Abstract
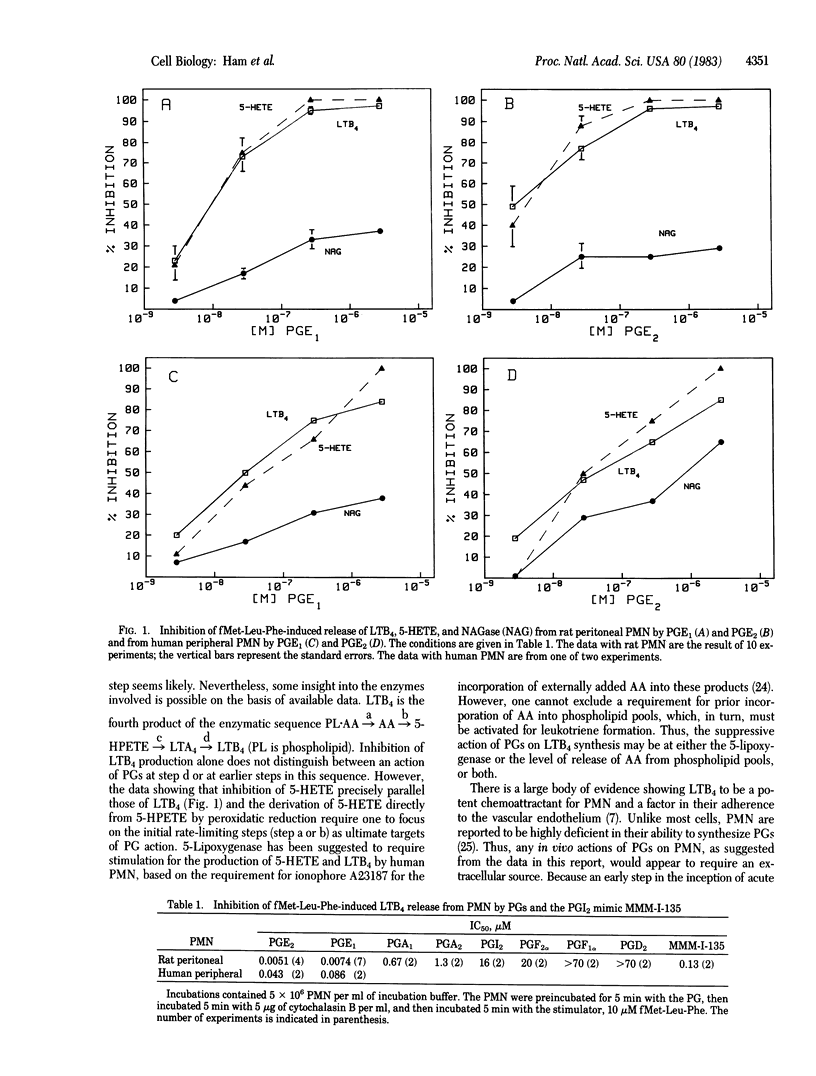
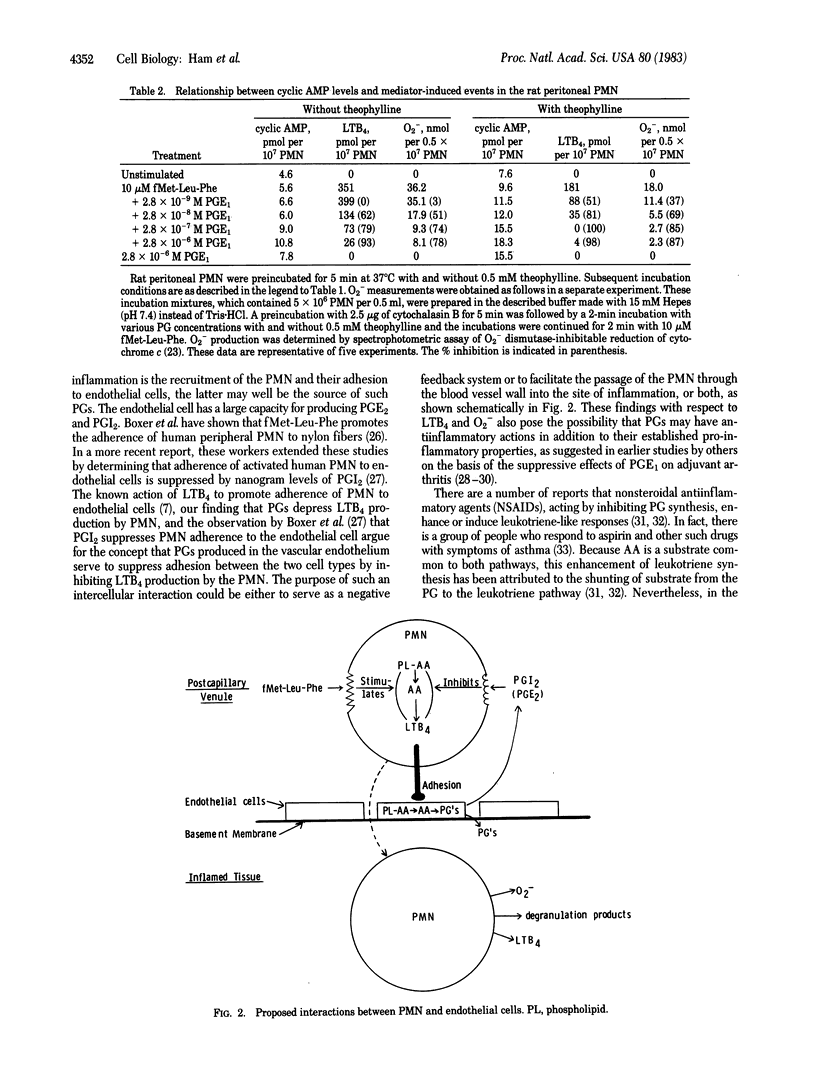
Chemoattractant N-formylmethionylleucylphenylalanine (fMet-Leu-Phe) in the presence of cytochalasin B stimulates the release of leukotriene B4 (LTB4), superoxide (O2-), and N-acetylglucosaminidase from elicited rat peritoneal and human peripheral neutrophils [PMN (polymorphonuclear leukocytes)]. Prostaglandins E1 and E2 (PGE1 and PGE2) inhibit LTB4 release from PMN in a dose-related manner with an IC50 of 1 X 10(-8) M. This action is associated with increased levels of cyclic AMP. The inhibitory activity of a variety of PGs on LTB4 production by rat peritoneal PMN parallels their affinity for PGE receptors in other tissues. O2- release is also suppressed by low levels of PGE1 and PGE2 in a dose-related manner and this inhibition is enhanced by theophylline. In contrast, lysosomal enzyme release is only minimally affected by physiological levels of PGs. These data are consistent with an action of PGs at the level of the PG receptor on LTB4 and O2- release from the fMet-Leu-Phe-stimulated rat peritoneal PMN. In addition, the fMet-Leu-Phe-induced adherence of PMN to endothelial cells and inhibition of this phenomenon by PGs may now be explained by PG-mediated inhibition of LTB4 formation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspinall R. L., Cammarata P. S. Effect of prostaglandin E2 on adjuvant arthritis. Nature. 1969 Dec 27;224(5226):1320–1321. doi: 10.1038/2241320a0. [DOI] [PubMed] [Google Scholar]
- Aswanikumar S., Corcoran B., Schiffmann E., Day A. R., Freer R. J., Showell H. J., Becker E. L. Demonstration of a receptor on rabbit neutrophils for chemotactic peptides. Biochem Biophys Res Commun. 1977 Jan 24;74(2):810–817. doi: 10.1016/0006-291x(77)90375-8. [DOI] [PubMed] [Google Scholar]
- Bentwood B. J., Henson P. M. The sequential release of granule constitutents from human neutrophils. J Immunol. 1980 Feb;124(2):855–862. [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: effects of ionophore A23187. Proc Natl Acad Sci U S A. 1979 May;76(5):2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Allen J. M., Schmidt M., Yoder M., Baehner R. L. Inhibition of polymorphonuclear leukocyte adherence by prostacyclin. J Lab Clin Med. 1980 May;95(5):672–678. [PubMed] [Google Scholar]
- Boxer L. A., Yoder M., Bonsib S., Schmidt M., Ho P., Jersild R., Baehner R. L. Effects of a chemotactic factor, N-formylmethionyl peptide, on adherence, superoxide anion generation, phagocytosis, and microtubule assembly of human polymorphonuclear leukocytes. J Lab Clin Med. 1979 Mar;93(3):506–514. [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Bryant R. E., Sutcliffe M. C. The effect of 3',5'-adenosine monophosphate on granulocyte adhesion. J Clin Invest. 1974 Nov;54(5):1241–1244. doi: 10.1172/JCI107868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burka J. F., Paterson N. A. Evidence for lipoxygenase pathway involvement in allergic tracheal contraction. Prostaglandins. 1980 Apr;19(4):499–515. doi: 10.1016/s0090-6980(80)80001-3. [DOI] [PubMed] [Google Scholar]
