Abstract
Numerous proinflammatory cytokines have been implicated in the reorganization of lower urinary tract function following cyclophosphamide (CYP)-induced cystitis. The present study investigated the functional profile of three pleiotropic transforming growth factor-β (TGF-β) isoforms and receptor (TβR) variants in the normal and inflamed (CYP-induced cystitis) rat urinary bladder. Our findings indicate that TGF-β (1, 2, and 3) and TβR (1, 2, and 3) transcript and protein expression were regulated to varying degrees in the urothelium or detrusor smooth muscle following intermediate (48 h; 150 mg/kg ip) or chronic (75 mg/kg ip; once every 3 days for 10 days), but not acute (4 h; 150 mg/kg ip), CYP-induced cystitis. Conscious, open-outlet cystometry was performed to determine whether aberrant TGF-β signaling contributes to urinary bladder dysfunction following intermediate (48 h) CYP-induced cystitis. TβR-1 inhibition with SB505124 (5 μM) significantly (p ≤ 0.001) decreased voiding frequency and increased bladder capacity (2.5-fold), void volume (2.6-fold), and intercontraction intervals (2.5-fold) in CYP-treated (48 h) rats. Taken together, these results provide evidence for 1) the involvement of TGF-β in lower urinary tract neuroplasticity following urinary bladder inflammation, 2) a functional role of TGF-β signaling in the afferent limb of the micturition reflex, and 3) urinary bladder TβR-1 as a viable target to reduce voiding frequency with cystitis.
Keywords: inflammation, transforming growth factor-β, urothelium, detrusor smooth muscle, cyclophosphamide
interstitial cystitis/bladder pain syndrome (IC/BPS) is defined by the presence of dysuric symptoms and unpleasant sensations (visceral pain and discomfort) perceived to be related to the urinary bladder lasting longer than 6 wk and in the absence of other clinically identifiable sources (25). While the etiology remains unknown, overlapping clinical signs and symptoms suggest an involvement of microbial infection (14), submucosal immune cell infiltration (10), disrupted urothelial cell permeability (34), or inflammation (44) (for a review, see Ref. 22). We propose that micturition dysfunction and visceral neurogenic pain associated with IC/BPS are partly mediated by the urinary bladder inflammatory response (18, 21, 41). Several studies have already established the role of proinflammatory cytokines and their downstream targets in the organizational (19, 31) and functional (3, 29) alterations in micturition reflex pathways following chemically (cyclophosphamide; CYP)-induced cystitis. Most recently, the inflammatory mediator transforming growth factor (TGF)-β has been implicated in the pathogenesis of CYP-induced cystitis (45, 55).
The TGF-β superfamily is composed of at least 35 structurally related pleiotropic proteins (28). In addition to classic TGF-β, the superfamily can be subdivided into bone morphogenetic proteins (BMPs), activins/inhibins, growth and differentiation factors (GDFs), anti-müllerian hormone (AMH), and nodal (for a review, see Ref. 30). In mammals, the prototypic TGF-β cytokine exists in three isoforms (TGF-β1, TGF-β2, and TGF-β3) and is synthesized as a 75-kDa homodimeric propeptide, in which the mature growth factor is noncovalently bound to a pro-domain (latency-associated protein; LAP) (1). During secretion, covalent interactions between LAP and a glycoprotein, latent TGF-β binding protein (LTBP), translocate the latent complex to the extracellular matrix where it can be activated through protease-dependent (54) or protease-independent (thrombospondins, integrins, reactive oxygen species and protons) (6, 35, 38, 47) mechanisms (for a review, see Ref. 1). The destabilization of TGF-β/LAP and LAP/LTBP releases an activated TGF-β dimer that forms a heterotetrameric receptor complex composed of TGF-β type I receptor (TβR-1) and TβR-2. Ligand binding can also be enhanced through a third coreceptor (TβR-3) that lacks an intracellular signaling domain (52). To propagate an intracellular signal, intrinsic serine/threonine kinase activity of TβR-2 transphosphorylates TβR-1 to initiate receptor-regulated SMAD-dependent or SMAD-independent transduction pathways (33).
The maintenance and modulation of TGF-β signaling is essential for epithelial cell cycle progression, proliferation, and apoptosis (26), as well as immune cell function and inflammation (7). The present study examined the transcriptional and translational regulation of TGF-β (1, 2, and 3) and TβR (1, 2, and 3) in the rat urinary bladder inflammatory response and the role of TGF-β signaling in urinary bladder dysfunction following CYP-induced cystitis. Cytotoxicity in the urinary bladder accompanying CYP metabolism increases urinary bladder permeability (16), urothelial hyperplasia, and voiding frequency (43), in addition to altering somatic sensitivity (23, 24), neurochemical (48, 50), and electrophysiological (53) properties of micturition reflex function. Therefore, CYP-induced cystitis permits a controlled, systematic identification of inflammatory mediators underlying urinary bladder dysfunction that would otherwise be inaccessible in a clinical population (18). With this in mind, we determined 1) the temporal and tissue (urothelium and detrusor smooth muscle) expression of TGF-β (1, 2, and 3) and TβR (1, 2, and 3) urinary bladder transcripts following CYP-induced cystitis (acute, intermediate, or chronic); 2) the temporal expression of TGF-β (1, 2, and 3) urinary bladder protein following CYP-induced cystitis; 3) the temporal and tissue (urothelium and detrusor smooth muscle) expression of TGF-β1 and TβR (1, 2, and 3) using immunohistochemistry following CYP-induced cystitis; and 4) the functional effects of pharmacological TβR-1 inhibition using conscious, open-outlet cystometry following intermediate (48 h) CYP-induced cystitis.
MATERIALS AND METHODS
Animals
Naïve adult female Wistar rats (200–375 g) purchased from Charles River Laboratories (Wilmington, MA) were housed two per cage and maintained in standard laboratory conditions with food and water available ad libitum. Estrous cycles were not determined at any point in these studies. Experimental procedures were approved by the University of Vermont Institutional Animal Care and Use Committee (protocol 08-085), and experimentation was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th ed.).
Induction of CYP-Induced Cystitis
Female Wistar rats (n = 4–8/condition) received either no treatment, acute (150 mg/kg), intermediate (150 mg/kg), or chronic (75 mg/kg) intraperitoneal (ip) injections of cyclophosphamide (CYP; Sigma-Aldrich, St. Louis, MO). Following CYP (150 mg/kg ip) treatment, rats were harvested either 4 (acute) or 48 (intermediate) h postinjection (5, 11, 31). For chronic CYP (75 mg/kg ip) treatment, rats received injections every third day for 10 days, and tissues were harvested on day 10 (5, 11, 31).
Antibodies
Polyclonal primary antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX): rabbit anti-TGF-β1 (sc-146, 1:1,000), rabbit anti-TβR-1 (sc-398, 1:5,000), rabbit anti-TβR-2 (sc-220, 1:2,000), and goat anti-TβR-3 (sc-6199, 1:1,000). Secondary antibodies were purchased from Jackson ImmunoResearch Labs (West Grove, PA): Cy3 AffiniPure Goat Anti-Rabbit IgG (H+L) (111-165-144, 1:500) and Cy3 AffiniPure Donkey Anti-Goat IgG (H+L) (705-166-147, 1:500). Primary antibodies were diluted in 0.1 M phosphate-buffered saline (PBS) containing either 1% goat or donkey serum.
Real-Time Quantitative RT-PCR
Female Wistar rats (n = 5–7/condition) were anesthetized with 2% isoflurane, and urinary bladders were harvested under RNase-free conditions following a thoracotomy. Components of the urinary bladder (urothelium and detrusor smooth muscle) were separated as previously described (9, 32). For transcript analyses, the term urothelium refers to both the urothelial cell layers (basal, intermediate, and apical) and accompanying suburothelial structures. Total RNA was extracted from the urothelium and detrusor smooth muscle using STAT-60 total RNA/mRNA isolation reagent (Tel-Test ‘B’, Friendswood, TX), and complementary DNA (cDNA) was synthesized using random hexamer and oligo dT primers with M-MLV reverse transcriptase (Promega, Madison, WI) (20). cDNA templates were assayed using HotStart-IT SYBR Green qPCR Master Mix (USB, Cleveland, OH) containing 5 mM MgCl2, 0.4 mM dATP, dGTP, dCTP, and dTTP, HotStart-IT Taq DNA polymerase, and 300 nM of each primer (Table 1) in a final 25-μl reaction volume (3, 20, 32). Quantitative (q) RT-PCR was performed on an Applied Biosystems 7500 Fast real-time PCR system (Foster City, CA) using previously defined conditions (3, 20, 32). Amplicons were subjected to a SYBR Green I melting curve analysis by ramping the reaction temperature from 60 to 95°C. A single hyperchromic effect was observed under these dissociation conditions demonstrating amplification of a specific product free of primer-dimers or other contaminants.
