Abstract
Organophosphates (OPs) are among the most used pesticides. Although some OPs have had their use progressively more restricted, other OPs are being used without sufficient investigation of their effects. Here, we investigated the immediate neurochemical and delayed neurochemical and behavioral actions of the OP methamidophos to verify whether there are concerns regarding exposure during early postnatal development. From the third to the nineth postnatal day (PN), Swiss mice were sc injected with methamidophos (1mg/kg). At PN10, we assessed cholinergic and serotonergic biomarkers in the cerebral cortex and brainstem. From PN60 to PN63, mice were submitted to a battery of behavioral tests and subsequently to biochemical analyses. At PN10, the effects were restricted to females and to the cholinergic system: Methamidophos promoted increased choline transporter binding in the brainstem. At PN63, in the brainstem, there was a decrease in choline transporter, a female-only decrease in 5HT1A and a male-only increase in 5HT2 receptor binding. In the cortex, choline acetyltransferase activity was decreased and 5HT2 receptor binding was increased both in males and females. Methamidophos elicited behavioral alterations, suggestive of increased depressive-like behavior and impaired decision making. There were no significant alterations on anxiety-related measures and on memory/learning. Methamidophos elicited cholinergic and serotonergic alterations that depended on brain region, sex, and age of the animals. These outcomes, together with the behavioral effects, indicate that this OP is deleterious to the developing brain and that alterations are indeed identified long after the end of exposure.
Key Words: organophosphate, AChE, ChAT, serotonin, mood disorders, depression, development.
Organophosphates (OPs) are among the most widely used class of pesticides in the world (FAOSTAT, 2010; Terry, 2012). The effects of exposure to high levels of OPs are well documented and mainly involve irreversible inhibition of the enzyme acetylcholinesterase (AChE), causing an accumulation of acetylcholine in synaptic clefts and, consequently, cholinergic hyperstimulation (Mileson et al., 1998). However, when the doses of exposure and AChE inhibition are low, the effects in other neurotransmitter systems may prevail and each pesticide in this class may substantially differ in their effects on brain function (Aldridge et al., 2005a; Gupta, 2004; Pope, 1999; Timofeeva et al., 2008). Subchronic or chronic exposures to doses devoid of systemic toxicity are more common in real life than acute exposure to high doses, making the former kinds of exposure a necessary topic of investigation.
Extensive evidence indicates that the developing brain is more vulnerable to OP exposure in that neurodevelopmental effects occur at doses below the threshold for systemic toxicity or even for AChE inhibition (Flaskos, 2012; Slotkin, 2004). During the “brain growth spurt,” which, in rodents, comprises the first 10 days of postnatal life (Bayer et al., 1993; Clancy et al., 2007; Quinn, 2005), the brain is highly vulnerable to several neurotoxic agents (Dribben et al., 2011; Nunes et al., 2011; Nunes-Freitas et al., 2011; Pohl-Guimaraes et al., 2011) including OPs (Aldridge et al., 2003, 2005a; Slotkin and Seidler, 2007, 2008). This susceptibility is thought to be due to events such as intense dendritic arborization, synaptogenesis, and the migration of multiple neuronal populations in most brain regions (Bandeira et al., 2009; Dobbing and Sands, 1979). It is also the period of entry of cholinergic fibers into the cortex and the period within which the expression of major components of the cholinergic system peak in several brain regions (for review: Abreu-Villaça et al., 2011; Dwyer et al., 2008).
It has been demonstrated that exposure to OPs such as diazinon, parathion, chlorpyrifos, and dichlorvos during the perinatal period elicits widespread abnormalities in indices of cholinergic, dopaminergic, and serotonergic (5HT) synaptic function (Aldridge et al., 2005b; Levin et al., 2010; Slotkin and Seidler, 2007, 2008; Slotkin et al., 2006b, 2008c), which are known to promote behavioral changes later in life (Ahlbom et al., 1995; Aldridge et al., 2005a; Dam et al., 2000; Levin et al., 2010; Slotkin et al., 2001). Accordingly, some OPs have suffered severe restrictions in several countries such as United States (U.S. EPA, 2002) and Brazil (ANVISA, 2004). Despite that, several other OPs are still used, mostly in developing countries (FAOSTAT, 2010), without sufficient investigation of their effects. One such case is the OP methamidophos. Its use has been restricted in several countries (ANVISA, 2011; Rotterdam Convention, 2010a), but it is still extensively used and, in fact, its use has been increasing in the last few years (Rotterdam Convention, 2010b). In our previous studies that investigated the effects of methamidophos at adulthood, we detected behavioral changes associated with depression (Lima et al., 2009) and changes in neurochemical markers of serotonin function (Lima et al., 2011) at exposure levels that caused low cholinesterase inhibition (15%) and even after recovery of cholinesterase activity. These results indicate that methamidophos is able to cause damage to the mature nervous system. However, we do not know whether exposure during critical periods of development elicits similar changes.
