Abstract
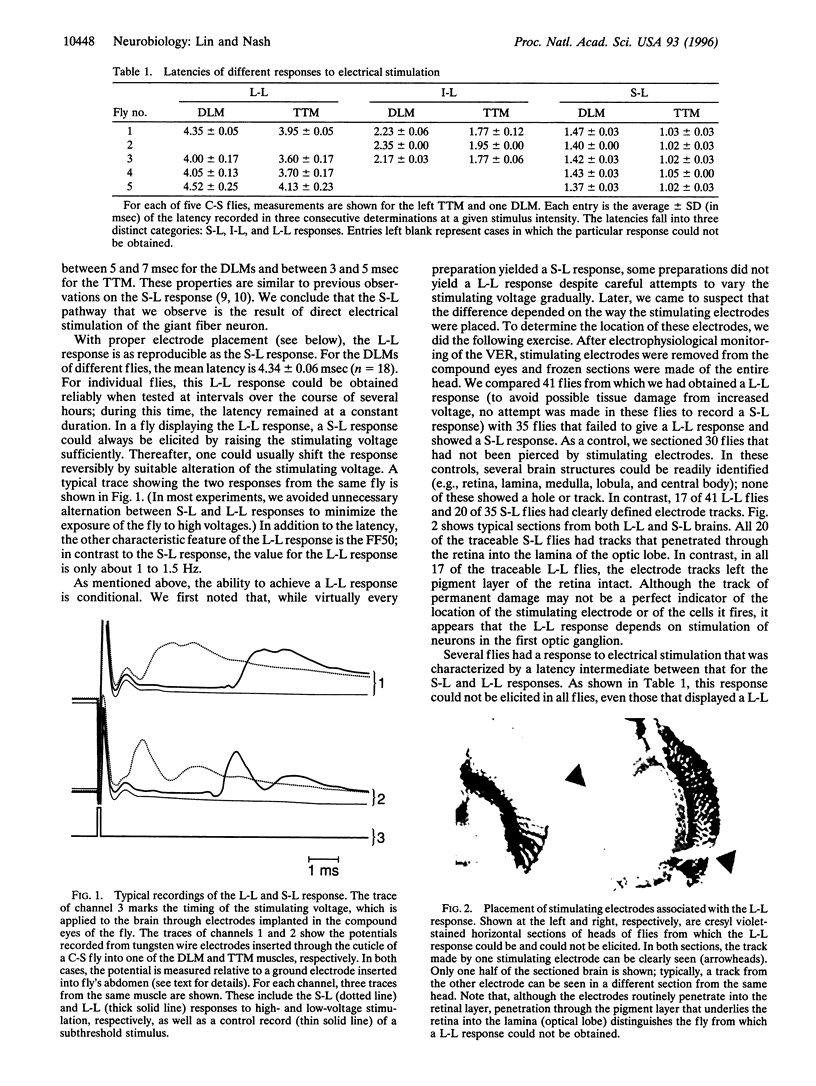
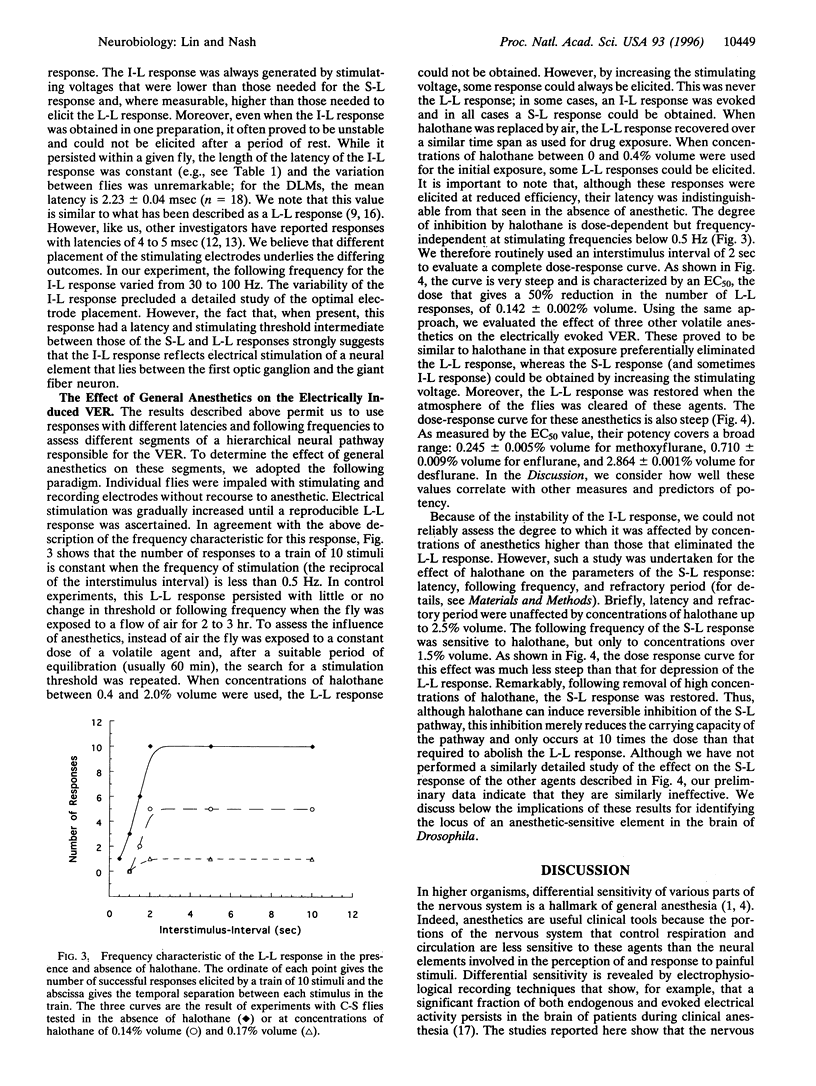
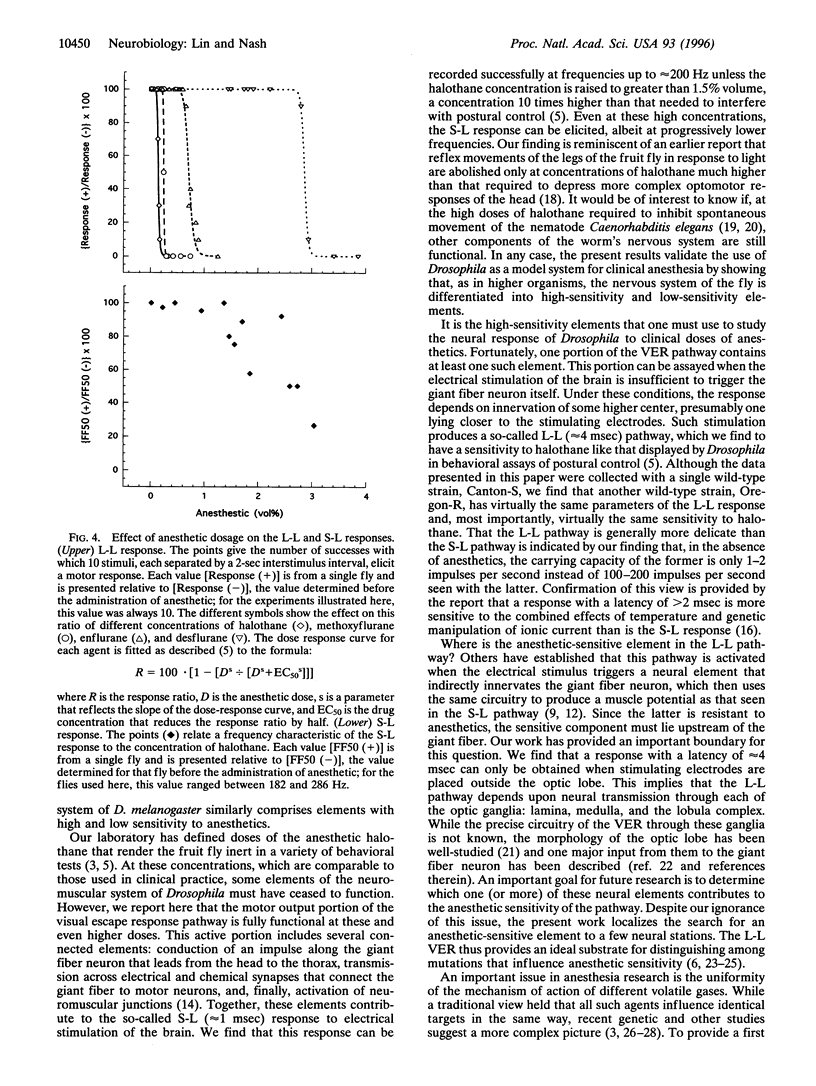
The neural pathway that governs an escape response of Drosophila to sudden changes in light intensity can be artificially induced by electrical stimulation of the brain and monitored by electrical recording from the effector muscles. We have refined previous work in this system to permit reliable ascertainment of two kinds of response: (i) a short-latency response that follows from direct excitation of a giant fiber neuron in the interior of the fly brain and (ii) a long-latency response in which electrical stimulation triggers neurons in the optic ganglia that ultimately impinge on the giant fiber neuron. The general anesthetic halothane is reported here to have very different potencies in inhibiting these two responses. The long-latency response is obliterated at concentrations similar to those that cause gross behavioral effects in adult flies, whereas the short-latency response is only partially inhibited at doses that are 10-fold higher. Three other volatile anesthetic agents show a similar pattern. Thus, as in higher organisms, the Drosophila nervous system is differentiated into components of high and low sensitivity to general anesthetics. Moreover, this work shows that one of the sensitive components of the nervous system lies in the optic lobe and is readily assayed by its effect on downstream systems; it should provide a focus for exploring the effects of genetic alteration of anesthetic sensitivity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allada R., Nash H. A. Drosophila melanogaster as a model for study of general anesthesia: the quantitative response to clinical anesthetics and alkanes. Anesth Analg. 1993 Jul;77(1):19–26. doi: 10.1213/00000539-199307000-00005. [DOI] [PubMed] [Google Scholar]