Table 1.
Primer sequences
| Primer | Sequence |
|---|---|
| rTGF-β1 F | 5′-AGATTCAAGTCAACTGTGGA-3′ |
| rTGF-β1 R | 5′-AGGTCCTTCCTAAAGTCAAT-3′ |
| rTGF-β2 F | 5′-TTTAGGAATGTGCAGGATAA-3′ |
| rTGF-β2 R | 5′-CCAGCACAGAAGTTAGCATT-3′ |
| rTGF-β3 F | 5′-ATTCAAAGGAGTGGACAACG-3′ |
| rTGF-β3 R | 5′-AGTCGGTGTGGAGGAATCAT-3′ |
| rTβR-1 F | 5′-CCAACTACAGGACCTTTTTC-3′ |
| rTβR-1 R | 5′-TGAATGACAGTGCGGTT-3′ |
| rTβR-2 F | 5′-TAATGAAGAATACACCACCA-3′ |
| rTβR-2 R | 5′-GTCTCAAACTGCTCTGAAGT-3′ |
| rTβR-3 F | 5′-TATGTTGAGGTGTCTGTCAC-3′ |
| rTβR-3 R | 5′-TCTCTTGGAGCTGTAGAACT-3′ |
rTGF-β, rat transforming growth factor-β; rTβR, rat TGF-β receptor; F, forward; R, reverse.
Analysis.
Data were analyzed at the termination of each assay using the Sequence Detection Software (version 1.3.1; Applied Biosystems, Norwalk, CT). A standard curve was constructed by amplification of serially diluted plasmids containing the target sequence. The increase in SYBR Green I fluorescence intensity (ΔRn) was plotted as a function of cycle number and the threshold cycle (CT) was the amplification cycle at which ΔRn intersects baseline (3, 20, 32). qRT-PCR data are expressed as relative quantity of the gene of interest normalized to the relative quantity of the ribosomal reference gene, L32 (19).
ELISAs
Female Wistar rats (n = 8/condition) underwent the aforementioned CYP treatment and tissue harvest. Individual whole urinary bladders were weighed and solubilized for immunoassays in tissue protein extraction (Pierce Biotechnology, Woburn, MA) solution, a mild zwitterionic dialyzable detergent, supplemented with a complete protease inhibitor cocktail (Roche, Indianapolis, IN) (36). Tissue was homogenized using a Kinematica Polytron homogenizer (Fischer Scientific, Pittsburgh, PA) and centrifuged (3,000 rpm at 10°C for 10 min). The supernatant was removed, and protein was quantified according to the manufacturer's instructions using a Coomassie Plus Protein Assay Kit (Pierce Biotechnology).
Ninety-six-well microtiter plates (R&D Systems, Minneapolis, MN) were coated overnight with a capture antibody specific to the analyte (DuoSet Development Systems, R&D Systems). Plates were washed (1× PBS with 0.05% Tween 20), blocked (1× PBS with 5% Tween 20), and then standards and samples were added in duplicate. Standards provided by the manufacturer consisted of a seven-point standard curve (r2 = 0.989–0.997) in Reagent Diluent (1% BSA in PBS) beginning at 2,000 pg/ml. Following a 2-h incubation, standards and samples were vigorously washed three times, and a biotinylated detection antibody specific to the analyte was added and allowed to incubate for 2 h. Plates were washed three times, and a detection reagent (streptavidin-horseradish peroxidase) was added and incubated for 20 min. Plates were washed three times, and a substrate solution (hydrogen peroxide and tetramethylbenzidine) was added. Following a 20-min incubation, reactions were terminated with 2 N sulfuric acid, and the optical density was read at 450 and 570 nm.
Analysis.
Absorbance values were corrected for optical imperfections by subtracting readings at 570 nm (49). Samples were diluted to bring absorbance values onto the linear portion of the standard curve. Samples did not fall below the minimum detection limits of the assay. Curve fitting of standards and sample were performed using least-squares fit regression analysis (49).
Immunohistochemistry
Female Wistar rats (n = 4/condition) underwent the aforementioned CYP treatment and tissue harvest. Urinary bladder tissue was immediately allowed to incubate in 4% paraformaldehyde for 24 h at 4°C and was then transferred to 30% sucrose in 0.1 M PBS overnight at 4°C. Tissue was embedded in optimal cutting temperature compound (Tissue-Tek, Batavia, IL), sectioned at 20 μm, and mounted on 0.5% gel-coated slides (5, 9, 11). Sections were incubated overnight in polyclonal primary antibody (rabbit anti-TGF-β1, rabbit anti-TβR-1, rabbit anti-TβR-2, and goat anti-TβR-3), washed (3 × 10 min), and then incubated in cyanine 3 (Cy3) AffiniPure conjugated secondary antibody for 2 h. Following washing (3 × 10 min), slides were mounted with an antifading media (Citifluor, Fischer Scientific) and coverslipped.
Visualization and semiquantitative analysis.
Immunoreactivity (IR) was captured using an Olympus fluorescent photomicroscope with a charge-coupled device (CCD) camera (MagnaFire SP, Optical Analysis, Nashua, NH) and LG-3 frame grabber (Scion, Frederick, MD). Each experimental series was processed on the same day with exposure time, brightness, and contrast held constant throughout image acquisition (5, 9). To visualize Cy3, the filter was set with an excitation range of 560–569 nm and an emission range of 610–655 nm (5, 9). Images (6/animal) were acquired, saved in a 24-bit RGB-tagged image file format, and imported into MetaMorph Image Analysis Software (v4.5r4, Microscope Imaging Center, Downingtown, PA) (5, 9).
The focus of our analyses was on the urothelium and detrusor smooth muscle, whereas other parts of the lower urinary tract including the suburothelial plexus and lumbosacral dorsal root ganglia (DRG) are the focus of a separate study. A free-hand drawing tool was used to trace and measure the total pixel area in the urothelium (basal, intermediate, apical) and excluded any suburothelial structures (11). For the detrusor smooth muscle, six rectangles of fixed dimensions (125 × 125 pixels) were placed without overlap according to random x and y coordinates (9, 32). As previously described (9, 32), a threshold encompassing an intensity range of 100–250 grayscale values was applied to the region of interest in the least immunoreactive condition first and was maintained throughout the series. The threshold was adjusted for each experimental series with negative controls as a guide for setting background fluorescence (9, 32).
Assessment of IR.
Immunohistochemistry and evaluation of TGF-β1 and TβR (1, 2, and 3) IR in the urothelium or detrusor smooth muscle were performed in control and experimental tissues simultaneously to reduce the incidence of staining variation that can occur between tissues processed on different days (5, 9, 11). Staining in experimental tissue was compared with that in experiment-matched negative controls. Negative controls following the same experimental procedures were processed in the absence of a polyclonal primary antibody to assess specificity and background staining. In the absence of a primary antibody, positive IR was not observed (data not shown). IR was considered positive only when intensity measurements of the target exceeded the established threshold. Percent IR above threshold as a function of total area selected was calculated and reported. For the detrusor smooth muscle, percent target expression above threshold was averaged across the six regions.
Cystometry
Intravesical catheter implant.
A dorsal (below scruff of the neck) incision and lower midline abdominal incision was performed under 2% isoflurane using aseptic techniques. The end of a polyethylene (PE-50; Clay Adams, Parsippany, NJ) tube was flared, tunneled, and inserted into the bladder dome. The proximal end was secured to the dome with a 6-0 nylon purse string suture, whereas the distal end was sealed, coiled, and stored in a dorsal subcutaneous pouch. Adult female Wistar rats (n = 4–5/condition) recovered for 72 h with postoperative analgesia (buprenorphine, 0.05 mg/kg sc) being maintained for 48 h. No animals met our exclusion criteria (3, 5).
Conscious, freely moving cystometry with an open outlet.
