Abstract
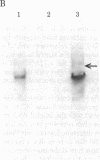
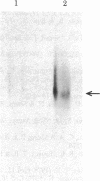
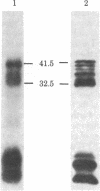
Transcription of the Bal I E restriction fragment of adenovirus DNA by RNA polymerase II in a HeLa cell extract produces a RNA transcript 1,712 nucleotides in length. This transcript contains the first two elements of the tripartite leader that, in vivo, is spliced onto the late mRNAs. We have found that this adenovirus 2 transcript forms a specific ribonucleoprotein complex (RNP) in this in vitro system. The RNP particle sediments in sucrose gradients as a monodisperse peak at 50 S and has a buoyant density of 1.34 g/cm3 in Cs2SO4, indicating the same 4:1 protein/RNA composition as native nuclear RNPs that contain pre-mRNA sequences (hnRNP). Moreover, the in vitro-assembled RNP is resistant to concentrations of NaCl that are known to dissociate nonspecific RNA-protein complexes. The adenovirus 2 transcript is precipitated by a monoclonal antibody for hnRNP core proteins. In addition, RNA-protein crosslinking of [alpha-32P]UTP-labeled transcript/RNP complexes reveals that the major proteins in contact with the RNA are the Mr 32,500-41,500 species known to be associated with hnRNA in vivo. These results demonstrate the in vitro assembly of a specific RNA polymerase II transcript into RNP. Moreover, because the 1,712-nucleotide adenovirus 2 transcript lacks poly(A) addition sites and because the leader sequences are not spliced appreciably in this in vitro system, it follows that RNP formation requires neither polyadenylylation nor splicing, nor is it sufficient to cause the latter.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Pettersson U. Sequence analysis of adenovirus DNA: complete nucleotide sequence of the spliced 5' noncoding region of adenovirus 2 hexon messenger RNA. Cell. 1979 Apr;16(4):841–850. doi: 10.1016/0092-8674(79)90099-0. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Huang A. S. Interaction of HeLa cell proteins with RNA. J Mol Biol. 1970 Feb 14;47(3):263–273. doi: 10.1016/0022-2836(70)90301-3. [DOI] [PubMed] [Google Scholar]
- Berg O. G., Winter R. B., von Hippel P. H. Diffusion-driven mechanisms of protein translocation on nucleic acids. 1. Models and theory. Biochemistry. 1981 Nov 24;20(24):6929–6948. doi: 10.1021/bi00527a028. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer A. L., Christensen M. E., Walker B. W., LeStourgeon W. M. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977 May;11(1):127–138. doi: 10.1016/0092-8674(77)90323-3. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Miller O. L., Jr, McKnight S. L. Ribonucleoprotein structure in nascent hnRNA is nonrandom and sequence-dependent. Cell. 1980 May;20(1):75–84. doi: 10.1016/0092-8674(80)90236-6. [DOI] [PubMed] [Google Scholar]
- Calvet J. P., Pederson T. Nucleoprotein organization of inverted repeat DNA transcripts in heterogeneous nuclear RNA-ribonucleoprotein particles from HeLa cells. J Mol Biol. 1978 Jul 5;122(3):361–378. doi: 10.1016/0022-2836(78)90195-x. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Hansen U., Handa H., Sharp P. A. Sequential transcription-translation of simian virus 40 by using mammalian cell extracts. Mol Cell Biol. 1981 Oct;1(10):919–931. doi: 10.1128/mcb.1.10.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R. The spliced structures of adenovirus 2 fiber message and the other late mRNAs. Cell. 1978 Oct;15(2):497–510. doi: 10.1016/0092-8674(78)90019-3. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Economidis I. V., Pederson T. Assembly of nuclear ribonucleoprotein particles during in vitro transcription. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1469–1473. doi: 10.1073/pnas.79.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidis I. V., Pederson T. Structure of nuclear ribonucleoprotein: heterogeneous nuclear RNA is complexed with a major sextet of proteins in vivo. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1599–1602. doi: 10.1073/pnas.80.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findly R. C., Pederson T. Regulated transcription of the genes for actin and heat-shock proteins in cultured Drosophila cells. J Cell Biol. 1981 Feb;88(2):323–328. doi: 10.1083/jcb.88.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Schibler U. Mouse beta-globin and adenovirus-2 major late transcripts are initiated at the cap site in vitro. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2283–2286. doi: 10.1073/pnas.78.4.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Kaufman R. J., Manley J., Gefter M., Sharp P. A. Transcription of Simian virus 40 DNA in a HeLa whole cell extract. J Biol Chem. 1981 Jan 10;256(1):478–482. [PubMed] [Google Scholar]
- Holland C. A., Mayrand S., Pederson T. Sequence complexity of nuclear and messenger RNA in HeLa cells. J Mol Biol. 1980 Apr 25;138(4):755–778. doi: 10.1016/0022-2836(80)90064-9. [DOI] [PubMed] [Google Scholar]
- Hough P. V., Mastrangelo I. A., Wall J. S., Hainfeld J. F., Simon M. N., Manley J. L. DNA-protein complexes spread on N2-discharged carbon film and characterized by molecular weight and its projected distribution. J Mol Biol. 1982 Sep 15;160(2):375–386. doi: 10.1016/0022-2836(82)90183-8. [DOI] [PubMed] [Google Scholar]
- Jones R. E., Okamura C. S., Martin T. E. Immunofluorescent localization of the proteins of nuclear ribonucleoprotein complexes. J Cell Biol. 1980 Jul;86(1):235–243. doi: 10.1083/jcb.86.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Vidali G., Boffa L. C., Allfrey V. G. Characterization of the non-histone nuclear proteins associated with rapidly labeled heterogeneous nuclear RNA. J Biol Chem. 1977 Oct 25;252(20):7307–7322. [PubMed] [Google Scholar]
- Keohavong P., Gattoni R., LeMoullec J. M., Jacob M., Stévenin J. The orderly splicing of the first three leaders of the adenovirus-2 major late transcript. Nucleic Acids Res. 1982 Feb 25;10(4):1215–1229. doi: 10.1093/nar/10.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinniburgh A. J., Martin T. E. Detection of mRNA sequences in nuclear 30S ribonucleoprotein subcomplexes. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2725–2729. doi: 10.1073/pnas.73.8.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole R., Weissman S. M. Accurate in vitro splicing of human beta-globin RNA. Nucleic Acids Res. 1982 Sep 25;10(18):5429–5445. doi: 10.1093/nar/10.18.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulguskin V. V., Krichevskaya A. A., Lukanidin E. M., Georgiev G. P. Studies on dissociation and reconstitution of nuclear 30-S ribonucleoprotein particles containing pre-mRNA. Biochim Biophys Acta. 1980 Oct 17;609(3):410–424. doi: 10.1016/0005-2787(80)90115-x. [DOI] [PubMed] [Google Scholar]
- Lukanidin E. M., Zalmanzon E. S., Komaromi L., Samarina O. P., Georgiev G. P. Structure and function of informofers. Nat New Biol. 1972 Aug 16;238(85):193–197. doi: 10.1038/newbio238193a0. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand S., Pederson T. Heat shock alters nuclear ribonucleoprotein assembly in Drosophila cells. Mol Cell Biol. 1983 Feb;3(2):161–171. doi: 10.1128/mcb.3.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand S., Pederson T. Nuclear ribonucleoprotein particles probed in living cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2208–2212. doi: 10.1073/pnas.78.4.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand S., Setyono B., Greenberg J. R., Pederson T. Structure of nuclear ribonucleoprotein: identification of proteins in contact with poly(A)+ heterogeneous nuclear RNA in living HeLa cells. J Cell Biol. 1981 Aug;90(2):380–384. doi: 10.1083/jcb.90.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Post-replicative nonribosomal transcription units in D. melanogaster embryos. Cell. 1979 Jul;17(3):551–563. doi: 10.1016/0092-8674(79)90263-0. [DOI] [PubMed] [Google Scholar]