- Campbell D. B., Nash H. A. Use of Drosophila mutants to distinguish among volatile general anesthetics. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2135–2139. doi: 10.1073/pnas.91.6.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins T., Ganetzky B. Conduction in the giant nerve fiber pathway in temperature-sensitive paralytic mutants of Drosophila. J Neurogenet. 1990 Aug;6(4):207–219. doi: 10.3109/01677069009107111. [DOI] [PubMed] [Google Scholar]
- Engel J. E., Wu C. F. Interactions of membrane excitability mutations affecting potassium and sodium currents in the flight and giant fiber escape systems of Drosophila. J Comp Physiol A. 1992 Aug;171(1):93–104. doi: 10.1007/BF00195964. [DOI] [PubMed] [Google Scholar]
- Gorczyca M., Hall J. C. Identification of a cholinergic synapse in the giant fiber pathway of Drosophila using conditional mutations of acetylcholine synthesis. J Neurogenet. 1984 Dec;1(4):289–313. doi: 10.3109/01677068409107093. [DOI] [PubMed] [Google Scholar]
- Hummon M. R., Costello W. J. Giant fiber activation of flight muscles in Drosophila: asynchrony in latency of wing depressor fibers. J Neurobiol. 1989 Sep;20(6):593–602. doi: 10.1002/neu.480200606. [DOI] [PubMed] [Google Scholar]
- Kirschfeld K., Baier-Rogowski V. The neuronal basis of the anesthetic state: a comparative physiological approach. II. The influence of anesthetics on various reactions in flies. Biol Cybern. 1988;58(1):1–11. doi: 10.1007/BF00363951. [DOI] [PubMed] [Google Scholar]
- Krishnan K. S., Nash H. A. A genetic study of the anesthetic response: mutants of Drosophila melanogaster altered in sensitivity to halothane. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8632–8636. doi: 10.1073/pnas.87.21.8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovitch B. A., Campbell D. B., Krishnan K. S., Nash H. A. Mutations that affect ion channels change the sensitivity of Drosophila melanogaster to volatile anesthetics. J Neurogenet. 1995 Apr;10(1):1–13. doi: 10.3109/01677069509083455. [DOI] [PubMed] [Google Scholar]
- Morgan P. G., Cascorbi H. F. Effect of anesthetics and a convulsant on normal and mutant Caenorhabditis elegans. Anesthesiology. 1985 Jun;62(6):738–744. doi: 10.1097/00000542-198506000-00007. [DOI] [PubMed] [Google Scholar]
- Morgan P. G., Sedensky M. M. Mutations conferring new patterns of sensitivity to volatile anesthetics in Caenorhabditis elegans. Anesthesiology. 1994 Oct;81(4):888–898. doi: 10.1097/00000542-199410000-00016. [DOI] [PubMed] [Google Scholar]
- Morgan P. G., Sedensky M., Meneely P. M. Multiple sites of action of volatile anesthetics in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2965–2969. doi: 10.1073/pnas.87.8.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Madison D. V. General anesthetics hyperpolarize neurons in the vertebrate central nervous system. Science. 1982 Sep 10;217(4564):1055–1057. doi: 10.1126/science.7112112. [DOI] [PubMed] [Google Scholar]
- Tanouye M. A., Wyman R. J. Motor outputs of giant nerve fiber in Drosophila. J Neurophysiol. 1980 Aug;44(2):405–421. doi: 10.1152/jn.1980.44.2.405. [DOI] [PubMed] [Google Scholar]
- Trimarchi J. R., Schneiderman A. M. Different neural pathways coordinate Drosophila flight initiations evoked by visual and olfactory stimuli. J Exp Biol. 1995 May;198(Pt 5):1099–1104. doi: 10.1242/jeb.198.5.1099. [DOI] [PubMed] [Google Scholar]
- Wyman R. J., Thomas J. B. What genes are necessary to make an identified synapse? Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):641–652. doi: 10.1101/sqb.1983.048.01.068. [DOI] [PubMed] [Google Scholar]