The dorsal subcutaneous polyethylene tubing was externalized under 2% isoflurane, and the animal was placed unrestrained into a Small Animal Cystometry System (Med Associates, St. Albans, VT) recording cage over a scale and pan used to quantify voided volume. The animals were each given a 10-min acclimation period before the continuous intravesical infusion of 0.9% sodium chloride (NaCl) injection, USP (Baxter, Deerfield, IL) at a rate of 10 ml/h. To avoid micturition variation resulting from circadian rhythms, functional tests were conducted at similar times of the day (15). Each animal was run for at least six reproducible micturition cycles (predrug baseline) after an initial stabilization period (30 min). Intravesical pressure (nonvoiding, filling, threshold, and peak micturition pressures), intercontraction interval, and infused and voided volume were recorded for each micturition cycle. Bladder capacity was quantified as the total infused 0.9% NaCl at the time micturition commenced (3, 5). Nonvoiding bladder contractions were defined as rhythmic intravesical pressure increases 0.69 kilopascals (kPa) above baseline without the release of fluid from the urethra and are not reported due to their infrequency in the current study (3, 5).
To determine the role of TGF-β on urinary bladder function in control (no inflammation) or CYP-treated rats, we intravesically instilled 1 ml of a TβR-1 small molecule inhibitor, SB505124 (5 μM; R&D Systems), under 2% isoflurane for 30 min immediately following the baseline measurements. SB505124 (5 μM) was chosen due to its in vitro potency and selectivity for TGF-β-activated activin receptor-like kinases (ALKs) (12). Intravesical instillation of 1 ml 0.01% DMSO in 0.9% NaCl under 2% isoflurane was used for the vehicle control conditions. Rats remained anesthetized during instillation to suppress the micturition reflex and prevent expulsion of SB505124 (5 μM) or 0.01% DMSO (2, 3, 5). At the conclusion of drug instillation, urinary bladder function tests were repeated and run for at least six reproducible micturition cycles (postdrug). The animals were euthanized following functional testing as described above.
Materials
SB505124 (R&D Systems) was reconstituted in DMSO (99.5%) and stored at −20°C. Before its use, stock solutions were diluted to a working concentration (5 μM) with 0.9% NaCl injection, USP (Baxter).
Statistical Analyses
All values represent means ± SE. Data were compared with one-way or repeated measures ANOVA where appropriate. When F (test statistic) exceeded the critical value (P ≤ 0.05), the Bonferroni or Newman-Keuls multiple comparisons test was used to compare group means.
RESULTS
TGF-β (1, 2, and 3) and TβR (1, 2, and 3) Transcript Expression in the Urothelium and Detrusor Smooth Muscle Following CYP-Induced Cystitis
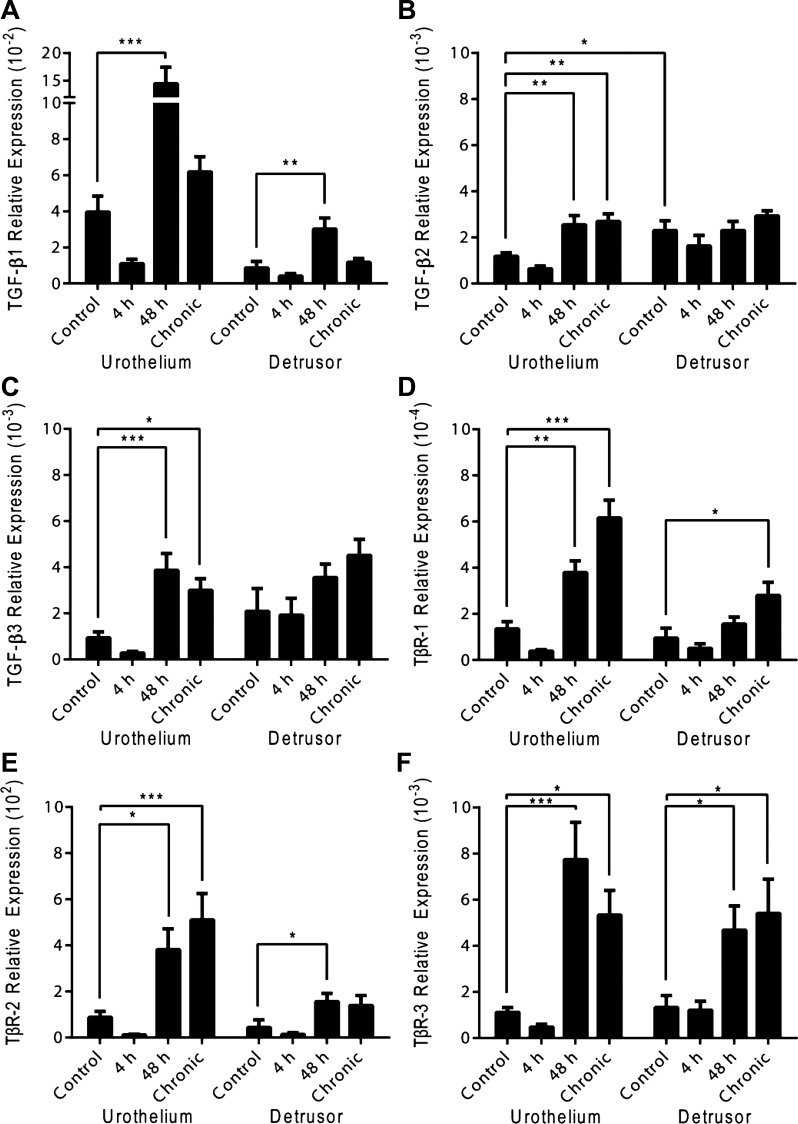
The regulation of TGF-β (1, 2, and 3) and TβR (1, 2, and 3) transcripts in the urothelium and detrusor smooth muscle was examined by qRT-PCR analyses (Fig. 1, A–F).
Fig. 1.
Time- and tissue-dependent regulation of transforming growth factor (TGF)-β (1, 2 and 3) and TGF-β receptor [(TβR) 1, 2, and 3] transcript expression following cyclophosphamide (CYP) treatment. TGF-β (1, 2, and 3) and TβR (1, 2, and 3) transcript expression was not significantly regulated following acute (4 h) induced cystitis. Intermediate (48 h) CYP-induced cystitis significantly increased TGF-β1 (A; urothelium/detrusor smooth muscle), TGF-β2 (B; urothelium), TGF-β3 (C; urothelium), TβR-1 (D; urothelium), TβR-2 (E; urothelium/detrusor smooth muscle), and TβR-3 (F; urothelium/detrusor smooth muscle) transcript expression relative to control. Chronic CYP-induced cystitis significantly increased TGF-β2 (B; urothelium), TGF-β3 (C; urothelium), TβR-1 (D; urothelium/detrusor smooth muscle), TβR-2 (E; urothelium), and TβR-3 (F; urothelium/detrusor smooth muscle) transcript expression relative to control. Values are means ± SE; n = 5–7/condition. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. control.
TGF-β1.
TGF-β1 transcript expression significantly increased relative to control in the urothelium (P ≤ 0.001) and detrusor smooth muscle (P ≤ 0.01) following intermediate (48 h) CYP-induced cystitis (Fig. 1A). Significant differences were not observed in the urothelium or detrusor smooth muscle following acute (4 h) or chronic CYP-induced cystitis (Fig. 1A).
TGF-β2.
Basal TGF-β2 transcript expression significantly (P ≤ 0.05) differed between the urothelium and detrusor smooth muscle under control conditions (Fig. 1B). TGF-β2 transcript expression in the urothelium significantly increased relative to control following intermediate (48 h; P ≤ 0.01) or chronic (P ≤ 0.01) CYP-induced cystitis, whereas no significant differences were observed following acute (4 h) CYP-induced cystitis (Fig. 1B). Acute (4 h), intermediate (48 h), or chronic CYP-induced cystitis did not significantly regulate TGF-β2 transcript expression in the detrusor smooth muscle (Fig. 1B).
TGF-β3.
TGF-β3 transcript expression significantly increased in the urothelium following intermediate (48 h; P ≤ 0.001) or chronic (P ≤ 0.05) CYP-induced cystitis relative to control, whereas no significant differences were observed following acute (4 h) CYP-induced cystitis (Fig. 1C). Acute (4 h), intermediate (48 h), or chronic CYP-induced cystitis did not significantly regulate TGF-β3 transcript expression in the detrusor smooth muscle (Fig. 1C).