Accordingly, here we sought to achieve two main objectives. The first one was to investigate the immediate neurochemical actions of early methamidophos exposure on the cholinergic and serotoninergic systems to clarify its interference on brain processes, particularly during the “brain growth spurt.” The second objective was to investigate the effects at adulthood, so as to verify whether there are concerns regarding long-term and/or late-emergent neurochemical alterations. We further extended our study to include a battery of behavioral tests at adulthood to verify whether our exposure protocol resulted in functional alterations. We hypothesize that methamidophos exposure would produce immediate and late-emergent cholinergic and serotonergic alterations in the brain, as well as behavioral effects at adulthood. To test this hypothesis, mice were subchronically exposed to methamidophos from the third to the ninth postnatal day. Both by the end of exposure and at adulthood, to analyze the cholinergic system, we assessed the binding of hemicholinium-3 to the high-affinity presynaptic choline transporter (Ch transporter) and the activities of choline acetyltransferase (ChAT) and AChE. For the serotonergic system analysis, we assessed the binding to the 5HT1A and 5HT2 receptors, as well as the binding to the presynaptic 5HT transporter. The analyses of the cholinergic and serotonergic systems were performed in the cerebral cortex and brainstem. The cerebral cortex contains major cholinergic and serotonergic projections, and the brainstem, in addition to dendritic arbors, contains the majority of the cholinergic and serotonergic cell bodies of pathways that ascend into the cerebral cortex, hippocampus, and other regions involved in affective disorders and cognition. Considering the relevant association between these neurotransmitter systems, cognitive function, and mood disorders, we chose the following tests to evaluate the behavior of methamidophos-exposed mice at adulthood: The anxiety-like behavior was assessed through the use of the elevated plus maze (EPM) and open field (OF) tests, the depressive-like behavior was evaluated through the forced-swimming test, the step-down passive avoidance test investigated memory and learning, and the time spent in the center of the EPM was used to assess decision making.
Materials and Methods
All experiments were carried out under institutional approval of the Animal Care and Use Committee of the Universidade do Estado do Rio de Janeiro (CEUA/006/2011), in accordance with the declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. All Swiss mice were bred and maintained in a temperature-controlled vivarium on a 12:12-h light/dark cycle (lights on at 1:00 a.m.). Access to food and water was ad lib. After mating, each female mice was placed in an individual cage with free access to water and food until delivery.
Methamidophos Dose Selection
Previous studies have found that developmental exposure to OPs such as chlorpyrifos, diazinon, and parathion, resulting in up to 20% reduction of AChE activity 1 day postexposure, differentially target distinct neurotransmitter systems and behaviors in the developing and adult brain of rodents (Slotkin and Seidler, 2007; Slotkin et al., 2006b, 2008c, 2009). In order to find a comparable dose of methamidophos, 63 animals from 11 litters were submitted to daily sc injections of methamidophos (1ml/kg, on the hindquarters) from postnatal day (PN)3 to PN9. Control mice (CT) received dimethyl sulfoxide (DMSO) as vehicle. For each litter, offspring were distributed into four groups: higher dose (HighD, 3mg/kg), intermediate dose (IntD, 1mg/kg), lower dose (LowD, 0.25mg/kg), and CT. No more than one male and one female from each litter were assigned to each group.
Mice were sacrificed at PN10 (24–26h after the last injection), the brains were dissected, and the regions of interest were immediately frozen and stored at −45°C for later analysis of AChE activity. Dissection was performed by a cut through the cerebellar peduncles, whereupon the cerebellum (including flocculi) was lifted from the underlying tissue. The cerebral cortex (forebrain with removal of the hippocampus) was separated from the brainstem (midbrain + pons + medulla) by a cut made rostral to the thalamus. AChE activity was measured by the spectrophotometric mode described by Ellman et al. (1961). The cerebral cortex and the brainstem of each animal were weighed and homogenized to approximately 90mg/ml in sodium phosphate buffer (0.12M, pH 7.6) using a homogenizer Ultra-Turrax T10 basic (IKA, São Paulo, Brazil). Each assay contained 0.1ml of diluted homogenate in a total volume of 1.16ml, with final concentrations of 102mM of sodium phosphate buffer (pH 7.6), 0.3mM of 5,5-dithiobis(2-nitrobenzoic) acid, and 1mM of acetylthiocholine iodide. Immediately after the addition of tissue, the duplicates were read at 412nm in kinetic mode every 30 s during 2min. Blank absorbances were subtracted from the final readings. To get the values of AChE activity in nmols/min, we used a previously built standard curve of L-cysteine. The activity was determined relative to tissue protein. Proteins were measured by bicinchoninic acid (BCA) protein assay.
As described in the Results section, the IntD of methamidophos (1mg/kg) elicited approximately 20% inhibition of AChE in the brain. Accordingly, for the next experiments, offspring from each litter was exposed either to this dose of methamidophos (MET group) or to vehicle (CT group). To further evaluate whether, during the period of exposure, methamidophos elicited higher levels of AChE inhibition, separate groups of mice received the IntD of methamidophos or vehicle and were sacrificed either 1 or 4h after the first (PN3, three litters, n = 24) or last (PN9, four litters, n = 24) injection. At each age, male and female mice were distributed into two treatment groups (CT and MET) and two time points (1 and 4h). As described in the Results section, AChE inhibition ranged from 29 to 50% at PN3 and from 52 to 67% at PN9.
