Abstract
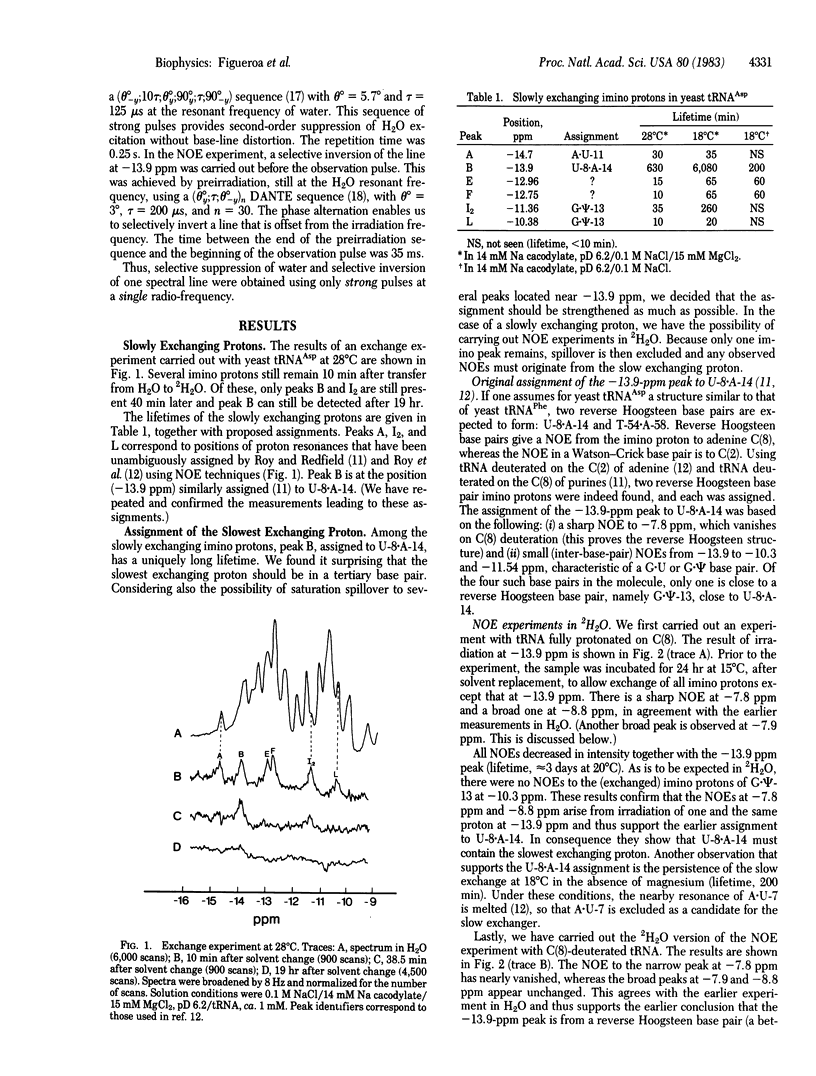
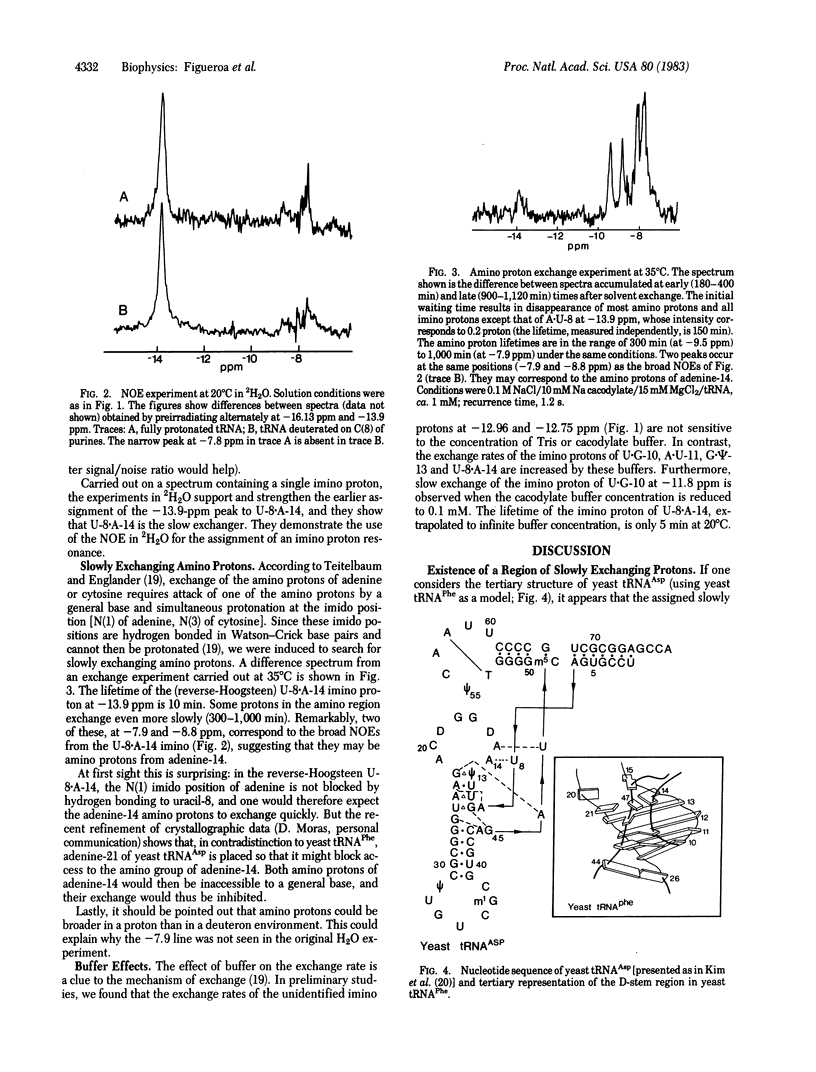
We have monitored the exchange of imino and amino protons by NMR after quick transfer of yeast tRNAAsp in 2H2O solvent. When the concentration of exchange-catalyzing buffer is not too high, one imino proton exchanges considerably more slowly than any other (e.g., 100 hr versus 4 hr for the second-slowest imino proton at 18 degrees C in 15 mM Mg). This provides excellent conditions for identification, by the nuclear Overhauser effect, of the slowest exchanging proton, which we show to be the imino proton of the U-8 . A-14 reverse Hoogsteen tertiary-structure base pair; other slowly exchanging protons are identified as imino protons from A . U-11 and G . psi-13. In preliminary experiments, we find that the exchange of these protons is catalyzed by cacodylate or Tris buffer. The lifetimes of two other imino protons, ca. 10 min at 28 degrees C, are buffer independent. Slowly exchanging amino protons have also been observed. Correlation with the exchange of the uracil-8 imino proton suggests that they may be from adenine-14.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Englander J. J., Kallenbach N. R., Englander S. W. Hydrogen exchange study of some polynucleotides and transfer RNA. J Mol Biol. 1972 Jan 14;63(1):153–169. doi: 10.1016/0022-2836(72)90527-x. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Englander J. J. HYDROGEN EXCHANGE STUDIES OF sRNA. Proc Natl Acad Sci U S A. 1965 Feb;53(2):370–378. doi: 10.1073/pnas.53.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander S. W., Englander J. J. Hydrogen--tritium exchange. Methods Enzymol. 1978;49:24–39. doi: 10.1016/s0076-6879(78)49005-6. [DOI] [PubMed] [Google Scholar]
- Goldstein R. N., Stefanovic S., Kallenbach N. R. On the conformation of transfer RNA in solution: dependence of denaturation temperature and structural parameters of mixed and formylmethionyl Escherichia coli transfer RNA on sodium ion concentration. J Mol Biol. 1972 Aug 21;69(2):217–236. doi: 10.1016/0022-2836(72)90227-6. [DOI] [PubMed] [Google Scholar]
- Guéron M., Leroy J. L. Significance and mechanism of divalent-ion binding to transfer RNA. Biophys J. 1982 Jun;38(3):231–236. doi: 10.1016/S0006-3495(82)84553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerschap A., Haasnoot C. A., Hilbers C. W. Nuclear magnetic resonance studies on yeast tRNAPhe I. Assignment of the iminoproton resonances of the acceptor and D stem by means of Nuclear Overhauser Effect experiments at 500 MHz. Nucleic Acids Res. 1982 Nov 11;10(21):6981–7000. doi: 10.1093/nar/10.21.