Abstract
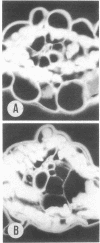
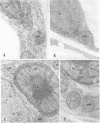
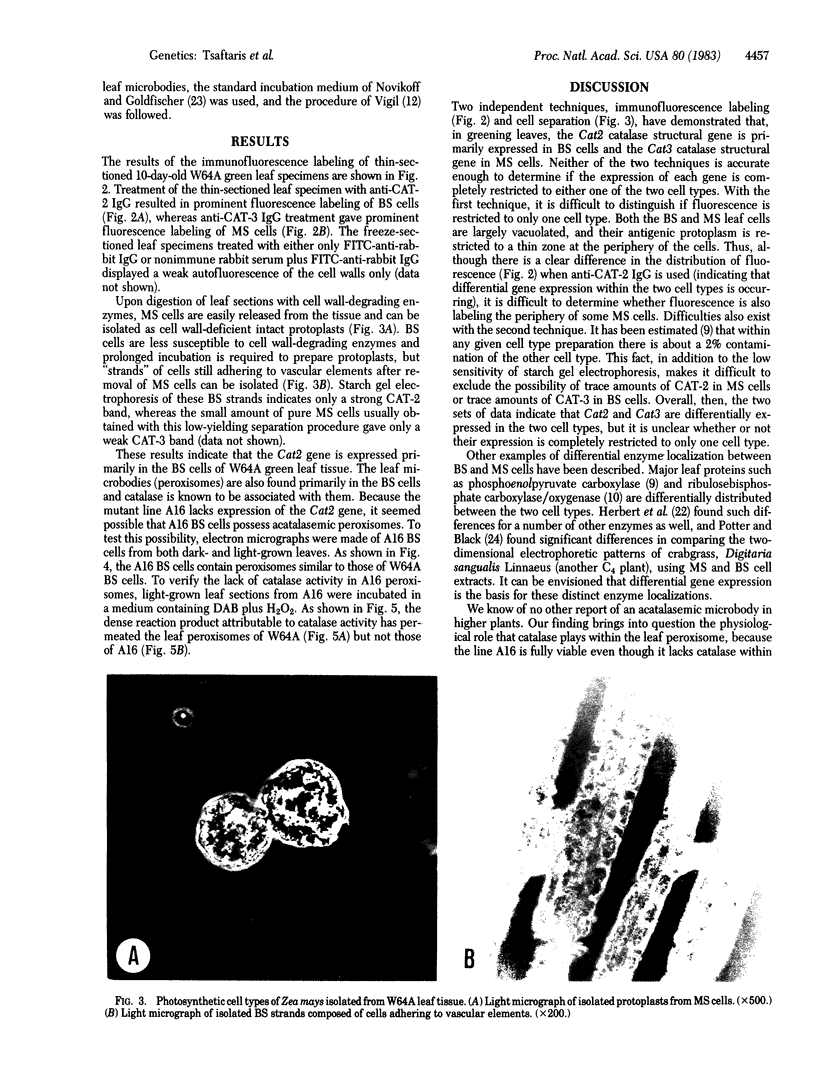
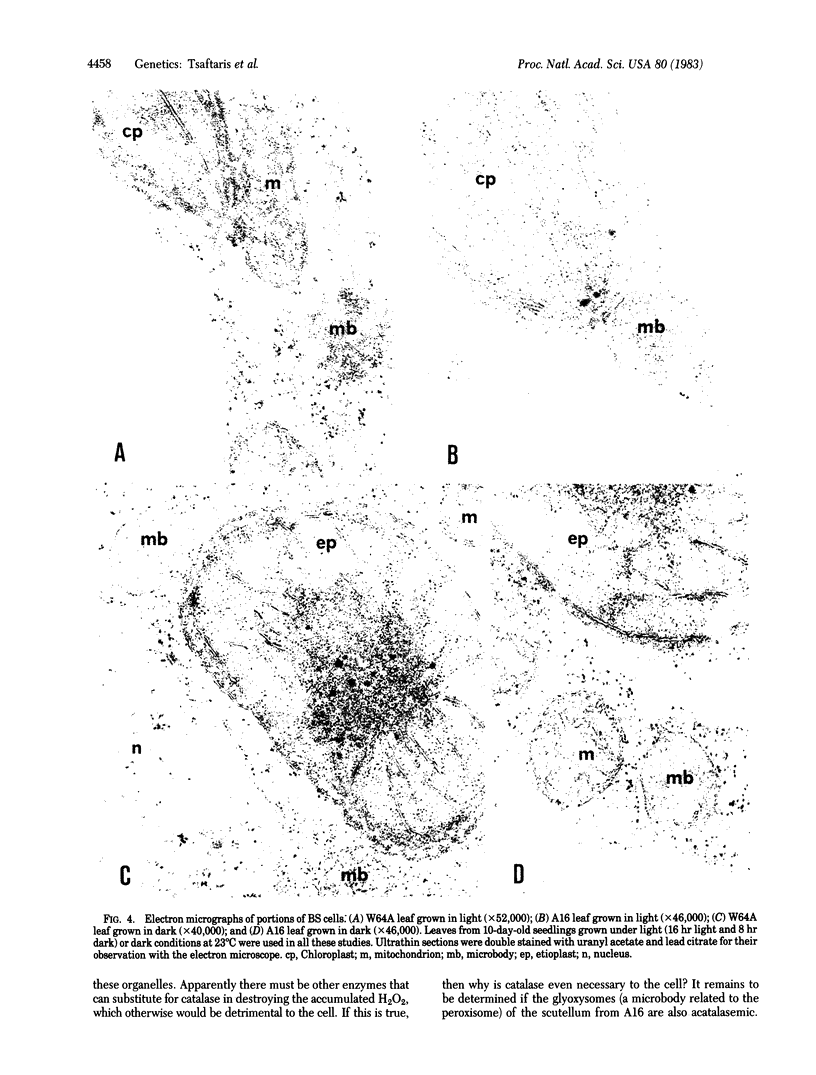
Cell separation studies in conjunction with immunocytochemical studies indicate that mesophyll cells and bundle-sheath cells, the dimorphic photosynthetic cell types in the leaves of the C4 plant Zea mays, differ in their catalase composition. In particular, catalase-2, the product of the Cat2 gene, is found primarily in the bundle-sheath cells, whereas catalase-3, the product of the Cat3 gene, is found primarily in the mesophyll cells. Electron microscopic observations reveal that bundle-sheath cells of A16, a mutant line lacking expression of the Cat2 gene in all tissues examined, contain numerous peroxisomes, but they are acatalasemic as determined by staining with 3,3′-diaminobenzidine. The significance of this mutant in physiological studies is discussed.
Keywords: maize microbodies, mesophyll cells, bundle-sheath cells
Full text
PDF

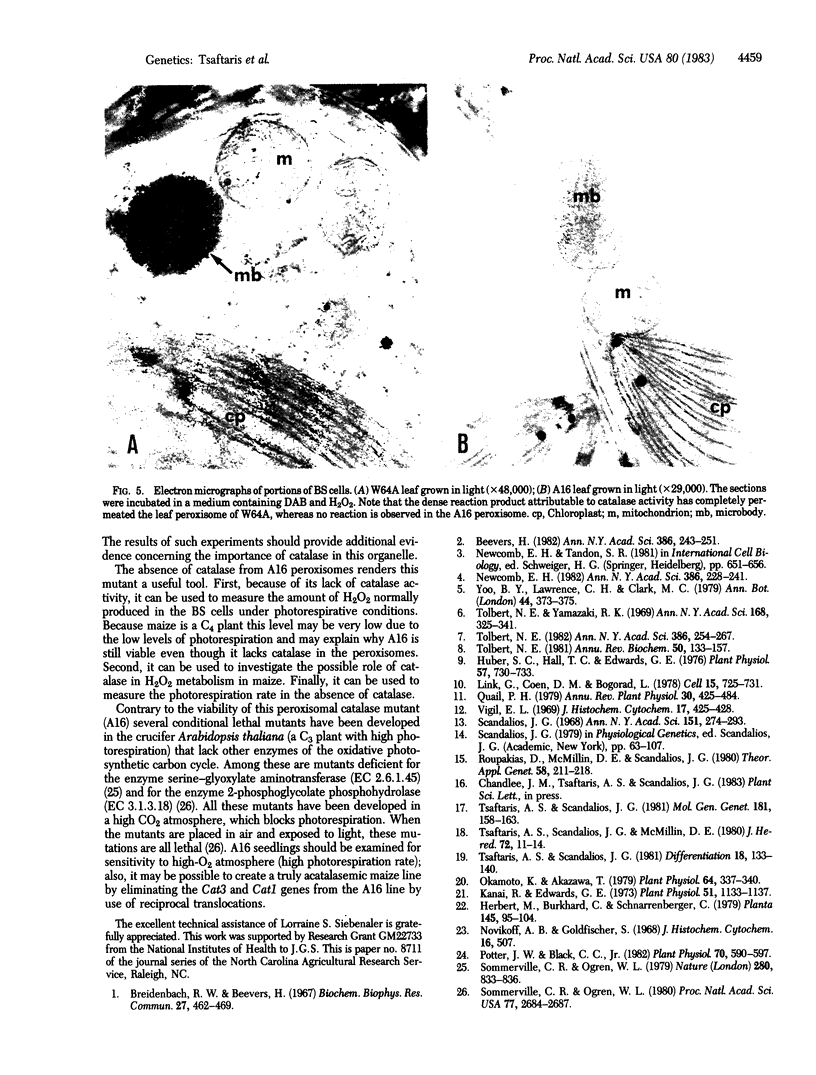
