Abstract
BACKGROUND
As of June 11, 2009, a total of 17,855 probable or confirmed cases of 2009 pandemic influenza A (H1N1) had been reported in the United States. Risk factors for transmission remain largely uncharacterized. We characterize the risk factors and describe the transmission of the virus within households.
METHODS
Probable and confirmed cases of infection with the 2009 H1N1 virus in the United States were reported to the Centers for Disease Control and Prevention with the use of a standardized case form. We investigated transmission of infection in 216 households — including 216 index patients and their 600 household contacts — in which the index patient was the first case patient and complete information on symptoms and age was available for all household members.
RESULTS
An acute respiratory illness developed in 78 of 600 household contacts (13%). In 156 households (72% of the 216 households), an acute respiratory illness developed in none of the household contacts; in 46 households (21%), illness developed in one contact; and in 14 households (6%), illness developed in more than one contact. The proportion of household contacts in whom acute respiratory illness developed decreased with the size of the household, from 28% in two-member households to 9% in six-member households. Household contacts 18 years of age or younger were twice as susceptible as those 19 to 50 years of age (relative susceptibility, 1.96; Bayesian 95% credible interval, 1.05 to 3.78; P = 0.005), and household contacts older than 50 years of age were less susceptible than those who were 19 to 50 years of age (relative susceptibility, 0.17; 95% credible interval, 0.02 to 0.92; P = 0.03). Infectivity did not vary with age. The mean time between the onset of symptoms in a case patient and the onset of symptoms in the household contacts infected by that patient was 2.6 days (95% credible interval, 2.2 to 3.5).
CONCLUSIONS
The transmissibility of the 2009 H1N1 influenza virus in households is lower than that seen in past pandemics. Most transmissions occur soon before or after the onset of symptoms in a case patient.
As of June 11, 2009, a total of 17,855 probable or confirmed cases of 2009 H1N1 virus infection, including 45 deaths, had been reported in the United States.1-3 The risk factors for transmission of this emerging virus remain largely uncharacterized, particularly in subgroups such as households.
Most people who have 2009 H1N1 influenza are advised to stay home until they have been afebrile for at least 24 hours.4 This practice puts other household members, who care for the patient when he or she is most infectious, at risk for infection. But so far, neither this risk nor the potential risk factors for transmission have been evaluated in the case of 2009 H1N1 influenza. In this article, we analyze data that describe patients with probable or confirmed cases of 2009 H1N1 influenza, and their household contacts, in the United States and characterize the risk factors for transmission in the household, as well as key transmission characteristics of the virus.
The household data that are considered here also provide an insight into the way that susceptibility to infection varies with age. Although 60% of the reported cases of 2009 H1N1 influenza in the United States have involved persons who were 18 years of age or younger,3 this age distribution might be partly explained by a potential case-ascertainment bias, since children may be tested more often than adults, or by the importance of school clusters in the early phase of the outbreak (with an expected spread to other age groups at a later stage).3 However, household contacts of patients with reported infection are expected to be less affected by such case-ascertainment bias.
Household studies are also perhaps the most reliable source of data for estimating the serial interval of the disease — the time between the onset of symptoms in a case patient and the onset of symptoms in the household contacts they infect (see the Supplementary Appendix, available with the full text of this article at NEJM.org). Serial-interval estimates are needed to characterize the likely speed at which an epidemic spreads, to inform recommendations with respect to the period of time that patients should stay home, and to estimate the effect of delays in treatment on transmission.
METHODS
DATA COLLECTION
We defined a case patient as a person with a body temperature of more than 37.8°C (100°F) and cough or sore throat who was positive for the 2009 H1N1 virus, as assessed with the use of a reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay (confirmed case) or who was positive for influenza A virus but negative for human H1 and H3 serotypes, as assessed with the use of RT-PCR (probable case).5 The RT-PCR assays used for characterizing cases as confirmed or probable were developed by the Centers for Disease Control and Prevention (CDC) (see the Supplementary Appendix).