TβR-1.
TβR-1 transcript expression significantly increased relative to control in the urothelium following intermediate (48 h; P ≤ 0.01) or chronic (P ≤ 0.001) CYP-induced cystitis (Fig. 1D). In the detrusor smooth muscle, significant increases in TβR-1 transcript expression were only observed following chronic (P ≤ 0.05) CYP-induced cystitis relative to control (Fig. 1D). Significant differences in detrusor smooth muscle TβR-1 transcript expression were not observed following acute (4 h) or intermediate (48 h) CYP-induced cystitis (Fig. 1D).
TβR-2.
Intermediate (48 h) CYP-induced cystitis significantly increased TβR-2 transcript expression in both the urothelium (P ≤ 0.05) and detrusor smooth muscle (P ≤ 0.05), whereas significant transcript increase following chronic CYP-induced cystitis was only seen in the urothelium (P ≤ 0.001) (Fig. 1E). Acute (4 h) CYP-induced cystitis did not significantly regulate urothelial or detrusor smooth muscle TβR-2 transcript expression (Fig. 1E).
TβR-3.
TβR-3 transcript expression significantly increased relative to control in the urothelium following intermediate (48 h; P ≤ 0.001) and chronic (P ≤ 0.05) CYP-induced cystitis (Fig. 1F). Significant transcript increases were similarly seen in the detrusor smooth muscle following intermediate (48 h; P ≤ 0.05) and chronic (P ≤ 0.05) CYP-induced cystitis (Fig. 1F). Acute (4 h) CYP-induced cystitis did not significantly regulate TβR-3 transcript expression in the urothelium or detrusor smooth muscle (Fig. 1F).
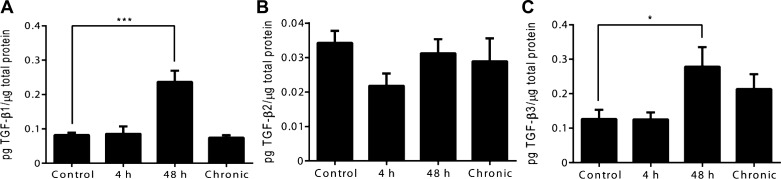
TGF-β (1, 2, and 3) Protein Expression in Whole Urinary Bladder Following CYP-Induced Cystitis
The regulation of TGF-β (1, 2, and 3) urinary bladder protein was examined by ELISAs (Fig. 2, A–C). The selection of analyte was limited by commercial availability. Intermediate (48 h) CYP-induced cystitis significantly increased urinary bladder TGF-β1 (P ≤ 0.001) and TGF-β3 (P ≤ 0.05) protein expression relative to control (Fig. 2, A and C). Significant differences relative to control were not observed in TGF-β1 and TGF-β3 urinary bladder protein expression following acute (4 h) or chronic CYP-induced cystitis (Fig. 2, A and C). TGF-β2 urinary bladder protein expression did not significantly differ relative to control across acute (4 h), intermediate (48 h), or chronic CYP treatments (Fig. 2B).
Fig. 2.
Time-dependent regulation of TGF-β (1, 2, and 3) protein expression in whole rat urinary bladder following CYP treatment. A: TGF-β1 urinary bladder protein expression was significantly increased following intermediate (48 h) CYP-induced cystitis. B: TGF-β2 urinary bladder protein expression did not significantly differ across any condition relative to control. C: TGF-β3 urinary bladder protein expression was significantly increased following intermediate (48 h) CYP-induced cystitis. Values are means ± SE; n = 8/condition. *P ≤ 0.05, ***P ≤ 0.001 vs. control.
TGF-β1 and TβR (1, 2, and 3) IR in the Urothelium and Detrusor Smooth Muscle Following CYP-Induced Cystitis
Based on the transcriptional and translational expression profiles observed, we focused immunostaining on TGF-β1 and TβR variants whose mRNA and protein exhibited tissue and temporal regulation by CYP treatment (Fig. 1, A and D–F, and Fig. 2A). Regional differences in IR were not observed in the dome, body, or neck of the rat urinary bladder.
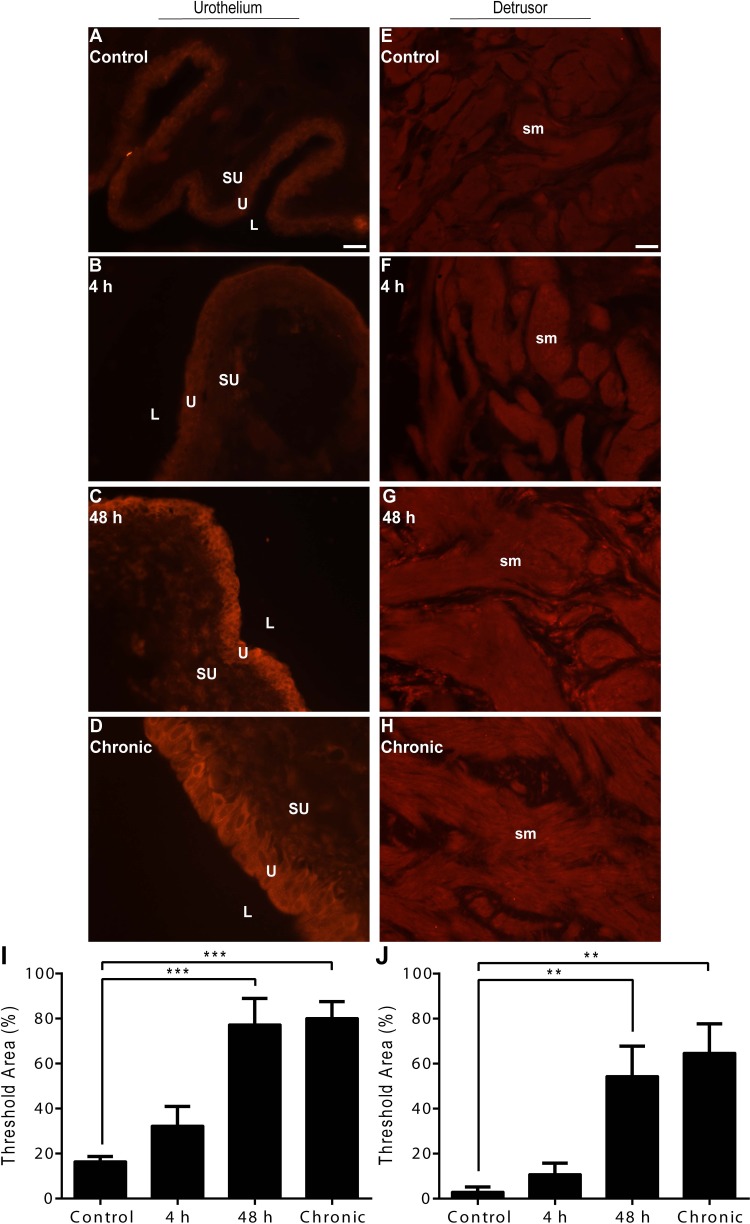
TGF-β1.
