 10.1016/0022-2836(76)90339-9. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Ascarelli R. The A* protein of phi X174 is an inhibitor of DNA replication. Nucleic Acids Res. 1981 Apr 24;9(8):1991–2002. doi: 10.1093/nar/9.8.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Griffith J., Kornberg A. phiX174 cistron A protein is a multifunctional enzyme in DNA replication. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3198–3202. doi: 10.1073/pnas.74.8.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Kornberg A. Purification and characterization of phiX174 gene A protein. A multifunctional enzyme of duplex DNA replication. J Biol Chem. 1979 Jun 25;254(12):5328–5332. [PubMed] [Google Scholar]
- Eisenberg S., Scott J. F., Kornberg A. Enzymatic replication of viral and complementary strands of duplex DNA of phage phiX174 proceeds by seprate mechanisms. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3151–3155. doi: 10.1073/pnas.73.9.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Cis-limited action of the gene-A product of bacteriophage phiX174 and the essential bacterial site (E. coli-electron microscopy-cis-acting protein-specifically-nicked RF). Proc Natl Acad Sci U S A. 1972 Feb;69(2):475–479. doi: 10.1073/pnas.69.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H., Hayashi M. Assembly of bacteriophage phi X174: identification of a virion capsid precursor and proposal of a model for the functions of bacteriophage gene products during morphogenesis. J Virol. 1977 Oct;24(1):303–313. doi: 10.1128/jvi.24.1.303-313.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H., Hayashi M. Functions of gene C and gene D products of bacteriophage phi X 174. J Virol. 1977 Feb;21(2):506–515. doi: 10.1128/jvi.21.2.506-515.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H., Hayashi M. Gene A product of phi X174 is required for site-specific endonucleolytic cleavage during single-stranded DNA synthesis in vivo. J Virol. 1976 Aug;19(2):416–424. doi: 10.1128/jvi.19.2.416-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H., Hayashi M. Two infectious forms of bacteriophage phi X 174. J Virol. 1977 Aug;23(2):439–442. doi: 10.1128/jvi.23.2.439-442.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H., Hayashi M. Viral DNA-synthesizing intermediate complex isolated during assembly of bacteriophage phi X174. J Virol. 1976 Aug;19(2):409–415. doi: 10.1128/jvi.19.2.409-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamatake R. K., Mukai R., Hayashi M. Role of DNA gyrase subunits in synthesis of bacteriophage phi X174 viral DNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1532–1536. doi: 10.1073/pnas.78.3.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda J. E., Yudelevich A., Hurwitz J. Isolation and characterization of the protein coded by gene A of bacteriophage phiX174 DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2669–2673. doi: 10.1073/pnas.73.8.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda J. E., Yudelevich A., Shimamoto N., Hurwitz J. Role of polymeric forms of the bacteriophage phi X174 coded gene A protein in phi XRFI DNA cleavage. J Biol Chem. 1979 Oct 10;254(19):9416–9428. [PubMed] [Google Scholar]
- Koths K., Dressler D. The rolling circle . capsid complex as an intermediate in phi X DNA replication and viral assembly. J Biol Chem. 1980 May 10;255(9):4328–4338. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McHenry C., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. Purification and resolution into subunits. J Biol Chem. 1977 Sep 25;252(18):6478–6484. [PubMed] [Google Scholar]
- Mukai R., Hamatake R. K., Hayashi M. Isolation and identification of bacteriophage phi X174 prohead. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4877–4881. doi: 10.1073/pnas.76.10.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai R., Hayashi M. Synthesis of infectious phiX-174 bacteriophage in vitro. Nature. 1977 Nov 24;270(5635):364–366. doi: 10.1038/270364a0. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Scott J. F., Kornberg A. Purification of the rep protein of Escherichia coli. An ATPase which separates duplex DNA strands in advance of replication. J Biol Chem. 1978 May 10;253(9):3292–3297. [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. Deoxyuridine triphosphatase of Escherichia coli. Purification, properties, and use as a reagent to reduce uracil incorporation into DNA. J Biol Chem. 1978 May 10;253(9):3305–3312. [PubMed] [Google Scholar]
- Shlomai J., Polder L., Arai K., Kornberg A. Replication of phi X174 dna with purified enzymes. I. Conversion of viral DNA to a supercoiled, biologically active duplex. J Biol Chem. 1981 May 25;256(10):5233–5238. [PubMed] [Google Scholar]
- Spindler K. R., Hayashi M. DNA synthesis in Escherichia coli cells infected with gene H mutants of bacteriophage phi X174. J Virol. 1979 Mar;29(3):973–982. doi: 10.1128/jvi.29.3.973-982.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Wolfson R., Eisenberg S. Escherichia coli host factor required specifically for the phi X174 stage III reaction: in vitro identification and partial purification. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5768–5772. doi: 10.1073/pnas.79.19.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mansfeld A. D., Langeveld S. A., Weisbeek P. J., Baas P. D., van Arkel G. A., Jansz H. S. Cleavage site of phiX174 gene-A protein in phiX and G4 RFI DNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):331–334. doi: 10.1101/sqb.1979.043.01.039. [DOI] [PubMed] [Google Scholar]