Evaluation of Neurochemical and Behavioral Effects of Methamidophos
Time Line of the Experiments
One hundred mice from 19 litters were used in this set of experiments. Each litter was distributed into either MET or CT groups. Daily exposure extended from PN3 to PN9. Body weights were measured daily during the period of exposure. Thirty-two animals were sacrificed at PN10. The brains were dissected as described above, and the cerebral cortex and brainstem were immediately frozen and stored at −45°C for later biochemical analysis. Sixty-eight mice were maintained in the vivarium until adulthood, at which time they were submitted to a battery of behavioral tests from PN60 to PN63 and subsequently sacrificed. From these, 32 had the brains dissected and stored for later analysis. No more than one male and one female from each litter were assigned to each treatment group/age.
Evaluation of Cholinergic and Serotonergic Systems
At PN10 and PN63, we evaluated three cholinergic and three serotonergic biomarkers. Regarding the cholinergic system, we evaluated ChAT activity, the binding of [3H]hemicholinium-3 to the Ch transporter, and AChE activity. ChAT, the enzyme that catalyses acetylcholine biosynthesis, is a constitutive marker for the cholinergic system, which reflects the concentration of cholinergic nerve terminals. Accordingly, ChAT increases during cholinergic synaptogenesis but does not change in response to stimuli that alter cholinergic neuronal activity (Aubert et al., 1996; Happe and Murrin, 1992; Zahalka et al., 1992). In contrast, the Ch transporter is responsive to neuronal activity (Klemm and Kuhar, 1979; Simon et al., 1976); moreover, because acetylcholine synthesis and release depend on the availability of choline, these events are indirectly dependent on the functionality of the transporter and AChE (for review: Ribeiro et al., 2006). For the serotonergic system analysis, we chose to assess the binding to the 5HT1A and 5HT2 receptors and the binding to the 5HT transporter. The function of these receptors is particularly important during the neonatal period due to their role in modulating both neuronal and glial proliferation and maturation (for review: Azmitia, 2001). In addition, these two receptors play major roles in 5HT-related mental disorders, especially depression (Arango et al., 2001; Fujita et al., 2000), whereas the presynaptic 5HT transporter is the primary target for antidepressant drugs (Maes and Meltzer, 1995; Nemeroff, 1998; Nutt, 2002).
Tissues were thawed and homogenized in ice-cold 50mM Tris (pH 7.4). Aliquots of this homogenate were withdrawn for measurements of total protein, ChAT, and AChE activities. The remaining homogenate was then sedimented by centrifugation at 39,000 × g for 15min. The pellet was resuspended in the original volume of buffer and resedimented, and the resultant pellet was resuspended in ¼ of the original volume using a smooth glass homogenizer fitted with a Teflon pestle. Aliquots of this last resuspension were withdrawn for measurements of binding to the Ch transporter, 5HT1A and 5HT2 receptor binding, 5HT transporter binding and for membrane protein. Proteins were measured by BCA protein assay. All assays have been described in detail in previous articles (Abreu-Villaça et al., 2003, 2004; Lima et al., 2011; Nunes-Freitas et al., 2011; Ribeiro-Carvalho et al., 2008, 2009) and will therefore be presented briefly. At PN60, AChE activity measurements came from the same homogenate used for the ChAT and total protein, whereas at PN10, the AChE activity assay was run in a separate group of mice, as described in the Methamidophos dose selection section.
ChAT activity.
Assays contained tissue homogenate diluted in phosphate buffer (pH =7.9) and a mixture with final concentrations of 200mM NaCl, 17mM MgCl2, 1mM EDTA, Triton X-100 0.2% in buffer, 0.12mM physostigmine, 0.6mg/ml bovine serum albumin, 20mM choline chloride, and 50mM [14C]acetyl-coenzyme A. Triplicate samples from each homogenate were preincubated for 15min at 4°C and then incubated for 30min at 37°C. Under these conditions, the enzymatic reaction took place and ChAT catalyzed the synthesis of acetylcholine. Labeled acetylcholine was then extracted and the activity determined relative to tissue protein.
High-affinity choline uptake.
It was assessed with the binding of [3H]hemicholinium-3 to the presynaptic high-affinity choline transporter. The binding of [3H]hemicholinium-3 was determined using a final ligand concentration of 2nM in the membrane fraction; incubations lasted for 20min at 20°C in a buffer consisting of 10nM NaKHPO4/150nM NaCl (pH 7.4), and unlabeled hemicholinium-3 (20μM) was used to displace specific binding for the cholinergic transporter. Incubations were stopped by the addition of excess of ice-cold incubation buffer, and the labeled membranes were trapped by rapid vacuum filtration onto glass fiber filters that were presoaked in 0.15% polyethyleneimine. The filters were then washed with incubation buffer, and radiolabeling was determined. Data were obtained by calculating the specific binding per milligrams of membrane protein.
Serotonin receptors and transporter.