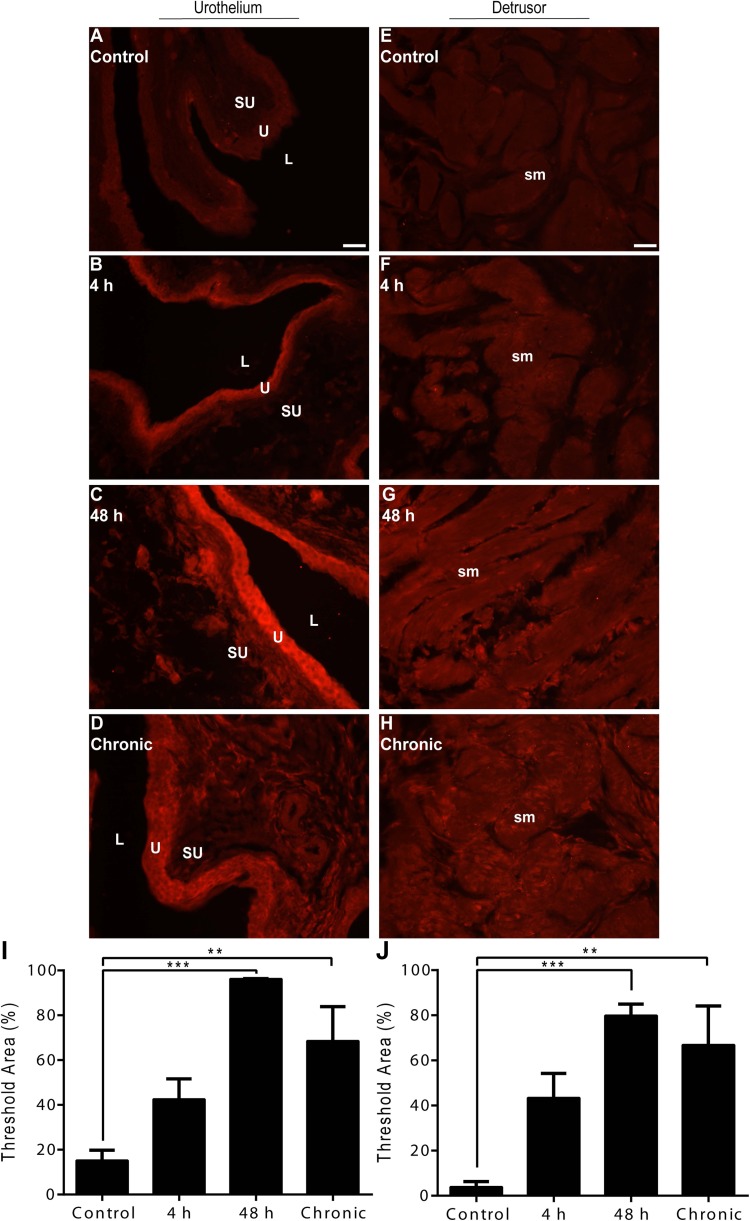
Low-intensity, basal TGF-β1-IR was present in all urothelial layers (basal, intermediate, and apical) and detrusor smooth muscle of control rat urinary bladders (Fig. 3, A and E). Acute (4 h) CYP-induced cystitis revealed no observable differences in urothelial or detrusor smooth muscle TGF-β1-IR relative to control (Fig. 3, B and F). Intermediate (48 h) or chronic CYP-induced cystitis, however, resulted in high-intensity TGF-β1-IR in both the urothelium and detrusor smooth muscle (Fig. 3, C and D and G and H). Semiquantitative analyses revealed a significant suprathreshold increase relative to control in the urothelium following intermediate (48 h; P ≤ 0.001) or chronic (P ≤ 0.001) CYP-induced cystitis (Fig. 3I). Similarly, semiquantitative analyses in the detrusor smooth muscle revealed a significant suprathreshold increase relative to control following intermediate (48 h; P ≤ 0.01) or chronic (P ≤ 0.01) CYP-induced cystitis (Fig. 3J).
Fig. 3.
Time- and tissue-dependent regulation of TGF-β1 immunoreactivity (IR) following CYP treatment. A–D: representative images of TGF-β1-IR in urothelium following control or CYP treatment paradigms. E–H: representative images of TGF-β1-IR in detrusor smooth muscle following control or CYP treatment paradigms. I: semiquantitative analyses in the urothelium revealed a significant suprathreshold increase following intermediate (48 h) or chronic CYP-induced cystitis. J: semiquantitative analyses in detrusor smooth muscle revealed a significant suprathreshold increase following intermediate (48 h) or chronic CYP-induced cystitis. L, lumen; U, urothelium; SU, suburothelium; sm, detrusor smooth muscle. Calibration bar represents 25 μm. Values are means ± SE; n = 4/condition. **P ≤ 0.01, ***P ≤ 0.001 vs. control.
TβR-1.
Low-intensity, basal TβR-1-IR was present in all urothelial layers (basal, intermediate, and apical) and detrusor smooth muscle of control rat urinary bladders (Fig. 4, A and E). Acute (4 h) CYP-induced cystitis resulted in moderate urothelial IR relative to control, whereas intermediate (48 h) or chronic CYP-induced cystitis resulted in high-intensity urothelial TβR-1-IR (Fig. 4, B–D). Semiquantitative analyses revealed a significant suprathreshold increase relative to control in the urothelium following intermediate (48 h; P ≤ 0.001) or chronic (P ≤ 0.01) CYP-induced cystitis (Fig. 4I). There were no observable differences in detrusor smooth muscle TβR-1-IR following acute (4 h) CYP-induced cystitis, while intermediate (48 h) or chronic CYP-induced cystitis resulted in moderate IR relative to control (Fig. 4, F–H). Semiquantitative analyses revealed an emerging trend of robust suprathreshold IR following intermediate (48 h) or chronic CYP-induced cystitis (Fig. 4J).
Fig. 4.
Time- and tissue-dependent regulation of TβR-1-IR following CYP treatment. A–D: representative images of TβR-1-IR in the urothelium following control or CYP treatment paradigms. E–H: representative images of TβR-1-IR in detrusor smooth muscle following control or CYP treatment paradigms. I: semiquantitative analyses in urothelium revealed a significant suprathreshold increase following intermediate (48 h) or chronic CYP-induced cystitis. J: semiquantitative analyses in the detrusor smooth muscle revealed a robust suprathreshold trend following intermediate (48 h) or chronic CYP-induced cystitis. Calibration bar represents 25 μm. Values are means ± SE; n = 4/condition. **P ≤ 0.01, ***P ≤ 0.001 vs. control.
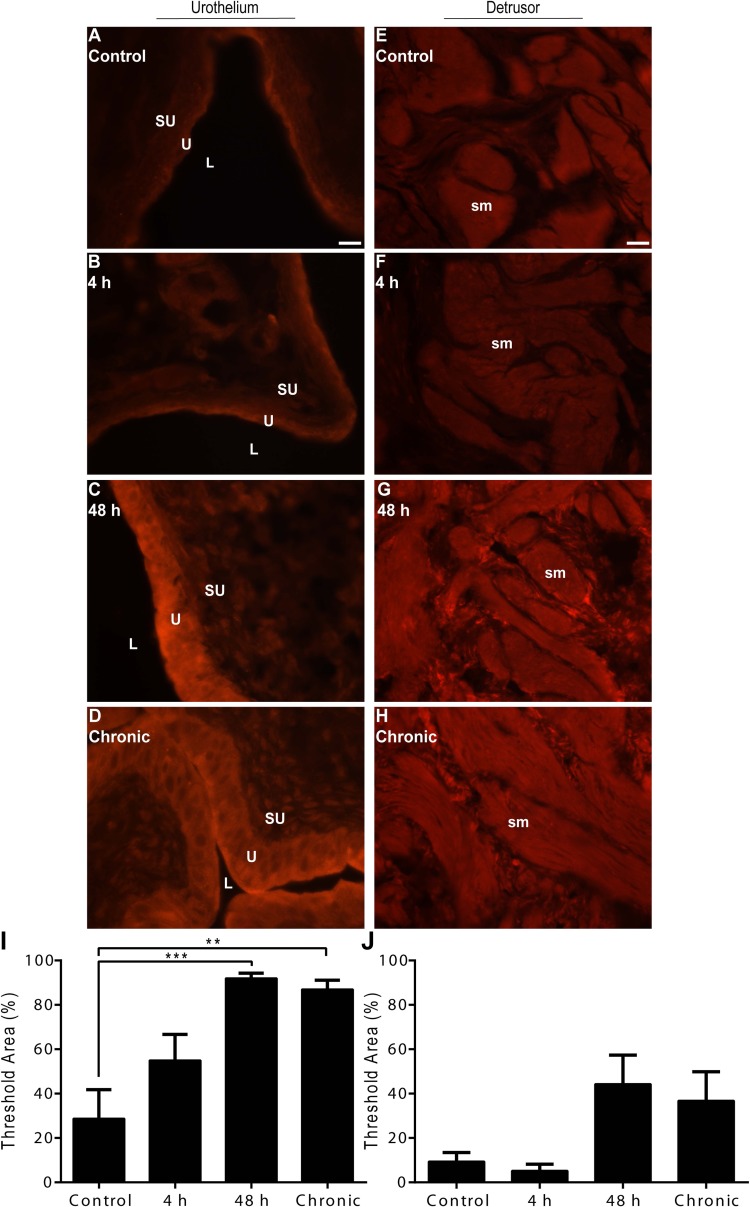
TβR-2.