In the early phase of the epidemic (April 29 to May 25, 2009), state health departments were asked to report all probable and confirmed cases to the CDC in Atlanta with the use of a standardized case-report form. Persons with illness that met the definition of a probable or confirmed case were contacted by staff members of state and local health departments and interviewed by telephone or in person with the use of the standardized case-report form. Case-report forms were faxed to the CDC or transmitted through the password-protected online reporting system developed by the CDC. The case-report form included the following data on the patient: age, symptoms (fever, defined as a body temperature >37.8°C, feverishness, cough, runny nose, sore throat, diarrhea, or vomiting), date of the onset of symptoms, and underlying conditions.
We defined the index patient as the person who was the focus of the case-report form. A section of the case-report form was dedicated to information on household members, who were defined as the index patient plus any person who had stayed overnight in the house at least one night within 7 days before or after the date of symptom onset in the index patient. The number of household members was documented, along with the following information on each household contact: age, date of the onset of symptoms, and the symptoms (fever, feverishness, cough, sore throat, runny nose, or diarrhea) that occurred within 7 days before or after the onset of symptoms in the index patient. Because 2009 H1N1 influenza is a nationally notifiable disease, written informed consent from case patients was not required.
CLINICAL OUTCOME
An acute respiratory illness was defined by the presence of at least two of the following symptoms: fever or feverishness, cough, sore throat, and runny nose. An influenza-like illness was defined by the presence of fever or feverishness plus cough, sore throat, or both. There was no systematic confirmation of cases of acute respiratory illness or influenza-like illness.
STATISTICAL ANALYSIS
We assumed that if an acute respiratory illness developed in a household contact of an index patient with 2009 H1N1 influenza, the household contact was infected with the 2009 H1N1 virus. Two statistical approaches were used to characterize the onset of acute respiratory illness in household contacts in the 7 days after the onset of symptoms in the index patient. The analysis was restricted to households of the most common size (two to six members) in which the index patient was the first case patient in the household and information on symptoms and age was complete for all household members.
Logistic models involving generalized estimating equations to account for household clustering were used to evaluate the potential risk factors for transmission: the age group of the index patient, the age group of the household contact, confirmed or probable influenza status of the index patient, the use or nonuse of antiviral medication by the index patient, household size, need or no need for hospitalization of the index patient, and symptoms in the index patient (cough, runny nose, sore throat, diarrhea, or vomiting). Households in which at least one household contact had an onset of symptoms on the same day as the onset of symptoms in the index patient (termed coprimary cases) were excluded from this analysis.
The logistic generalized-estimating-equation model makes the assumption that all the sick contacts were infected by the index patients. However, sick contacts may have been infected through contact with members of the wider community (community infections) or by other sick household members (tertiary infections). In addition, analyses are complicated by censoring, in that the follow-up of the household may have ended before secondary cases with long serial intervals could be identified. A statistical model that was specifically developed to correct for those potential biases was used to estimate the transmission characteristics of the virus (see the detailed description of this model in the Supplementary Appendix). The approach uses the sequence of symptom onset along with household demographic characteristics to infer serial intervals, community hazards of infection, and person-to-person hazards of transmission and to investigate the way in which the hazard of transmission may be affected by the following covariates: the age group of the case patient, the age group of the household contact, household size, and symptoms in the case patient (fever, cough, runny nose, sore throat, or diarrhea). It is assumed that there is a time lag of at least 1 day between the onset of symptoms in a patient and the onset of symptoms in the people they infect. The results of an extensive sensitivity analysis investigating the robustness of the results with respect to modeling assumptions are presented in the Supplementary Appendix.
The parameters of the transmission model were estimated with the use of Bayesian Markov chain Monte Carlo sampling. We report the posterior median and 95% credible interval of the parameters. The likelihood-ratio test and the deviance information criterion6 were used for the comparison of models and assumption testing. The adequacy of the model was also tested with the use of a simulation-based chi-square test comparing observed and expected distributions of the number of cases per household size.
In the descriptive analysis, relative risks were computed with the use of a log-binomial generalized-estimating-equation model accounting for household clustering. All reported P values are two-sided and were not adjusted for multiple testing.
The secondary attack rate was defined as the proportion of household contacts in whom the onset of symptoms occurred within 7 days after the onset of symptoms in the index patient. These analyses were planned during the time of data collection.