- Claesson H. E., Lundberg U., Malmsten C. Serum-coated zymosan stimulates the synthesis of leukotriene B4 in human polymorphonuclear leukocytes. Inhibition by cyclic AMP. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1230–1237. doi: 10.1016/0006-291x(81)90751-8. [DOI] [PubMed] [Google Scholar]
- Cunningham F. M., Smith M. J., Ford-Hutchinson A. W., Walker J. R. Migration of peritoneal polymorphonuclear leucocytes in the rat. J Pathol. 1979 May;128(1):15–20. doi: 10.1002/path.1711280104. [DOI] [PubMed] [Google Scholar]
- Dahlén S. E., Björk J., Hedqvist P., Arfors K. E., Hammarström S., Lindgren J. A., Samuelsson B. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: in vivo effects with relevance to the acute inflammatory response. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3887–3891. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer D. M., Jose P. J., Piper P. J., Tippins J. R. Modulation of slow-reacting substance of anaphylaxis and histamine release by prostacyclin and thromboxanes [proceedings]. J Physiol. 1978 Aug;281:42P–42P. [PubMed] [Google Scholar]
- Fried J., Mitra D. K., Nagarajan M., Mehrotra M. M. 10,10-Difluoro-13-dehydroprostacyclin: a chemically and metabolically stabilized potent prostacyclin. J Med Chem. 1980 Mar;23(3):234–237. doi: 10.1021/jm00177a003. [DOI] [PubMed] [Google Scholar]
- Higgs G. A., Bax C. M., Moncada S. Inflammatory properties of lipoxygenase products and the effects of indomethacin and BW755C on prostaglandin production, leukocyte migration, and plasma exudation in rabbit skin. Adv Prostaglandin Thromboxane Leukot Res. 1982;9:331–339. [PubMed] [Google Scholar]
- Humes J. L., Sadowski S., Galavage M., Goldenberg M., Subers E., Bonney R. J., Kuehl F. A., Jr Evidence for two sources of arachidonic acid for oxidative metabolism by mouse peritoneal macrophages. J Biol Chem. 1982 Feb 25;257(4):1591–1594. [PubMed] [Google Scholar]
- Issekutz A. C., Movat H. Z. The effect of vasodilator prostaglandins on polymorphonuclear leukocyte infiltration and vascular injury. Am J Pathol. 1982 Jun;107(3):300–309. [PMC free article] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Humes J. L. Direct evidence for a prostaglandin receptor and its application to prostaglandin measurements (rat-adipocytes-antagonists-analogues-mouse ovary assay). Proc Natl Acad Sci U S A. 1972 Feb;69(2):480–484. doi: 10.1073/pnas.69.