Low-intensity, basal TβR-2-IR was present in all urothelial layers (basal, intermediate, and apical) and detrusor smooth muscle of control rat urinary bladders (Fig. 5, A and E). Acute (4 h) CYP-induced cystitis resulted in moderate urothelial and detrusor smooth muscle IR relative to control, while intermediate (48 h) or chronic CYP-induced cystitis resulted in a high-intensity TβR-2-IR (Fig. 5, B–D and F–H). Semiquantitative analyses revealed a significant suprathreshold increase relative to control in the urothelium following intermediate (48 h; P ≤ 0.001) or chronic (P ≤ 0.01) CYP-induced cystitis (Fig. 5I). Similarly, semiquantitative analyses in the detrusor smooth muscle revealed a significant suprathreshold increase relative to control following intermediate (48 h; P ≤ 0.001) or chronic (P ≤ 0.01) CYP-induced cystitis (Fig. 5J).
Fig. 5.
Time- and tissue-dependent regulation of TβR-2-IR following CYP treatment. A–D: representative images of TβR-2-IR in the urothelium following control or CYP treatment paradigms. E–H: representative images of TβR-2-IR in the detrusor smooth muscle following control or CYP treatment paradigms. I: semiquantitative analyses in the urothelium revealed a significant suprathreshold increase following intermediate (48 h) or chronic CYP-induced cystitis. J: semiquantitative analyses in the detrusor smooth muscle revealed a significant suprathreshold increase following intermediate (48 h) or chronic CYP-induced cystitis. Calibration bar represents 25 μm. Values are means ± SE; n = 4/condition. **P ≤ 0.01, ***P ≤ 0.001 vs. control.
TβR-3.
Low-intensity, basal TβR-3-IR was present in the detrusor smooth muscle of control rat urinary bladders (Fig. 6A). Acute (4 h) CYP-induced cystitis resulted in moderate IR relative to control, while intermediate (48 h) or chronic CYP-induced cystitis resulted in high-intensity detrusor smooth muscle TβR-3-IR (Fig. 6, B–D). Semiquantitative analyses revealed a significant suprathreshold increase relative to control in the detrusor smooth muscle following intermediate (48 h; P ≤ 0.01) or chronic (P ≤ 0.01) CYP-induced cystitis (Fig. 6E). TβR-3-IR was not present in the urothelium following any control or CYP treatment paradigm (data not shown).
Fig. 6.
Time- and tissue-dependent regulation of TβR-3-IR following CYP treatment. A–D: representative images of TβR-3-IR in the detrusor smooth muscle following control or CYP treatment paradigms. E: semiquantitative analyses in the detrusor smooth muscle revealed a significant suprathreshold increase following intermediate (48 h) or chronic CYP-induced cystitis. Calibration bar represents 25 μm. Values are means ± SE; n = 4/condition. **P ≤ 0.01 vs. control.
Urinary Bladder Function Following TβR-1 Inhibition in Control (No Inflammation) or Intermediate (48 h) CYP-Induced Cystitis Rats
Urinary bladder function was determined using open-outlet, continuous cystometry in conscious, freely moving control or intermediate (48 h) CYP-induced cystitis rats (Table 2). Intermediate (48 h) CYP-induced cystitis was chosen for analysis due to a robust increase in urinary bladder TGF-β ligand and receptor transcript and protein expression.
Table 2.
Cystometrogram recordings for control (no inflammation) or intermediate (48 h) CYP-induced cystitis rats pre- and post-SB505124 (5 μM) or 0.01% DMSO instillation
| Infused Volume, ml | Void Volume, ml | Intercontraction Interval, s | Filling Pressure, kPa | Threshold Pressure, kPa | Peak Micturition Pressure, kPa | |
|---|---|---|---|---|---|---|
| Control | ||||||
| Pre-DMSO | 1.3 ± 0.1 | 1.3 ± 0.1 | 478 ± 53 | 1.2 ± 0.2 | 1.5 ± 0.2 | 4.4 ± 0.5 |
| Pre-SB505124 | 1.3 ± 0.2 | 1.3 ± 0.2 | 483 ± 76 | 1.1 ± 0.1 | 1.3 ± 0.1 | 4.9 ± 0.5 |
| Post-DMSO | 1.7 ± 0.3 | 1.7 ± 0.2 | 603 ± 92 | 1.1 ± 0.1 | 1.7 ± 0.2 | 4.6 ± 0.7 |
| Post-SB505124 | 1.8 ± 0.3* | 1.8 ± 0.3* | 641 ± 101* | 1.1 ± 0.1 | 1.5 ± 0.2 | 4.3 ± 0.8 |
| 48-h CYP | ||||||
| Pre-DMSO | 0.2 ± 3 × 10−2 | 0.2 ± 3 × 10−2 | 75 ± 11 | 1.9 ± 0.2 | 1.9 ± 0.3 | 7.1 ± 1.6 |
| Pre-SB505124 | 0.2 ± 3 × 10−2 | 0.2 ± 3 × 10−2 | 88 ± 10 | 2.2 ± 0.5 | 2.1 ± 0.3 | 5.4 ± 0.5 |
| Post-DMSO | 0.2 ± 4 × 10−2 | 0.2 ± 4 × 10−2 | 89 ± 17 | 1.9 ± 0.3 | 1.9 ± 0.3 | 8.3 ± 1.6 |
| Post-SB505124 | 0.6 ± 0.1† | 0.6 ± 0.1† | 217 ± 35† | 1.8 ± 0.4 | 2.0 ± 0.3 | 5.3 ± 0.6 |
Values are means ± SE; n = 4–5/condition. Intravesical instillation of SB505124 (5 μM) significantly decreased voiding frequency and increased bladder capacity (total infused 0.9% NaCl at the time micturition commenced), void volume, and intercontraction intervals relative to pre-SB505124 baseline measurements in both control (no inflammation) and intermediate (48 h) cyclophosphamide (CYP)-induced cystitis rats.
P ≤ 0.05,
P ≤ 0.001 vs. pre-SB505124 baseline.
Control (no inflammation).
Intravesical instillation of SB505124 (5 μM) significantly (P ≤ 0.05) decreased voiding frequency and increased bladder capacity (1.3-fold), void volume (1.4-fold), and intercontraction intervals (1.3-fold) relative to predrug baseline measurements (Table 2). Intravesical pressure (filling, threshold, peak) was not significantly affected following SB505124 (5 μM) instillation (Table 2). Residual volume before or after SB505124 (5 μM) instillation was minor (≤35 μl), and the effects of treatment persisted throughout the entirety of the experiment (1.5–2 h).
Intermediate (48 h) CYP-induced cystitis.
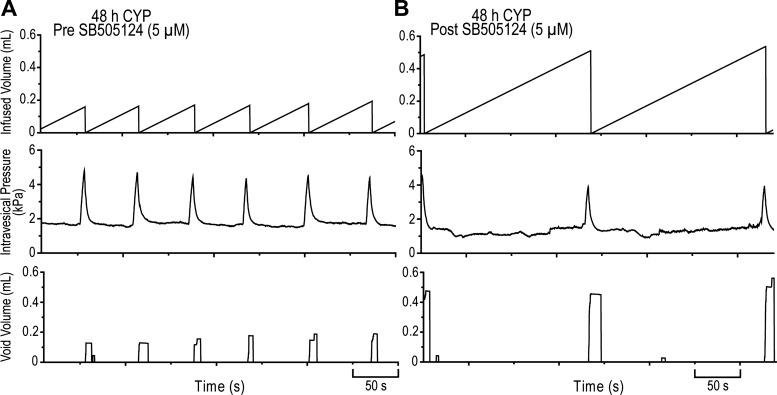
As previously demonstrated (3, 5, 29), intermediate (48 h) CYP-induced cystitis increased voiding frequency and intravesical pressure (filling, threshold, peak) and decreased bladder capacity, void volume, and intercontraction intervals relative to control (no inflammation) rats (Table 2). Intravesical instillation of SB505124 (5 μM) significantly (P ≤ 0.001) decreased voiding frequency and increased bladder capacity (2.5-fold), void volume (2.6-fold), and intercontraction intervals (2.5-fold) relative to predrug baseline measurements (Table 2, Fig. 7). SB505124 (5 μM) did not significantly affect intravesical pressure (filling, threshold, peak) in CYP-treated (48 h) rats (Table 2, Fig. 7). Residual volume before or after SB505124 (5 μM) instillation was minor (≤20 μl), and the effects of treatment persisted throughout the entirety of the experiment (1.5–2 h).