RESULTS
INDEX PATIENTS
As of May 28, 2009, a total of 938 case reports of persons with probable or confirmed 2009 H1N1 influenza, along with information on 2085 household contacts, had been received by the CDC. Demographic and clinical characteristics of the index patients are shown in Table 1. Information on age was collected from 865 index patients (92%). The median age of the index patients was 15 years (range, 0 to 86). A total of 488 index patients (52%) were from six states: 270 patients (29% of all index patients) were from Arizona, 67 (7%) from Connecticut, 54 (6%) from Pennsylvania, 49 (5%) from Texas, and 48 (5%) from Delaware. A total of 455 patients (53% of those with reported age) were 5 to 18 years of age and only 331 patients (38% of those with reported age) were 19 years of age or older.
Table 1.
Baseline Characteristics of the Index Patients, According to Age Group.*
| Characteristic | Total (N = 938) |
0–23 Mo (N = 26) |
2–4 Yr (N = 53) |
5–18 Yr (N = 455) |
19–50 Yr (N = 295) |
≥51 Yr (N = 36) |
|---|---|---|---|---|---|---|
| Male sex — no./total no. with data (%) |
462/938 (49) | 11/26 (42) | 30/53 (57) | 235/455 (52) | 145/295 (49) | 12/36 (33) |
| 2009 H1N1 influenza — no. (%) |
||||||
| Probable | 389 (41) | 9 (35) | 24 (45) | 193 (42) | 111 (38) | 17 (47) |
| Confirmed | 549 (59) | 17 (65) | 29 (55) | 262 (58) | 184 (62) | 19 (53) |
| Clinical symptoms — no. (%) | ||||||
| Fever or feverishness | 860 (92) | 26 (100) | 51 (96) | 422 (93) | 268 (91) | 28 (78) |
| Cough | 806 (86) | 21 (81) | 43 (81) | 378 (83) | 266 (90) | 34 (94) |
| Sore throat | 554 (59) | 9 (35) | 16 (30) | 294 (65) | 179 (61) | 16 (44) |
| Runny nose | 452 (48) | 14 (54) | 25 (47) | 218 (48) | 147 (50) | 15 (42) |
| Vomiting | 204 (22) | 10 (38) | 13 (25) | 119 (26) | 40 (14) | 8 (22) |
| Diarrhea | 162 (17) | 6 (23) | 7 (13) | 76 (17) | 50 (17) | 7 (19) |
| Hospitalization — no./total no. with data (%) |
65/902 (7) | 6/26 (23) | 4/52 (8) | 19/443 (4) | 25/286 (9) | 5/34 (15) |
| Admission to ICU — no. (%) | 8 (1) | 0 | 0 | 1 (<1) | 5 (2) | 1 (3) |
| Asthma — no. (%) | 110 (12) | 2 (8) | 6 (11) | 63 (14) | 27 (9) | 5 (14) |
Data on age were available for 865 patients. The median age of the index patients was 15 years. ICU denotes intensive care unit
HOUSEHOLD CONTACTS
A total of 533 of the 938 households with index patients (57%) had two to six household members (74 had seven or more); 331 index patients listed no household contacts. In 216 of these 533 households (41%), the index patient was the first case patient in the household and there was no missing information on symptoms or age for any household member. Table 2 shows the demographic and clinical characteristics of the household contacts for this subgroup of households.
Table 2.