The 5HT receptors binding was evaluated by using two radioligands: 1nM [3H]8-hydroxy-2-(di-n-propylamino)tetralin for 5HT1A receptors and 0.4nM [3H]ketanserin for 5HT2 receptors. Binding to the presynaptic 5HT transporter was evaluated with 85pM [3H]paroxetine. For 5HT1A receptors, incubations lasted for 30min at 25°C in a buffer consisting of 50mM Tris (pH 8), 0.5mM MgCl2, and 0.5mM sodium ascorbate; 100μM 5HT was used to displace specific binding. For 5HT2 receptors, incubations lasted for 15min at 37°C in 50nM Tris (pH 7.4) and specific binding was displaced with 10μM methysergide. For binding to the presynaptic 5HT transporter, incubations lasted for 120min at 20°C in a buffer consisting of 50mM Tris (pH 7.4), 120mM NaCl, and 5mM KCl; 100μM 5HT was used to displace specific binding. Incubations were stopped by the addition of excess of ice-cold incubation buffer, and the labeled membranes were trapped by rapid vacuum filtration onto glass fiber filters that were presoaked in 0.15% polyethyleneimine. The filters were then washed with incubation buffer, and radiolabeling was determined. Data were obtained by calculating the specific binding per milligrams of membrane protein.
Behavioral Tests
From PN60 to PN63, mice were submitted to the four behavioral tests described below. Due to the presence of technical problems with the video data in some of the tests, the sample size used for the quantitative analysis (indicated between parentheses) varied from test to test. On the first day, anxiety levels and decision making were assessed through the use of the EPM (n = 67). This test was performed between 2:00 and 4:00 p.m. On the next day, mice were submitted to the OF test (n = 63) in the morning (between 09:00 and 11:00 a.m.) and to the forced swimming test (n = 48) in the afternoon (between 2:00 and 4:00 p.m.). The OF was used to assess both locomotor activity and anxiety levels, whereas the forced swimming investigated the depressive-like behavior. Finally, memory and learning was assessed in the morning of the fourth day of testing through the use of the step-down passive avoidance test (n = 68). Because the EPM and forced swimming tests are classically used to investigate emotional reactivity, both tests were performed in the same period of the circadian cycle of the mice (dark phase). The other tests were performed in the light phase. All behavioral tests were performed in a testing room next to our vivarium and with lights on (60W fluorescent light bulb, 3 m high). All animals were allowed to habituate for 10min in the testing room before each behavioral test.
EPM.
The anxiety-like behavior was initially investigated by using the EPM test. The test procedure is described in detail elsewhere (Abreu-Villaça et al., 2008). The EPM is shaped like a plus sign and consists of two “open” (no walls, 5×28.5cm) and two “closed” (5×28.5×14cm) arms, arranged perpendicularly and elevated 50cm above the floor. The test began with the animal being placed on the center of the equipment, facing a closed arm. Each test lasted 10min in a sound attenuated room. All tests were videotaped, and the percentage of time spent in the open arms (%Time OA: the time spent in open arms divided by time spent in open + closed arms) and the percentage of open arms entries (%Entries OA: the number of entries in open arms divided by number of entries in open + closed arms) were used as anxiety measures (Rodgers and Dalvi, 1997). Increased %Time OA and/or %Entries OA correspond to decreased anxiety-like behavior and vice versa (Rodgers and Dalvi, 1997). The number of closed arms entries (Entries CA) was used as a measure of activity, and the time spent in the center of the maze (Time Cen) was used as a measure of decision making (Fraga et al., 2011; Rodgers et al., 1997). All the variables were scored using the video images of the tests.
OF.
The OF arena consists of an transparent acrylic box (46cm length × 46cm width × 43cm height) that was equipped with 2 arrays of 16 infrared beams each, positioned at 1.5cm above the floor to measure horizontal spontaneous locomotor activity. Interruptions of photocell beams were detected by a computer system, and the location of the animal was calculated by the software with a 0.1-s resolution. Each mouse was individually placed in the center of the arena, and spontaneous locomotor activity was determined. Total ambulation (Ambulation OF) was quantified on the basis of the traveled distance. In addition, considering that measures of central exploration are often regarded as anxiety-related indices (Filgueiras et al., 2009; Prut and Belzung, 2003), the time spent in the center (Time Cen OF) was used as a measure of anxiety-like behavior. Increased Time Cen OF corresponds to decreased anxiety-like behavior and vice versa (Filgueiras et al., 2009; Prut and Belzung, 2003).
Forced swimming test.
Each mouse was submitted to a 10-min forced swimming testing (FST) session. The test procedure is described in detail elsewhere (Filgueiras et al., 2006). Briefly, each mouse was placed in a plastic container (diameter = 21cm, height = 23cm) filled with 16cm of water at about 25°C. The animal’s behavior was continuously recorded throughout the testing session with an overhead video camera. Animals were considered to be immobile when they remained floating with all limbs and tail motionless. The time the animals spent in this condition was considered to be the measure of immobility (Immob Time) and was used as depressive-like measure. Increased Immob Time corresponds to increased depressive-like behavior.
Step-down passive avoidance test.
The test apparatus contained one chamber, 25×25×25cm (length × width × height). The test procedure is described in detail elsewhere (Abreu-Villaça et al., 2013). Mice were submitted to two testing sessions: Initially, in a training/acquisition session, subjects were placed in a circular platform (diameter = 6.5cm) and allowed up to 3min to descend from it, whereupon they received a mild foot shock (0.3 mA/3 s). Three hours later, the animals were retested and allowed up to 3min to descend from the platform (shock was not administered). The latency (L) to descend from the platform on the first (L0) and second (L3) sessions was registered. The learning/memory component of the passive avoidance task is expressed as an increase in the time the animal takes to descend from the platform from the first to the second session. Therefore, in order to visualize more clearly differences between groups, the learning/memory component of the task was evaluated by calculating a memory/learning index as follows: (L3 − L0)/L0.