Fig. 7.
Cystometrogram traces of TβR-1 inhibition following intermediate (48 h) CYP-induced cystitis. A: representative pre-SB505124 baseline traces. B: representative post-SB505124 (5 μM) traces. Intravesical instillation of SB505124 (5 μM; B) significantly (P ≤ 0.001) decreased voiding frequency and increased bladder capacity (total infused 0.9% NaCl at the time micturition commenced; 2.5-fold), void volume (2.6-fold), and intercontraction intervals (2.5-fold) relative to pre-SB505124 baseline measurements (A). Representative traces (A and B) were from the same rat.
DISCUSSION
The present study characterized the expression of urinary bladder TGF-β (1, 2, and 3) and TβR (1, 2, and 3) following CYP-induced cystitis of varying durations and established a role for TGF-β signaling in micturition reflex function. We confirmed the presence of a delayed TGF-β proinflammatory phenotype following acute urinary bladder inflammation and extended previous findings (45, 55) by demonstrating the functional expression and chronicity of urinary bladder TGF-β. These results provide evidence for a novel molecular component of the urinary bladder inflammatory response that, when inhibited, reduces voiding frequency with urinary bladder dysfunction.
The expression of TGF-β ligands in the urinary bladder proinflammatory cytokine milieu has been previously suggested to reflect the inflammatory state of the urinary bladder (55). Zhang and Qiao (55) have observed a stepwise TGF-β1 transcript upregulation following acute (8 h) and intermediate (48 h) CYP-induced cystitis, while Tyagi et al. (45) observed significant upregulation of TGF-β1 urinary bladder protein following acute (24 h) CYP-induced cystitis. Similarly, the present study demonstrates an upregulation of TGF-β ligand and receptor expression following intermediate (48 h) or chronic CYP-induced cystitis, but not acute (4 h) CYP-induced cystitis. The temporal regulation of TGF-β transcript and protein expression may aid in monitoring the urinary bladder inflammatory response and suggests an involvement in the resolution of urinary bladder inflammation to initiate tissue remodeling (45).
In the remedial stages of inflammation, the urinary bladder undergoes alterations characterized by a deposition of extracellular matrix constituents and proliferation of activated fibroblasts (13). Although the precise contribution of TGF-β to pathological tissue remodeling is unknown, TGF-β is capable of promoting deposition of profibrotic extracellular matrix proteins and preventing matrix degradation by inhibiting expression of metalloproteinases and plasminogen-activators (8). While neither the present nor past studies address the role of TGF-β in urinary bladder remodeling, our results suggest the profibrotic profile may be partly mediated by a delayed, long-lasting TGF-β upregulation.
Chronic urinary bladder inflammation is characterized by increased mononuclear cell infiltration, mucosal ulceration, and the systemic release of proinflammatory cytokines and chemokines from distinct tissue compartments (40, 42). The present study not only demonstrated the temporal regulation of TGF-β but also the differential distribution of TGF-β ligands and its cognate receptors. The regulation of TGF-β1 and TGF-β3 transcript expression in the urothelium appeared to contribute most to the global translational profiles observed in our conditions. However, significant upregulation of TGF-β2 transcript in the urothelium was not sufficient to increase urinary bladder protein at any CYP-treatment duration. These differences may reflect the elevated basal TGF-β2 transcript expression in the detrusor smooth muscle relative to the urothelium. TβR (1, 2, and 3) transcript expression in the urothelium and detrusor smooth muscle was, in general, significantly increased following intermediate (48 h) or chronic CYP-induced cystitis. These profiles were maintained in TβR-1 and TβR-2 antigen immunoreactivity in the urothelium, as well as TβR (1, 2, and 3) immunoreactivity in the detrusor smooth muscle. The absence of urothelial TβR-3 immunoreactivity in control or CYP-treated animals may reflect transcript expression and regulation in the suburothelium, transcript instability (27), decreased translation efficiency, and/or increased receptor internalization (17).
While the clinical presentation of IC/BPS is diverse, there remain histopathological and immunological features that may contribute to urinary bladder dysfunction. Cystoscopic and bladder biopsy examinations indicate IC/BPS patients may present with a constellation of signs and symptoms, including an exaggerated inflammatory response, intrafascicular fibrosis, and detrusor mastocytosis (37, 39, 46). Our findings, combined with the established role of TGF-β in cellular proliferation, differentiation, and migration, suggest TGF-β ligand overexpression and subsequent intracellular signaling may contribute, in part, to the pathophysiology of our experimental cystitis model of IC/BPS. To determine whether aberrant TGF-β signaling contributes to urinary bladder dysfunction, we intravesically instilled a competitive ATP small-molecule inhibitor, SB505124 (5 μM). Due to the structural similarities between TGF-β-activated ALKs, it is possible for SB505124 to have off-target effects on activin A receptor, type IB (ACVR1B, ALK4). While further studies are necessary to define the specific role(s) of ACVR1B and TβR-1 on urinary bladder dysfunction, SB505124 demonstrates 2.5-fold less potency in vitro on ACVR1B SMAD phosphorylation, suggesting it may have a greater influence on TGF-β signaling in vivo (12, 51).
Instillation of SB505124 (5 μM) decreased voiding frequency in both control (no inflammation) and intermediate (48 h) CYP-induced cystitis rats; however, differences in fold-change suggest TGF-β signaling has a more prominent role in micturition reflex function following urinary bladder inflammation. These results not only remain consistent with the transcriptional and translational overexpression profiles observed following intermediate (48 h) CYP-induced cystitis but also demonstrate a novel, albeit small, role for TGF-β in normal micturition reflex function. Cystometrogram recordings in control (no inflammation) rats following SB505124 (5 μM) instillation revealed a role for basal urothelial TGF-β signaling in micturition reflex function due to the barrier properties provided by intact junction complexes and specialized apical proteins (4). In addition, the absence of intravesical pressure changes suggests little to no effect on the urethral outlet and efferent limb of the micturition reflex. Similar parameters (void frequency, infused and voided volume, intercontraction intervals) were altered following intermediate (48 h) CYP-induced cystitis and SB505124 (5 μM), suggesting a role for aberrant TGF-β signaling in the afferent limb of the micturition reflex. Specific tissues or cell types in the urinary bladder that contribute to the functional effects are less clear due to increases in urinary bladder permeability after CYP metabolism (16) and global TβR-1 overexpression. Future studies can address suburothelial-specific overexpression of TβR-1 and/or determine the contributions of urothelial and detrusor smooth muscle TGF-β signaling to resolve our observed phenotypes.
The combination of systemic proinflammatory cytokine release, mononuclear cell infiltration, and the profibrotic resolution of inflammation may contribute to peripheral and central sensitization that may facilitate viscerosomatic hypersensitivity and bladder hyperreflexia observed in IC/BPS (42). Given the biological contributions TGF-β may have to urinary bladder dysfunction (45, 55) and its recently established role in peripheral sensitization (56), the present study characterized TGF-β transcript and protein expression at the level of the urinary bladder and provided evidence for a functional role of TGF-β signaling in the afferent limb of the micturition reflex. These studies demonstrate that targeting one component of the urinary bladder inflammatory response may be an effective strategy to reduce voiding frequency in an experimental cystitis model of IC/BPS.
GRANTS
This work was funded by National Institutes of Health (NIH) Grants DK-051369, DK-065989, and DK-060481. NIH Grant P20 RR-16435 from the Centers of Biomedical Research Excellence Program of the National Center also supported the project with research resources.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.J.G., B.M.G., and M.A.V. provided conception and design of research; E.J.G. and B.M.G. performed experiments; E.J.G. and B.M.G. analyzed data; E.J.G., B.M.G., and M.A.V. interpreted results of experiments; E.J.G. and B.M.G. prepared figures; E.J.G. drafted manuscript; E.J.G., B.M.G., and M.A.V. edited and revised manuscript; E.J.G., B.M.G., and M.A.V. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical expertise and support provided by Susan Malley, Abbey Peterson, and the Vermont Cancer Center DNA Analysis Facility.