Baseline Characteristics of the Household Contacts, According to Age Group.*
| Characteristic | Total (N = 600) |
0–4 Yr (N = 40) |
5–18 Yr (N = 184) |
19–50 Yr (N = 328) |
≥51 Yr (N = 48) |
|---|---|---|---|---|---|
| Clinical symptoms — no. (%) | |||||
| Fever or feverishness | 76 (13) | 11 (28) | 32 (17) | 31 (9) | 2 (4) |
| Cough | 95 (16) | 10 (25) | 36 (20) | 48 (15) | 1 (2) |
| Sore throat | 50 (8) | 5 (12) | 15 (8) | 28 (9) | 2 (4) |
| Runny nose | 42 (7) | 7 (18) | 16 (9) | 17 (5) | 2 (4) |
| Diarrhea | 18 (3) | 3 (8) | 8 (4) | 7 (2) | 0 |
| Influenza-like illness† | |||||
| Affected contacts — no. (%) | 60 (10) | 9 (23) | 27 (15) | 23 (7) | 1 (2) |
| Relative risk (95% CI) | 3.18 (1.66–6.10) | 2.20 (1.39–3.51) | 1 | 0.35 (0.07–1.84) | |
| P value for relative risk | <0.001 | <0.001 | 0.22 | ||
| Acute respiratory illness‡ | |||||
| Affected contacts — no. (%) | 78 (13) | 10 (25) | 31 (17) | 35 (11) | 2 (4) |
| Relative risk (95% CI) | 2.33 (1.30–4.18) | 1.65 (1.12–2.43) | 1 | 0.37 (0.09–1.50) | |
| P value for relative risk | 0.005 | 0.01 | 0.16 |
The data are for household contacts from the 216 households with two to six members in which the index patient was the first case patient in the household and information on symptoms and age was complete for all household members.The median age of the household contacts was 26 years. CI denotes confidence interval.
Influenza-like illness was defined as fever or feverishness plus cough, sore throat, or both.
Acute respiratory illness was defined as the presence of at least two of the following signs: fever or feverishness, cough, sore throat, and runny nose.
Among the 600 household contacts of the 216 index patients included in the analysis, 78 (13%) had an acute respiratory illness and 60 (10%) had an influenza-like illness. There were 156 households (72%) in which an acute respiratory illness developed in none of the household contacts, 46 households (21%) in which an acute respiratory illness developed in one contact, and 14 households (6%) in which an acute respiratory illness developed in more than one contact (Table 3). The descriptive analysis suggests that household contacts who were 4 years of age or younger and those who were 5 to 18 years of age were at significantly higher risk for acute respiratory illness and for influenza-like illness than were contacts who were 19 to 50 years of age; the risk did not differ significantly between those who were older than 50 years of age and those who were 19 to 50 years of age (Table 1). Whereas the median age of household contacts was 26 years, the median age of patients with secondary cases of acute respiratory illness was 16.5 years, and the median age of patients with secondary cases of influenza-like illness was 14.5 years.
Table 3.
Number of Household Contacts with Diagnosis of Acute Respiratory Illness, According to the Number of Household Members.*
| No. of Household Members |
No. of Household Contacts with Diagnosis of Acute Respiratory Illness | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |||||||
| no. |
no. expected from
transmission model (95% credible interval) |
no. |
no. expected from
transmission model (95% credible interval) |
no. |
no. expected from
transmission model (95% credible interval) |
no. |
no. expected from
transmission model (95% credible interval) |
no. |
no. expected from
transmission model (95% credible interval) |
no. |
no. expected from
transmission model (95% credible interval) |
|
| 2 | 28 | 28.2 (21–34) | 11 | 10.8 (5–18) | ||||||||
| 3 | 34 | 32.6 (24–40) | 9 | 11.4 (5–18) | 4 | 3.1 (0–8) | ||||||
| 4 | 52 | 50.6 (41–59) | 13 | 13.4 (6–21) | 2 | 3.6 (1–8) | 2 | 1.5 (1–3) | ||||
| 5 | 31 | 30.7 (23–38) | 9 | 10.1 (5–16) | 4 | 2.7 (0–7) | 1 | 1.4 (0–3) | 0 | 0.2 (0–1) | ||
| 6 | 11 | 10.7 (7–14) | 4 | 3.4 (1–7) | 0 | 0.7 (0–3) | 1 | 1.0 (0–2) | 0 | 0.2 (0–1) | 0 | 0 (0–0) |
The number expected from the transmission model is the posterior mean.