Materials
Radioisotopically labeled compounds came from PerkinElmer Life Sciences (Boston, MA): [14C] Acetyl-CoA (specific activity, 4.0 Ci/mmol), [3H]hemicholinium-3 (specific activity, 170 Ci/mmol), [3H]8-hydroxy-2-(di-n-propylamino)tetralin (specific activity, 170.2 Ci/mmol), [3H]ketanserin (specific activity, 67.0 Ci/mmol), and [3H]paroxetine (specific activity, 24.4 Ci/mmol). Sigma Chemical Co. (St Louis, MO) was the source for bovine albumin, BCA kit, eserine hemisulfate salt, 3-heptanone, sodium tetraphenylborate, Triton X-100, methamidophos, serotonin, acetyltiocholine, methylsergide, and polyethyleneimine. VETEC Química Fina Ltda (Rio de Janeiro, RJ) was the source for all other reagents.
Statistical Analysis
AChE Activity
At PN10, the data were evaluated by ANOVA. Dose (HighD, IntD, LowD, and CT), Brain Region (cerebral cortex and brainstem), and Sex were used as between-subject factors. At PN3, PN9 (1 and 4h after injection) and PN63, separate ANOVAs on all factors—Treatment (MET and CT), Brain Region (cerebral cortex and brainstem), and Sex—were carried out. For PN3 and PN9 data, Time (1 and 4h after injection) was also a factor in the analysis.
Body Weight
A repeated-measures ANOVA (rANOVA) was carried out. Treatment (MET and CT) and Sex were used as factors. Day (PN3–PN9) was considered the within-subject factor. Within each treatment, animals from the same litter were considered as n = 1.
Cholinergic and Serotonergic Markers
Results were evaluated first by two rANOVAs on all factors: Treatment (MET and CT), Brain Region (cerebral cortex and brainstem), Age (PN10 and PN63), and Sex. For the first rANOVA, cholinergic measures (ChAT and Ch transporter) were considered the within-subject factor. For the second rANOVA, serotonergic measures (5HT1A receptor, 5HT2 receptor, and 5HT transporter) were considered the within-subject factor.
Behavioral Tests
Results were evaluated by ANOVAs. Treatment (MET and CT) and Sex were used as between-subject factors. For the elevated plus maze, EPM measures (%Time OA and %Entries OA) were considered the within-subject factor. Separate ANOVAs on Entries CA and Time Cen were carried out. For the open field, OF measures (Ambulation OF, Time Cen OF) were considered the within-subject factor. For the FST, an ANOVA on Immob Time was carried out. For the passive avoidance, an ANOVA on the (L3 − L0)/L0 index was carried out.
Whenever the ANOVAs indicated treatment effects that differed among the different within-subject factors, brain regions, ages, and/or sexes, data were then re-examined separately using lower order ANOVAs. All data were compiled as means and standard errors. Data were log transformed whenever variance was heterogeneous. All statistical results were described in the Results section. However, to avoid repetition, only results from the lower order tests were provided in the figures. Figures were segmented by sex only when significant Treatment × Sex interactions were observed. Significance was assumed at the level of p < 0.05 for main effects; however, for interactions at p < 0.1, we also examined whether lower order main effects were detectable after subdivision of the interactive factors (Snedecor and Cochran, 1967). The criterion for interaction terms was not used to assign significance to the effects but rather to identify interactive factors requiring subdivision for lower order tests of main effects of Treatment, the factor of chief interest (Snedecor and Cochran, 1967). The effects of MET exposure were presented as the percentage change from the corresponding CT group, but statistical evaluations involved only the original data; for reference, the CT values for all variables are provided in Table 1.
Table 1.