REFERENCES
- 1.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci 116: 217–224, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Arms L, Girard BM, Malley SE, Vizzard MA. Expression and function of CCL2/CCR2 in rat micturition reflexes and somatic sensitivity with urinary bladder inflammation. Am J Physiol Renal Physiol 305: F111–F122, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arms L, Girard BM, Vizzard MA. Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 298: F589–F600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arms L, Girard BM, Vizzard MA. Role of the bladder urothelium in voiding dysfunction. Curr Bladder Dysfunct Rep 4: 227–233, 2009 [Google Scholar]
- 5.Arms L, Vizzard MA. Role for pAKT in rat urinary bladder with cyclophosphamide (CYP)-induced cystitis. Am J Physiol Renal Physiol 301: F252–F262, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 10: 1077–1083, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer 6: 506–520, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Branton MH, Kopp JB. TGF-beta and fibrosis. Microbes Infect 1: 1349–1365, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Cheppudira BP, Girard BM, Malley SE, Schutz KC, May V, Vizzard MA. Upregulation of vascular endothelial growth factor isoform VEGF-164 and receptors (VEGFR-2, Npn-1, and Npn-2) in rats with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295: F826–F836, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christmas TJ. Lymphocyte sub-populations in the bladder wall in normal bladder, bacterial cystitis and interstitial cystitis. Br J Urol 73: 508–515, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R125–R134, 2007 [DOI] [PubMed] [Google Scholar]
- 12.DaCosta Byfield S, Major C, Laping NJ, Roberts AB. SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol Pharmacol 65: 744–752, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Deveaud CM, Macarak EJ, Kucich U, Ewalt DH, Abrams WR, Howard PS. Molecular analysis of collagens in bladder fibrosis. J Urol 160: 1518–1527, 1998 [PubMed] [Google Scholar]
- 14.Domingue GJ, Ghoniem GM, Bost KL, Fermin C, Human LG. Dormant microbes in interstitial cystitis. J Urol 153: 1321–1326, 1995 [PubMed] [Google Scholar]
- 15.Dorr W. Cystometry in mice—influence of bladder filling rate and circadian variations in bladder compliance. J Urol 148: 183–187, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Eichel L, Scheidweiler K, Kost J, Shojaie J, Schwarz E, Messing E, Wood R. Assessment of murine bladder permeability with fluorescein: validation with cyclophosphamide and protamine. Urology 58: 113–118, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Finger EC, Lee NY, You HJ, Blobe GC. Endocytosis of the type III transforming growth factor-beta (TGF-beta) receptor through the clathrin-independent/lipid raft pathway regulates TGF-beta signaling and receptor down-regulation. J Biol Chem 283: 34808–34818, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girard BM, Cheppudira BP, Malley SE, Schutz KC, May V, Vizzard MA. Increased expression of interleukin-6 family members and receptors in urinary bladder with cyclophosphamide-induced bladder inflammation in female rats. Front Neurosci 5: 20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girard BM, Malley SE, Vizzard MA. Neurotrophin/receptor expression in urinary bladder of mice with overexpression of NGF in urothelium. Am J Physiol Renal Physiol 300: F345–F355, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girard BM, May V, Bora SH, Fina F, Braas KM. Regulation of neurotrophic peptide expression in sympathetic neurons: quantitative analysis using radioimmunoassay and real-time quantitative polymerase chain reaction. Regul Pept 109: 89–101, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Girard BM, Tompkins JD, Parsons RL, May V, Vizzard MA. Effects of CYP-induced cystitis on PACAP/VIP and receptor expression in micturition pathways and bladder function in mice with overexpression of NGF in urothelium. J Mol Neurosci 48: 730–743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grover S, Srivastava A, Lee R, Tewari AK, Te AE. Role of inflammation in bladder function and interstitial cystitis. Ther Adv Urol 3: 19–33, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerios SD, Wang ZY, Bjorling DE. Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett 392: 193–197, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol 295: R111–R122, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanno PM, Burks DA, Clemens JQ, Dmochowski RR, Erickson D, FitzGerald MP, Forrest JB, Gordon B, Gray M, Mayer RD, Newman D, Nyberg L, Jr, Payne CK, Wesselmann U, Faraday MM. AUA Guideline for the Diagnosis and Treatment of Interstitial Cystitis/Bladder Pain Syndrome. J Urol 185: 2162–2170, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heldin CH, Landstrom M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol 21: 166–176, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Hempel N, How T, Cooper SJ, Green TR, Dong M, Copland JA, Wood CG, Blobe GC. Expression of the type III TGF-beta receptor is negatively regulated by TGF-beta. Carcinogenesis 29: 905–912, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horbelt D, Denkis A, Knaus P. A portrait of Transforming Growth Factor beta superfamily signalling: Background matters. Int J Biochem Cell Biol 44: 469–474, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Regul Integr Comp Physiol 284: R574–R585, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Kingsley DM. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev 8: 133–146, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Klinger MB, Dattilio A, Vizzard MA. Expression of cyclooxygenase-2 in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R677–R685, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295: F1778–F1789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lantero A, Tramullas M, Diaz A, Hurle MA. Transforming growth factor-beta in normal nociceptive processing and pathological pain models. Mol Neurobiol 45: 76–86, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Lokeshwar VB, Selzer MG, Cerwinka WH, Gomez MF, Kester RR, Bejany DE, Gousse AE. Urinary uronate and sulfated glycosaminoglycan levels: markers for interstitial cystitis severity. J Urol 174: 344–349, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol 106: 1659–1665, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malley SE, Vizzard MA. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol Genomics 9: 5–13, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Martin P. Wound healing–aiming for perfect skin regeneration. Science 276: 75–81, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev 11: 59–69, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Peeker R, Enerback L, Fall M, Aldenborg F. Recruitment, distribution and phenotypes of mast cells in interstitial cystitis. J Urol 163: 1009–1015, 2000 [PubMed] [Google Scholar]
- 40.Saban R. Gene-regulation during bladder neurogenic inflammation. Urology 57: 103, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Sant GR, Hanno PM. Interstitial cystitis: current issues and controversies in diagnosis. Urology 57: 82–88, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Sant GR, Kempuraj D, Marchand JE, Theoharides TC. The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology 69: 34–40, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Stewart FA. Mechanism of bladder damage and repair after treatment with radiation and cytostatic drugs. Br J Cancer Suppl 7: 280–291, 1986 [PMC free article] [PubMed] [Google Scholar]
- 44.Theoharides TC, Sant GR, el-Mansoury M, Letourneau R, Ucci AA, Jr, Meares EM., Jr Activation of bladder mast cells in interstitial cystitis: a light and electron microscopic study. J Urol 153: 629–636, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Tyagi P, Tyagi V, Yoshimura N, Witteemer E, Barclay D, Loughran PA, Zamora R, Vodovotz Y. Gender-based reciprocal expression of transforming growth factor-beta1 and the inducible nitric oxide synthase in a rat model of cyclophosphamide-induced cystitis. J Inflamm (Lond) 6: 23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van de Merwe JP, Nordling J, Bouchelouche P, Bouchelouche K, Cervigni M, Daha LK, Elneil S, Fall M, Hohlbrugger G, Irwin P, Mortensen S, van Ophoven A, Osborne JL, Peeker R, Richter B, Riedl C, Sairanen J, Tinzl M, Wyndaele JJ. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol 53: 60–67, 2008 [DOI] [PubMed] [Google Scholar]
- 47.van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res 305: 285–298, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat 21: 125–138, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Vizzard MA. Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol 278: R1027–R1039, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 161: 273–284, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Vogt J, Traynor R, Sapkota GP. The specificities of small molecule inhibitors of the TGFBs and BMP pathways. Cell Signal 23: 1831–1842, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Wharton K, Derynck R. TGFbeta family signaling: novel insights in development and disease. Development 136: 3691–3697, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci 19: 4644–4653, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 14: 163–176, 2000 [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang QL, Qiao LY. Regulation of IGF-1 but not TGF-beta1 by NGF in the smooth muscle of the inflamed urinary bladder. Regul Pept 177: 73–78, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu Y, Colak T, Shenoy M, Liu L, Mehta K, Pai R, Zou B, Xie XS, Pasricha PJ. Transforming growth factor beta induces sensory neuronal hyperexcitability, and contributes to pancreatic pain and hyperalgesia in rats with chronic pancreatitis. Mol Pain 8: 65, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]