ANALYSIS OF SECONDARY CASES
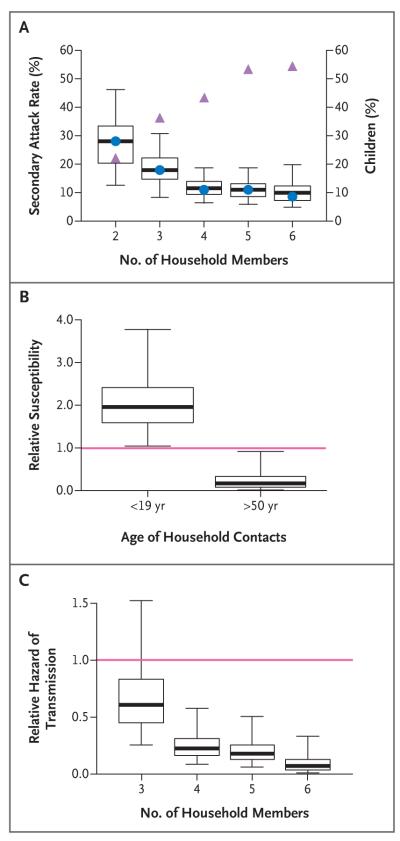
The effects of age and household size are confounded: the secondary attack rate for acute respiratory illness decreased with the size of the household, from 28% among households with two members to 9% among households with six members (Fig. 1A), and the secondary attack rate for influenza-like illness decreased with the size of the household, from 23% among two-member households to 4% among six-member households, although the proportion of children increased with the household size (Fig. 1A). The transmission model was used to disentangle the separate effects of the covariates. It was estimated that household members who were 18 years of age or younger were more susceptible by a factor of two than those who were 19 to 50 years of age (relative susceptibility to acute respiratory illness, 1.96; 95% credible interval, 1.05 to 3.78; P = 0.005) and that household members who were older than 50 years of age were significantly less susceptible than adults who were 50 years of age or younger (relative susceptibility to acute respiratory illness, 0.17; 95% credible interval, 0.02 to 0.92; P = 0.03) (Fig. 1B, and Table SM1 in the Supplementary Appendix). In addition, children and older adults 2nd were found to be as infectious as younger adults (P = 0.13) (Table SM9 in the Supplementary Appendix). Fever, cough, sore throat, runny nose, and diarrhea were not significantly associated with increased infectivity (Table SM9 in the Supplementary Appendix). The analysis also showed that the person-to-person hazard of transmission in the household decreased substantially with the size of the household (Fig. 1C). The fit of the model to the data, with respect to the numbers of observed and expected cases, was good (P = 0.98) (Table 3).
Figure 1. Characteristics of the Transmission of Acute Respiratory Illness in Households.
Panel A shows the secondary attack rates (i.e., the proportion of household contacts in whom acute respiratory illness developed); the circles indicate the observed rate, the box plot shows the distribution expected by the transmission model, and the triangles indicate the proportion of children according to the size of the household. Panel B shows the relative susceptibility of household contacts who were younger than 19 years of age and of those who were older than 50 years of age (the reference group [red line] was contacts who were 19 to 50 years of age). Panel C shows the relative hazard of person-to-person transmission with household size (the reference group [red line] was households with two members). The box plots show the posterior distribution estimated with the transmission model. The horizontal lines within the boxes represent the medians, the lower and upper bounds of the boxes represent the 25th and 75th percentiles, and the I bars represent the 2.5th and 97.5th percentiles.
These findings were consistent with the results of logistic-regression models. A logistic generalized-estimating-equation model built with a forward-selection procedure identified the age of the household member and the size of the household as the key risk factors for the onset of acute respiratory illness in exposed household members (Table 4). After adjustment for these variables, the inclusion of the other potential risk factors that were considered did not significantly improve the fit of the model to the data.
Table 4.
Odds Ratio for Onset of Acute Respiratory Illness in Household Contacts.*
| Variable | Estimated Odds Ratio (95% CI) |
P Value |
|---|---|---|
| Age of household contact | ||
| 0–4 yr | 3.52 (1.55–7.97) | 0.003 |
| 5–18 yr | 2.01 (1.11–3.63) | 0.03 |
| 19–50 yr | 1.00 | |
| ≥51 yr | 0.41 (0.08–2.04) | 0.28 |
| Doubling of household size† | 0.27 (0.14–0.52) | <0.001 |
The results were estimated with the use of a logistic generalized-estimatingequation model that included the age group of the household contact and log-transformed household size. CI denotes confidence interval.
The odds ratio is for each increase in household size by a factor of 2.