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Hall T. C., Edwards G. E. Differential Localization of Fraction I Protein between Chloroplast Types. Plant Physiol. 1976 May;57(5):730–733. doi: 10.1104/pp.57.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link G., Coen D. M., Bogorad L. Differential expression of the gene for the large subunit of ribulose bisphosphate carboxylase in maize leaf cell types. Cell. 1978 Nov;15(3):725–731. doi: 10.1016/0092-8674(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Akazawa T. Enzymic Mechanism of Starch Breakdown in Germinating Rice Seeds: 8. Immunohistochemical Localization of beta-Amylase. Plant Physiol. 1979 Aug;64(2):337–340. doi: 10.1104/pp.64.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J. W., Black C. C. Differential Protein Composition and Gene Expression in Leaf Mesophyll Cells and Bundle Sheath Cells of the C(4) Plant Digitaria sanguinalis (L.) Scop. Plant Physiol. 1982 Aug;70(2):590–597. doi: 10.1104/pp.70.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios J. G. Genetic control of multiple molecular forms of catalase in maize. Ann N Y Acad Sci. 1968 Jun 14;151(1):274–293. doi: 10.1111/j.1749-6632.1968.tb11896.x. [DOI] [PubMed] [Google Scholar]
- Somerville C. R., Ogren W. L. Photorespiration mutants of Arabidopsis thaliana deficient in serine-glyoxylate aminotransferase activity. Proc Natl Acad Sci U S A. 1980 May;77(5):2684–2687. doi: 10.1073/pnas.77.5.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Metabolic pathways in peroxisomes and glyoxysomes. Annu Rev Biochem. 1981;50:133–157. doi: 10.1146/annurev.bi.50.070181.001025. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K. Leaf peroxisomes and their relation to photorespiration and photosynthesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):325–341. doi: 10.1111/j.1749-6632.1969.tb43119.x. [DOI] [PubMed] [Google Scholar]
- Vigil E. L. Intracellular localization of catalase (peroxidatic) activity in plant microbodies. J Histochem Cytochem. 1969 Jun;17(6):425–428. doi: 10.1177/17.6.425. [DOI] [PubMed] [Google Scholar]