Control Values for Cholinergic and Serotonergic Biomarkers and for Behavioral Measures
| Cortex | ||||||
|---|---|---|---|---|---|---|
| PN10 | PN60 | |||||
| Female | Male | Combined | Female | Male | Combined | |
| AChE | 295.0±24.0 | 292.2±9.2 | 293.2±9.6 | 840.9±26.1 | 795.1±23.8 | 819.5±18.2 |
| ChAT | 0.23±0.02 | 0.22±0.01 | 0.22±0.01 | 1.40±0.05 | 1.26±0.06 | 1.33±0.04 |
| ChT | 43.4±1.6 | 44.2±1.5 | 43.8±1.1 | 103.5±4.6 | 111.2±3.7 | 107.3±3.0 |
| 5HT1A | 32.8±2.8 | 27.2±0.6 | 29.7±1.6 | 45.3±0.8 | 39.4±1.1 | 42.3±1.0 |
| 5HT2 | 29.3±1.5 | 23.2±0.9 | 25.9±1.3 | 59.6±1.7 | 56.4±2.0 | 58.0±1.3 |
| 5HTT | 139.3±3.6 | 141.1±7.6 | 140.3±4.3 | 183.2±5.3 | 172.0±7.2 | 177.6±4.5 |
| Brainstem | ||||||
| PN10 | PN60 | |||||
| Female | Male | Combined | Female | Male | Combined | |
| AChE | 432.7±16.7 | 385.9±4.9 | 401.5±8.5 | 100.5±3.5 | 97.5±2.2 | 98.9±2.0 |
| ChAT | 0.45±0.01 | 0.47±0.03 | 0.46±0.02 | 0.63±0.02 | 0.57±0.02 | 0.60±0.02 |
| ChT | 34.5±1.5 | 41.9±1.4 | 38.7±1.4 | 24.3±0.6 | 25.6±0.5 | 25.0±0.4 |
| 5HT1A | 12.9±1.2 | 14.7±0.7 | 14.1±0.7 | 23.2±0.7 | 22.1±0.7 | 22.7±0.5 |
| 5HT2 | 27.7±3.0 | 26.7±2.1 | 27.0±1.7 | 7.5±0.2 | 4.9±0.4 | 6.2±0.4 |
| 5HTT | 211.4±5.4 | 219.7±13.1 | 216.9±8.7 | 109.6±4.3 | 108.7±4.6 | 109.2±3.1 |
| Behavior | ||||
| Female | Male | Combined | ||
| EPM | %Time OA | 4.1±1.2 | 1.7±0.5 | 2.8±0.7 |
| %Entries OA | 10.5±2.9 | 6.8±2.1 | 8.6±1.8 | |
| Time Cen | 82.8±10.9 | 82.5±9.7 | 82.6±7.2 | |
| Entries CA | 9.6±1.2 | 8.9±0.9 | 9.2±0.7 | |
| OF | Ambulation OF | 1716.9±149.7 | 1830.5±151.8 | 1763.2±106.7 |
| Time Cen OF | 32.4±4.5 | 31.3±6.3 | 31.9±3.6 | |
| FST | Immob Time | 7.8±4.6 | 22.9±8.5 | 15.7±5.2 |
| PA | (L3 − L0)/L0 | 2.3±0.7 | 1.3±0.3 | 1.9±0.5 |
Note. Neurochemical biomarkers: AChE (nmoles/min/mg protein); ChAT (fmol/mg protein/min); ChT, 5HT1A, 5HT2, and 5HTT (fmol/mg protein). Behavioral measures: Time Cen (s); Ambulation (cm); Immob Time (s). PA, passive avoidance test; %Time OA, percentage of time spent in the open arms; %Entries OA, percentage of entries in the open arms; EntriesCA, number of entries in the closed arms; Time Cen, time spent in the center; Ambulation, traveled distance; Immob Time, immobility time; (L3 − L0)/L0, memory/learning index.
Results
Methamidophos Dose Selection (Table 2)
Table 2.
Acetylcholinesterase Activity During Methamidophos Dose Selection (sc Injected) and at Different Time Points After the Selection of the IntD for the Remainder of the Study
| Dose selection (AChE inhibition at PN10) | |||
|---|---|---|---|
| LowD | IntD | HighD | |
| Cortex | −3.5±3.1 | −19.9±1.7*** | −33.4±1.6*** |
| Brainstem | −9.8±2.9** | −18.7±2.3*** | −26.2±2.1*** |
| AChE inhibition using the IntD | |||||
| PN3 (first injection) | PN9 (last injection) | PN63 (adulthood) | |||
| 1h after injection | 4h after injection | 1h after injection | 4h after injection | ||
| Cortex | −43.4±6.6** | −41.6±4.3** | −53.8±10.3** | −67.2±6.8** | −4.5±2.1 |
| Brainstem | −29.3±7.6** | −50.2±3.6*** | −52.3±5.4*** | −53.5±3.6** | −3.1±2.7 |
Note. Data presented as percent change from control values. Bold indicates the dose chosen to be used in the next set of experiments. HighD (3mg/kg), IntD (1mg/kg), LowD (0.25mg/kg).
**p < 0.01, ***p < 0.001, versus respective control group.
The ANOVA on all factors (Dose, Brain Region, and Sex) identified a dose-dependent increase in inhibition of AChE at PN10 (Dose: F 3,109 = 61.0, p < 0.001). The IntD and HighD elicited significant inhibition compared with the CT group in the brainstem and cortex (p < 0.001 for all comparisons), whereas the LowD elicited a significant inhibition only in the brainstem (p = 0.005). The IntD produced 20.0% of AChE inhibition in the cerebral cortex and 18.7% inhibition in the brainstem. There were no differences in AChE inhibition between males and females. Accordingly, the IntD of methamidophos was chosen to be used in the next set of experiments, in which other aspects of the cholinergic system, the serotonergic system, and animals behavior were evaluated.
The analysis of AChE inhibition 1 or 4h after the first (PN3) or last (PN9) injection of the IntD of methamidophos elicited higher levels of AChE inhibition during the period of exposure. At PN3 and PN9, the ANOVAs on all factors (Treatment, Brain Region, Time, and Sex) indicated that methamidophos elicited comparable inhibition in both brain regions, at the two moments after injection and in both sexes (PN3—Treatment: F 1,32 = 76.5, p < 0.001; PN9—Treatment: F 1,32 = 43.9, p < 0.001). At PN3, in the cerebral cortex, AChE inhibition was of 43.4% 1h after the first injection and 41.6% 4h after the first injection (p < 0.01 for both comparisons). In the brainstem, 1h after the injection, the mean inhibition was of 29.3% and reached 50.2% at 4h (p < 0.01 and p < 0.001, respectively). At PN9, 1h after the last injection, AChE inhibition in the cerebral cortex was of 53.8% and reached 67.2% at 4h (p < 0.01 for both comparisons). In the brainstem, AChE inhibition was of 52.3% 1h after the last injection and 53.5% 4h after the injection (p < 0.001 and p < 0.01, respectively).