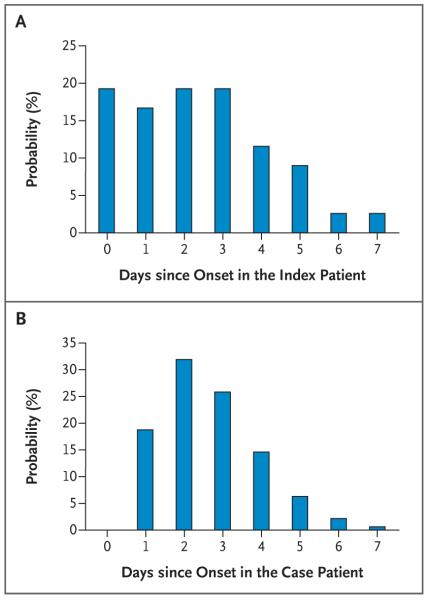
Empirically, the mean interval between the onset of symptoms in the index patient and the onset of symptoms in the household contacts in whom acute respiratory illness developed was 2.9 days if coprimary cases were excluded and 2.4 days if they were included (Fig. 2A). With the transmission model that accounted for tertiary and community cases, the mean serial interval (i.e., the interval between the onset of symptoms in a case patient and the onset of symptoms in the household contacts who were infected by that patient) was 2.6 days (95% credible interval, 2.2 to 3.5), with a standard deviation of 1.3 days (95% credible interval, 0.9 to 2.4) (Fig. 2B).
Figure 2. Serial Interval of 2009 H1N1 Influenza.
Panel A shows the observed probability that symptoms of acute respiratory illness will develop in a household member, according to the number of days after the onset of symptoms in the index patient. Household members in whom symptoms developed on the same day that symptoms developed in the index patient (i.e., 0 days since onset in the index patient) are termed coprimary case patients. Panel B shows the distribution of the serial interval (i.e., the interval between the onset of symptoms in a case patient and the onset of symptoms in the household contacts infected by that patient). This interval was estimated with the transmission model, which adjusted for tertiary infections, community infections, and censoring of the data. The estimated distribution is based on the assumption that the minimum time between the onset of symptoms in a case patient and the onset of symptoms in household contacts infected by that patient was 1 day.
DISCUSSION
We found that children were twice as susceptible to infection with the 2009 H1N1 virus from a household member as adults 19 to 50 years of age and that adults older than 50 years of age were less susceptible than younger adults. This suggests that the young age distribution that was observed among reported cases in the community (the index patients in our study) was not an artifact resulting from case-ascertainment bias. In addition, our findings are consistent with serologic analyses of the 2009 H1N1 virus suggesting that there are some preexisting pandemic H1N1 immune responses in the elderly; these are present to a lesser extent in younger adults but are rarely present in children.7 Susceptibility as measured in this analysis captures social and hygienic, as well as biologic, determinants.
Previous studies showed that infectivity was greater among children than among adults during seasonal influenza outbreaks8-11 and during the 1957 pandemic,12-14 but the difference was not as pronounced during the 1968 pandemic.13 However, our analysis did not provide evidence that infectivity was associated with the age of the patient, although the index patients in our analysis may not represent an unbiased sample of all community patients. No symptom was found to be significantly associated with increased infectivity; but the power to detect a difference was low in the case of some symptoms, since some of the symptoms were highly prevalent; for example, almost all cases (92%) involved cough.
The average secondary attack rates (13% for acute respiratory illness and 10% for influenza-like illness) were at the lower end of the range of rates that are seen with seasonal influenza (for which the range is 10 to 40%8,15-19), but in households with two members, the rates could be as high as 28% for acute respiratory illness and 23% for influenza-like illness. Our estimates of transmissibility in households, which are lower than estimates from previous pandemics12-14 and show a strong association with age, are consistent with and complement findings from analyses of transmissibility in the early phase of the epidemic in Mexico, which were based on aggregate population data.20
In a household study of seasonal influenza in France, the secondary attack rate was found to be approximately constant with household size.8 In our study, there was a relatively sharp reduction in secondary attack rates between households with two members and those with four members, after which secondary attack rates were approximately constant (Fig. 1A). These differences are intriguing and highlight the fact that the sociologic, environmental, and biologic mechanisms available to explain the relationship between secondary attack rates and household size are still limited.
