Abstract
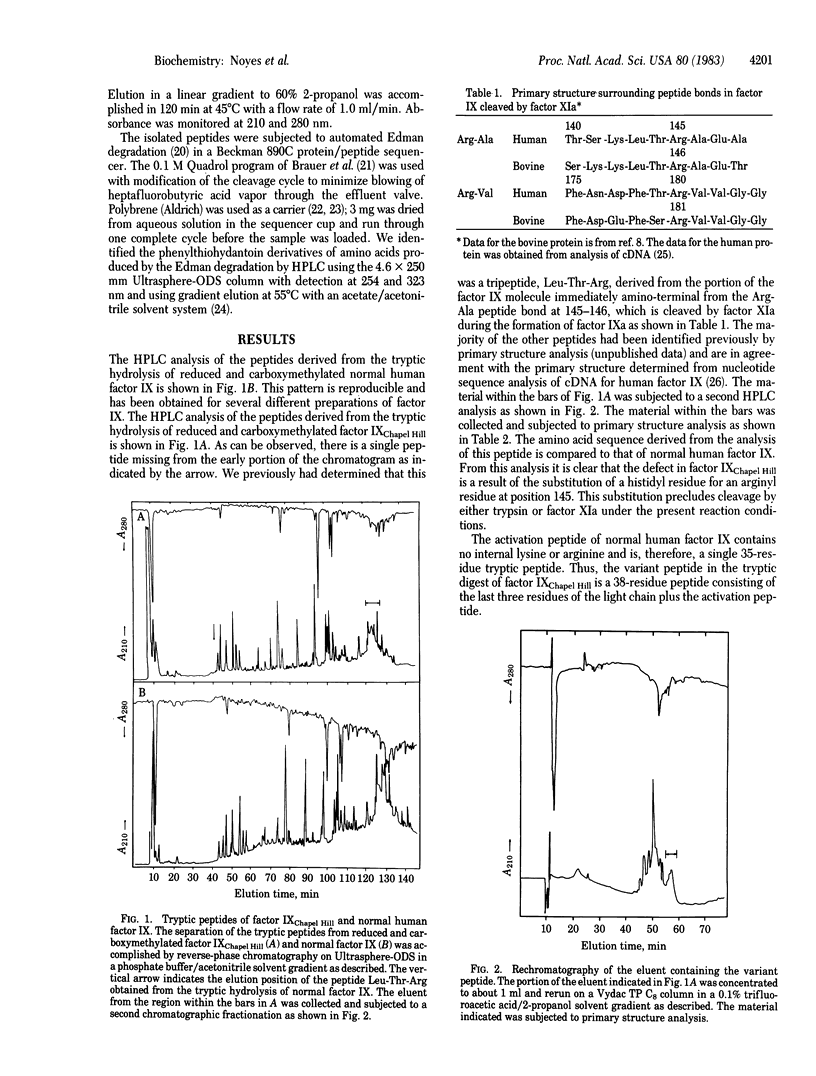
Hemophilia B Chapel Hill is a mild hereditary hemorrhagic disorder in which the factor IX antigen is present in normal amounts but factor IX biological activity is markedly reduced. Previous studies have demonstrated that purified factor IX Chapel Hill has 8% of the activity of normal human factor IX and that the activation of factor IX Chapel Hill is defective in that only one of the two peptide bonds hydrolyzed during activation of normal factor IX is cleaved. The tryptic peptides from normal human factor IX and factor IX Chapel Hill were subjected to analysis by high-performance liquid chromatography. Comparison of the elution profile of the peptides obtained from factor IX Chapel Hill and normal factor IX demonstrated that the tripeptide Leu-Thr-Arg, which is derived from the normal molecule (positions 143-145) immediately amino-terminal from the Arg-Ala peptide bond at 145-146 that is cleaved during the activation of factor IX with factor XIa, was absent in the digest obtained from factor factor IX Chapel Hill. The elongated "activation peptide" from factor factor IX Chapel Hill was obtained by further high-performance liquid chromatographic fractionation and subjected to primary structure analysis. The following sequence, corresponding to positions 143-147, was obtained: Leu-Thr-His-Ala-Glu. Thus, the primary molecular defect in factor factor IX Chapel Hill is the substitution of histidine for arginine at position 145. This substitution precludes cleavage by factor XIa at this peptide bond, and the activation peptide region remains associated with the light chain of factor IXa Chapel Hill.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENESCH R. E., LARDY H. A., BENESCH R. The sulfhydryl groups of crystalline proteins. I. Some albumins, enzymes, and hemoglobins. J Biol Chem. 1955 Oct;216(2):663–676. [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Braunstein K. M., Noyes C. M., Griffith M. J., Lundblad R. L., Roberts H. R. Characterization of the defect in activation of factor IX Chapel Hill by human factor XIa. J Clin Invest. 1981 Dec;68(6):1420–1426. doi: 10.1172/JCI110393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne R., Link R. P., Castellino F. J. A kinetic evaluation of activated bovine blood coagulation factor IX toward synthetic substrates. J Biol Chem. 1980 Jun 10;255(11):5336–5341. [PubMed] [Google Scholar]
- CANFIELD R. E., ANFINSEN C. B. CHROMATOGRAPHY OF PEPSIN AND CHYMOTRYPSIN DIGESTS OF EGG WHITE LYSOZYME ON PHOSPHOCELLULOSE. J Biol Chem. 1963 Aug;238:2684–2690. [PubMed] [Google Scholar]
- Chung K. S., Madar D. A., Goldsmith J. C., Kingdon H. S., Roberts H. R. Purification and characterization of an abnormal factor IX (Christmas factor) molecule. Factor IX Chapel Hill. J Clin Invest. 1978 Nov;62(5):1078–1085. doi: 10.1172/JCI109213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Scipio R. G., Kurachi K., Davie E. W. Activation of human factor IX (Christmas factor). J Clin Invest. 