AChE activity had returned to control levels at adulthood.
Effects on Body Mass (Fig. 1)
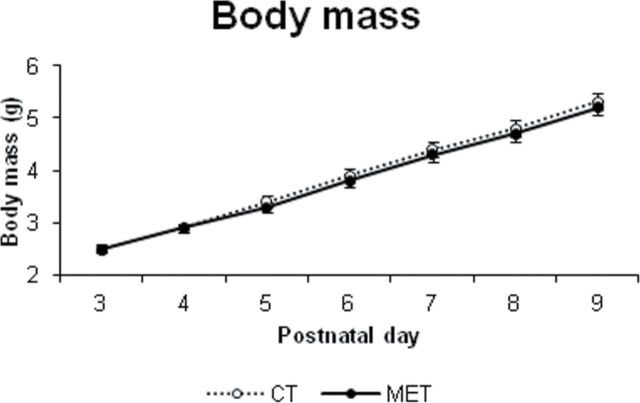
Fig. 1.
Effects of neonatal methamidophos exposure (1mg/kg/day sc) on body mass during the period of exposure. Values are means ± SEM. MET, methamidophos exposure group.
Body mass significantly increased throughout the exposure period (Day: F 6,342 = 927.1; p < 0.001). However, the rANOVA did not indicate significant Treatment effects or interactions.
Overall Analysis of Cholinergic and Serotonergic Biomarkers
The rANOVA across cholinergic biomarkers (ChAT and Ch transporter), treatments, brain regions, ages, and sexes identified interactions of Treatment × Age (F 1,142 = 4.2; p = 0.04), Treatment × Cholinergic measure × Sex (F1,142 = 4.9; p = 0.03), and Treatment × Cholinergic measure × Region × Age (F1,142 = 2.9; p = 0.09). The rANOVA across the serotonergic biomarkers (5HT1A receptor, 5HT2 receptor, and 5HT transporter), treatments, brain regions, ages and sexes identified interactions of Treatment × Age × Sex (F 1,119 = 3.8; p = 0.05), Treatment × Region × Age × Sex (F 1,119 = 3.3, p = 0.07), Treatment × Serotonergic measure (F 2,238 = 4.3, p = 0.02), Treatment × Serotonergic measure × Age × Sex (F 2,238 = 2.9; p = 0.05), and Treatment × Serotonergic measure × Region × Age × Sex (F 2,238 = 3.7; p = 0.03). Given the interactions, identified in both rANOVAs, we separated the data into the individual cholinergic and serotonergic measures, brain regions, and ages and then re-examined the results. After subdividing the data, we kept the factor Sex in the analysis.
Effects on the Cholinergic and Serotonergic Systems During Early Postnatal Development (Fig. 2)
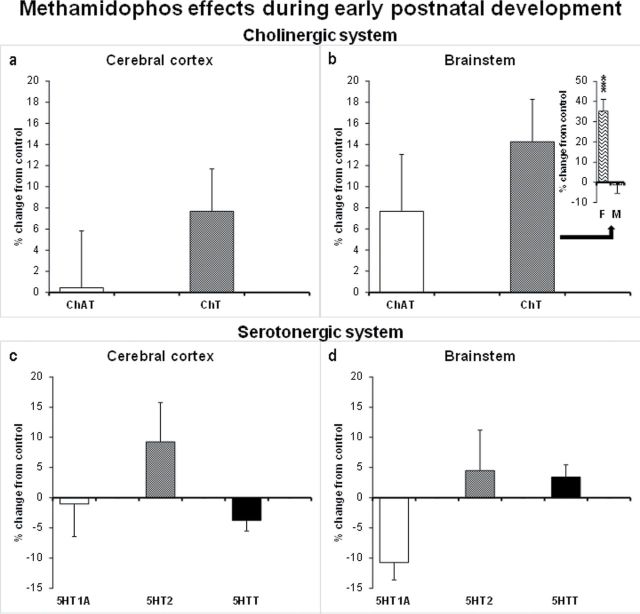
Fig. 2.
Effects of neonatal methamidophos exposure (1mg/kg/day sc, from PN3 to PN9) during early postnatal development presented as percent change from control values. ChAT activity and Ch transporter binding in the cerebral cortex (a) and brainstem (b). In the inset, the data pertaining to Ch transporter binding are separated by sex. 5HT1A, 5HT2, and 5HT transporter in the cerebral cortex (c) and brainstem (d). Values are means ± SEM. For each treatment group, 16 animals were examined, equally divided into males and females. 5HT1A; serotonin receptor subtype 1A; 5HT2 serotonin receptor subtype 2; 5HTT, serotonin presynaptic transporter; F, female; M, male. ***p < 0.001, versus respective control group.