In a sensitivity analysis (see the Supplementary Appendix), we found that key findings were robust with respect to changes in the main modeling assumptions. The age patterns observed in the data cannot be explained solely by a difference between community risks for adults and children. The key findings would be unchanged if the case definition were influenza-like illness rather than acute respiratory illness, although the transmission rates would be somewhat lower.
Studies that rely on the identification of an index patient in the household have important limitations. First, index patients may have more severe symptoms than are usual with the illness, and given that severity may be predictive of transmission, estimates may not be representative of typical cases. Second, ascertainment of households may be subject to selection bias; households may be more likely to enter a study if they have more cases, a factor that would also upwardly bias the secondary attack rate. To reduce the potential of this bias to affect the estimates in our study, we excluded households in which the index patient was not the first case patient in the household. Although our analysis controls for a range of covariates, other important covariates may be missing (e.g., coexisting conditions or antiviral treatment or prophylaxis in household contacts). If any of these covariates are correlated with age, for example, there might be a confounding of the effects of age on the risk of transmission.
Censoring of the data is another limitation. In our study, the duration of household follow-up was only 7 days. Although our estimation procedure accounts for this censoring, it does so by assuming a functional form for the serial interval distribution, which determines the probability associated with the (unobserved) tail of the distribution. Our estimates of the serial interval should be interpreted in this light.
Although these limitations are important, it would be difficult to investigate household transmission at such an early stage of the pandemic with alternative “community study” designs, in which a cohort of households is recruited before infection of any of their members and is followed throughout the course of the pandemic. Such a study would require very large numbers of households to achieve sufficient power for inference.
Another limitation of our study is that secondary cases were not confirmed by laboratory testing. It is therefore likely that some of the secondary cases were not cases of 2009 H1N1 influenza. Conversely, some of the household contacts with symptoms that did not meet the definition of acute respiratory illness or influenza-like illness were likely to have had 2009 H1N1 influenza. In general, the positive predictive value of acute respiratory illness for 2009 H1N1 influenza infection in persons who are not epidemiologically linked to a case patient is expected to be low. However, the probability that an acute respiratory illness is caused by the 2009 H1N1 virus is expected to be higher when the onset of symptoms occurs only a few days after the onset of symptoms in a household case patient with laboratory-confirmed 2009 H1N1 influenza. It is possible that the lack of laboratory confirmation of secondary cases biases the estimates of susceptibility among children, for example, if children are more likely than adults to receive a false positive clinical diagnosis of 2009 H1N1 influenza.
The epidemiologic factors estimated here should inform recommendations regarding the isolation and quarantine of infected patients and permit the effect of early treatment versus delayed treatment to be estimated.21,22 Our estimates of age-specific susceptibility also provide useful information for guiding public health policies that target specific age groups — policies such as school closures and vaccination efforts. In particular, our results underscore the critical role children play in the unfolding pandemic.
Supplementary Material
Acknowledgments
Supported by grants from the Medical Research Council, the Bill and Melinda Gates Foundation, the European Union Framework 7 program (FluModCont), and the Models of Infectious Disease Agent Study initiative of the National Institute of General Medical Sciences (all for the work at Imperial College), the Royal Society (to Dr. Fraser), and Research Councils U.K. (to Dr. Cauchemez).
We thank Anna Bramley, Jennifer Michalove, and Mackenzie Nowell for their assistance in collecting and analyzing the data, and staff members at the state and local health departments for the collection of case-report data.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Dr. Cauchemez reports receiving consulting fees from Sanofi Pasteur MSD.