1978 Jun;61(6):1528–1538. doi: 10.1172/JCI109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Legaz M. E., Kato H., Davie E. W. The mechanism of activation of bovine factor IX (Christmas factor) by bovine factor XIa (activated plasma thromboplastin antecedent). Biochemistry. 1974 Oct 22;13(22):4508–4516. doi: 10.1021/bi00719a006. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Thompson A. R., Legaz M. E., Meyer R. G., Davie E. W. Isolation and characterization of bovine factor IX (Christmas factor). Biochemistry. 1973 Nov 20;12(24):4938–4945. doi: 10.1021/bi00748a019. [DOI] [PubMed] [Google Scholar]
- Furie B., Bing D. H., Feldmann R. J., Robison D. J., Burnier J. P., Furie B. C. Computer-generated models of blood coagulation factor Xa, factor IXa, and thrombin based upon structural homology with other serine proteases. J Biol Chem. 1982 Apr 10;257(7):3875–3882. [PubMed] [Google Scholar]
- Herman A. C., Vanaman T. C. Automated micro procedures for peptide separations. Methods Enzymol. 1977;47:220–236. doi: 10.1016/0076-6879(77)47027-7. [DOI] [PubMed] [Google Scholar]
- Higgins D. L., Shafer J. A. Fibrinogen Petoskey, a dysfibrinogenemia characterized by replacement of Arg-A alpha 16 by a histidyl residue. Evidence for thrombin-catalyzed hydrolysis at a histidyl residue. J Biol Chem. 1981 Dec 10;256(23):12013–12017. [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Katayama K., Ericsson L. H., Enfield D. L., Walsh K. A., Neurath H., Davie E. W., Titani K. Comparison of amino acid sequence of bovine coagulation Factor IX (Christmas Factor) with that of other vitamin K-dependent plasma proteins. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4990–4994. doi: 10.1073/pnas.76.10.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper D. G., Wilde C. E., 3rd, Capra J. D. Automated amino acid sequence of small peptides utilizing Polybrene. Anal Biochem. 1978 Mar;85(1):126–131. doi: 10.1016/0003-2697(78)90282-8. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Isolation and characterization of a cDNA coding for human factor IX. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6461–6464. doi: 10.1073/pnas.79.21.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist P. A., Fujikawa K., Davie E. W. Activation of bovine factor IX (Christmas factor) by factor XIa (activated plasma thromboplastin antecedent) and a protease from Russell's viper venom. J Biol Chem. 1978 Mar 25;253(6):1902–1909. [PubMed] [Google Scholar]
- McRae B. J., Kurachi K., Heimark R. L., Fujikawa K., Davie E. W., Powers J. C. Mapping the active sites of bovine thrombin, factor IXa, factor Xa, factor XIa, factor XIIa, plasma kallikrein, and trypsin with amino acid and peptide thioesters: development of new sensitive substrates. Biochemistry. 1981 Dec 8;20(25):7196–7206. doi: 10.1021/bi00528a022. [DOI] [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5260–5264. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts H. R., Grizzle J. E., McLester W. D., Penick G. D. Genetic variants of hemophilia B: detection by means of a specific PTC inhibitor. J Clin Invest. 1968 Feb;47(2):360–365. doi: 10.1172/JCI105732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]