- Merlino G. T., Vogeli G., Yamamoto T., de Crombrugghe B., Pastan I. Accurate in vitro transcriptional initiation of the chick alpha 2 (Type I) collagen gene. J Biol Chem. 1981 Nov 10;256(21):11251–11258. [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978 Dec;15(4):1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Nowak L., Marvil D. K., Thomas J. O., Boublik M., Szer W. A single-stranded nucleic acid-binding protein from Artemia salina. II. Interaction with nucleic acids. J Biol Chem. 1980 Jul 10;255(13):6473–6478. [PubMed] [Google Scholar]
- Ohlendorf D. H., Anderson W. F., Fisher R. G., Takeda Y., Matthews B. W. The molecular basis of DNA-protein recognition inferred from the structure of cro repressor. Nature. 1982 Aug 19;298(5876):718–723. doi: 10.1038/298718a0. [DOI] [PubMed] [Google Scholar]
- Ohlsson R. I., van Eekelen C., Philipson L. Non-random localization of ribonucleoprotein (RNP) structures within an adenovirus mRNA precursor. Nucleic Acids Res. 1982 May 25;10(10):3053–3068. doi: 10.1093/nar/10.10.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T., Davis N. G. Messenger RNA processing and nuclear structure: isolation of nuclear ribonucleoprotein particles containing beta-globin messenger RNA precursors. J Cell Biol. 1980 Oct;87(1):47–54. doi: 10.1083/jcb.87.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T. Messenger RNA biosynthesis and nuclear structure. Am Sci. 1981 Jan-Feb;69(1):76–84. [PubMed] [Google Scholar]
- Pederson T., Munroe S. H. Ribonucleoprotein organization of eukaryotic RNA. XV. Different nucleoprotein structures of globin messenger RNA sequences in nuclear and polyribosomal ribonucleoprotein particles. J Mol Biol. 1981 Aug 25;150(4):509–524. doi: 10.1016/0022-2836(81)90377-6. [DOI] [PubMed] [Google Scholar]
- Pederson T. Proteins associated with heterogeneous nuclear RNA in eukaryotic cells. J Mol Biol. 1974 Feb 25;83(2):163–183. doi: 10.1016/0022-2836(74)90386-6. [DOI] [PubMed] [Google Scholar]
- Prosvirnin V. V., Ruzidic S., Samarina O. P. Cross-linked informofers. Nucleic Acids Res. 1979 Nov 24;7(6):1649–1661. doi: 10.1093/nar/7.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan T. J., Billings P. B., Martin T. E. Nuclear ribonucleoprotein complexes containing polyadenylate from mouse ascites cells. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2632–2636. doi: 10.1073/pnas.71.7.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. O., Raziuddin, Sobota A., Boublik M., Szer W. A RNA helix-destabilizing protein is a major component of Artemia salina nuclear ribonucleoproteins. Proc Natl Acad Sci U S A. 1981 May;78(5):2888–2892. doi: 10.1073/pnas.78.5.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil P. A., Luse D. S., Segall J., Roeder R. G. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. doi: 10.1016/0092-8674(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Wilt F. H., Anderson M., Ekenberg E. Centrifugation of nuclear ribonucleoprotein particles of sea urchin embryos in cesium sulfate. Biochemistry. 1973 Feb 27;12(5):959–966. doi: 10.1021/bi00729a027. [DOI] [PubMed] [Google Scholar]
- Winter R. B., Berg O. G., von Hippel P. H. Diffusion-driven mechanisms of protein translocation on nucleic acids. 3. The Escherichia coli lac repressor--operator interaction: kinetic measurements and conclusions. Biochemistry. 1981 Nov 24;20(24):6961–6977. doi: 10.1021/bi00527a030. [DOI] [PubMed] [Google Scholar]
- Winter R. B., von Hippel P. H. Diffusion-driven mechanisms of protein translocation on nucleic acids. 2. The Escherichia coli repressor--operator interaction: equilibrium measurements. Biochemistry. 1981 Nov 24;20(24):6948–6960. doi: 10.1021/bi00527a029. [DOI] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]