REFERENCES
- 1.2009 H1N1 flu: situation update. Centers for Disease Control and Prevention; Atlanta: [Accessed December 4, 2009]. http://www.cdc.gov/h1n1flu/update.htm. [Google Scholar]
- 2.Hospitalized patients with novel influenza A (H1N1) virus infection — California, April–May, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:536–41. [PubMed] [Google Scholar]
- 3.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]; N Engl J Med. 2009;361:102. Erratum. [Google Scholar]
- 4.CDC recommendations for the amount of time persons with influenza-like illness should be away from others. Centers for Disease Control and Prevention; Atlanta: [Accessed December 4, 2009]. http://www.cdc.gov/H1N1flu/guidance/exclusion.htm. [Google Scholar]
- 5.H1N1 flu: clinical and public health guidance. Centers for Disease Control and Prevention; Atlanta: [Accessed December 4, 2009]. http://www.cdc.gov/h1n1flu/casedef.htm. [Google Scholar]
- 6.Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc B. 2002;64:583–639. [Google Scholar]
- 7.Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb Mortal Wkly Rep. 2009;58:521–4. [PubMed] [Google Scholar]
- 8.Cauchemez S, Carrat F, Viboud C, Valleron AJ, Boëlle PY. A Bayesian MCMC approach to study transmission of influenza: application to household longitudinal data. Stat Med. 2004;23:3469–87. doi: 10.1002/sim.1912. [DOI] [PubMed] [Google Scholar]
- 9.Frank AL, Taber LH, Wells CR, Wells JM, Glezen WP, Paredes A. Patterns of shedding of myxoviruses and paramyxoviruses in children. J Infect Dis. 1981;144:433–41. doi: 10.1093/infdis/144.5.433. [DOI] [PubMed] [Google Scholar]
- 10.Hall CB, Douglas RG, Jr, Geiman JM, Meagher MP. Viral shedding patterns of children with influenza B infection. J Infect Dis. 1979;140:610–3. doi: 10.1093/infdis/140.4.610. [DOI] [PubMed] [Google Scholar]
- 11.Rampey AH, Jr, Longini IM, Jr, Haber M, Monto AS. A discrete-time model for the statistical analysis of infectious disease incidence data. Biometrics. 1992;48:117–28. [PubMed] [Google Scholar]
- 12.Chin TD, Foley JF, Doto IL, Gravelle CR, Weston J. Morbidity and mortality characteristics of Asian strain influenza. Public Health Rep. 1960;75:149–58. [PMC free article] [PubMed] [Google Scholar]
- 13.Davis LE, Caldwell GG, Lynch RE, Bailey RE, Chin TD. Hong Kong influenza: the epidemiologic features of a high school family study analyzed and compared with a similar study during the 1957 Asian influenza epidemic. Am J Epidemiol. 1970;92:240–7. doi: 10.1093/oxfordjournals.aje.a121203. [DOI] [PubMed] [Google Scholar]
- 14.Woodall J, Rowson KE, Mc Donald JC. Age and Asian influenza, 1957. Br Med J. 1958;2:1316–8. doi: 10.1136/bmj.2.5108.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayden FG, Belshe R, Villanueva C, et al. Management of influenza in households: a prospective, randomized comparison of oseltamivir treatment with or without postexposure prophylaxis. J Infect Dis. 2004;189:440–9. doi: 10.1086/381128. [DOI] [PubMed] [Google Scholar]
- 16.MacIntyre CR, Cauchemez S, Dwyer DE, et al. Face mask use and control of respiratory virus transmission in households. Emerg Infect Dis. 2009;15:233–41. doi: 10.3201/eid1502.081167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welliver R, Monto AS, Carewicz O, et al. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA. 2001;285:748–54. doi: 10.1001/jama.285.6.748. [DOI] [PubMed] [Google Scholar]
- 18.Carrat F, Sahler C, Rogez S, et al. Influenza burden of illness: estimates from a national prospective survey of household contacts in France. Arch Intern Med. 2002;162:1842–8. doi: 10.1001/archinte.162.16.1842. [DOI] [PubMed] [Google Scholar]
- 19.Longini IM, Jr, Koopman JS, Monto AS, Fox JP. Estimating household and community transmission parameters for influenza. Am J Epidemiol. 1982;115:736–51. doi: 10.1093/oxfordjournals.aje.a113356. [DOI] [PubMed] [Google Scholar]
- 20.Fraser C, Donnelly CA, Cauchemez S, et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324:1557–61. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–52. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Germann TC, Kadau K, Longini IM, Jr, Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci U S A. 2006;103:5935–40. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.