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P. D., Figueroa N., Redfield A. G. Real-time solvent exchange studies of the imino and amino protons of yeast phenylalanine transfer RNA by Fourier transform NMR. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3130–3134. doi: 10.1073/pnas.76.7.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Suddath F. L., Quigley G. J., McPherson A., Sussman J. L., Wang A. H., Seeman N. C., Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974 Aug 2;185(4149):435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Rich A. Structural domains of transfer RNA molecules. Science. 1976 Nov 19;194(4267):796–806. doi: 10.1126/science.790568. [DOI] [PubMed] [Google Scholar]
- Richarz R., Sehr P., Wagner G., Wüthrich K. Kinetics of the exchange of individual amide protons in the basic pancreatic trypsin inhibitor. J Mol Biol. 1979 May 5;130(1):19–30. doi: 10.1016/0022-2836(79)90549-7. [DOI] [PubMed] [Google Scholar]
- Roy S., Papastavros M. Z., Redfield A. G. Nuclear overhauser effect study of yeast aspartate transfer ribonucleic acid. Biochemistry. 1982 Nov 23;21(24):6081–6088. doi: 10.1021/bi00267a009. [DOI] [PubMed] [Google Scholar]
- Roy S., Redfield A. G. Assignment of imino proton spectra of yeast phenylalanine transfer ribonucleic acid. Biochemistry. 1983 Mar 15;22(6):1386–1390. doi: 10.1021/bi00275a010. [DOI] [PubMed] [Google Scholar]
- Roy S., Redfield A. G. Nuclear Overhauser effect study and assignment of D stem and reverse-Hoogsteen base pair proton resonances in yeast tRNAAsp. Nucleic Acids Res. 1981 Dec 21;9(24):7073–7083. doi: 10.1093/nar/9.24.7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P. R., Redfield A. G. Transfer RNA in solution: selected topics. Annu Rev Biophys Bioeng. 1980;9:181–221. doi: 10.1146/annurev.bb.09.060180.001145. [DOI] [PubMed] [Google Scholar]
- Sánchez V., Redfield A. G., Johnston P. D., Tropp J. Nuclear Overhauser effect in specifically deuterated macromolecules: NMR assay for unusual base pairing in transfer RNA. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5659–5662. doi: 10.1073/pnas.77.10.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum H., Englander S. W. Open states in native polynucleotides. I. Hydrogen-exchange study of adenine-containing double helices. J Mol Biol. 1975 Feb 15;92(1):55–78. doi: 10.1016/0022-2836(75)90091-1. [DOI] [PubMed] [Google Scholar]
- Webb P. K., Fresco J. R. Tritium exchange studies of transfer RNA in native and denaturated conformations. J Mol Biol. 1973 Mar 5;74(3):387–402. doi: 10.1016/0022-2836(73)90379-3. [DOI] [PubMed] [Google Scholar]