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Humes J. L., Tarnoff J., Cirillo V. J., Ham E. A. Prostaglandin receptor site: evidence for an essential role in the action of luteinizing hormone. Science. 1970 Aug 28;169(3948):883–886. doi: 10.1126/science.169.3948.883. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Showell H. S., Becker E. L., Ward P. A. Desensitization of the neutrophil aggregation response to chemotactic factors. Am J Pathol. 1978 Dec;93(3):693–706. [PMC free article] [PubMed] [Google Scholar]
- Oien H. G., Babiarz E. M., Soderma D. D., Ham E. A., Kuehl F. A., Jr Evidence for a PGE-receptor in the rat kidney. Prostaglandins. 1979 Apr;17(4):525–542. doi: 10.1016/0090-6980(79)90005-4. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Ford-Hutchinson A. W., Bray M. A. Leukotriene B: a potential mediator of inflammation. J Pharm Pharmacol. 1980 Jul;32(7):517–518. doi: 10.1111/j.2042-7158.1980.tb12985.x. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. Initial kinetics of lysosomal enzyme secretion and superoxide anion generation by human polymorphonuclear leukocytes. Inflammation. 1980 Jun;4(2):145–163. doi: 10.1007/BF00914161. [DOI] [PubMed] [Google Scholar]
- Van Arman C. G. Anti-inflammatory drugs. Clin Pharmacol Ther. 1974 Nov;16(5 Pt 2):900–904. doi: 10.1002/cpt1974165part2900. [DOI] [PubMed] [Google Scholar]
- WOOLLEN J. W., HEYWORTH R., WALKER P. G. Studies on glucosaminidase. 3. Testicular N-acetyl-beta-glucosaminidase and N-acetyl-beta-galactosaminidase. Biochem J. 1961 Jan;78:111–116. doi: 10.1042/bj0780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. E., Waite B. M., Thomas M. J., DeChatelet L. R. Release and metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1981 Jul 25;256(14):7228–7234. [PubMed] [Google Scholar]
- Weissmann G., Smolen J. E., Korchak H. Prostaglandins and inflammation: receptor/cyclase coupling as an explanation of why PGEs and PGI2 inhibit functions of inflammatory cells. Adv Prostaglandin Thromboxane Res. 1980;8:1637–1653. [PubMed] [Google Scholar]
- Williams T. J. Chemical mediators of vascular responses in inflammation: a two mediator hypothesis [proceedings]. Br J Pharmacol. 1977 Nov;61(3):447P–448P. [PMC free article] [PubMed] [Google Scholar]
- Williams T. J. Prostaglandin E2, prostaglandin I2 and the vascular changes of inflammation. Br J Pharmacol. 1979 Mar;65(3):517–524. doi: 10.1111/j.1476-5381.1979.tb07860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Suppression of acute and chronic inflammation in adrenalectomized rats by pharmacologic amounts of prostaglandins. Arthritis Rheum. 1973 Sep-Oct;16(5):606–618. doi: 10.1002/art.1780160505. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Quagliata F. Effect of prostaglandin E 1 on adjuvant arthritis. Nature. 1971 Dec 3;234(5327):304–305. doi: 10.1038/234304a0. [DOI] [PubMed] [Google Scholar]