Methamidophos exposure elicited sex-dependent effects on Ch transporter binding in the brainstem at PN10 (Treatment: F 1,27 = 11.5, p = 0.002; Treatment × Sex: F 1,27 = 13.6, p = 0.001). Separate analyses for males and females indicated significant effects for females only: Methamidophos increased Ch transporter binding (F 1,13 = 21.6, p < 0.001). There were no significant effects in the cortex. As for ChAT activity and the serotonergic measures, there were no significant effects.
Effects on the Cholinergic and Serotonergic Systems at Adulthood (Fig. 3)
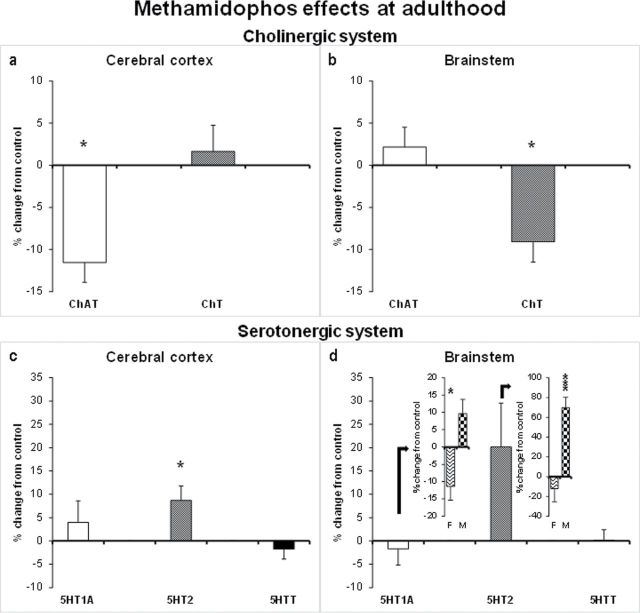
Fig. 3.
Effects of neonatal methamidophos exposure (1mg/kg/day sc, from PN3 to PN9) at adulthood presented as percent change from control values. ChAT activity and Ch transporter binding in the cerebral cortex (a) and brainstem (b). 5HT1A, 5HT2, and 5HT transporter in the cerebral cortex (c) and brainstem (d). In the inset, the data pertaining to 5HT1A and 5HT2 are separated by sex. Values are means ± SEM. For each treatment group, 16 animals were examined, equally divided into males and females. 5HT1A; serotonin receptor subtype 1A; 5HT2 serotonin receptor subtype 2; 5HTT, serotonin presynaptic transporter; F, female; M, male. *p < 0.05, ***p < 0.001, versus respective control group.
Early methamidophos exposure elicited a late-emergent decrease in ChAT activity in the cortex (Treatment: F 1,27 = 8.7, p = 0.006) but not in the brainstem. Regarding Ch transporter, there was a decrease in binding in the brainstem (Treatment: F 1,28 = 9.7, p = 0.004) but not in the cortex.
There were sex-dependent effects on 5HT1A receptor binding in the brainstem (Treatment × Sex: F 1,27 = 8.5, p = 0.007). Separate analyses for males and females indicated a significant methamidophos-elicited decrease for females (F 1,14 = 5.2, p = 0.03) and a trend toward increased binding for males (F 1,13 = 3.3, p = 0.08). There were no significant effects in the cortex. As for 5HT2 receptor binding, there were significant increases both in the cortex (Treatment: F 1,27 = 4.8, p = 0.03) and brainstem (Treatment: F 1,28 = 4.2, p = 0.04; Treatment × Sex: F 1,28 = 12.7, p = 0.001). However, in the brainstem, the increase in binding was restricted to males (F 1,14 = 28.8, p < 0.001). As for the 5HT transporter, there were no significant effects at adulthood.
Effects on Behavior at Adulthood (Fig. 4)
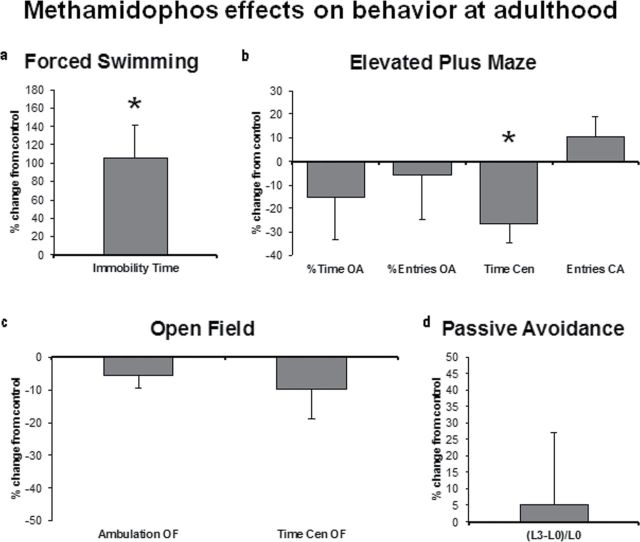
Fig. 4.
Effects of neonatal methamidophos exposure (1mg/kg/day sc, from PN3 to PN9) on behavior at adulthood presented as percent change from control values. In (a), immobility time in the forced swimming test. In (b), anxiety-like measures (%Time OA and % Entries OA), decision making (Time Cen), and activity (Entries OA) measures in the EPM. In (c), locomotor activity (Ambulation OF) and anxiety-like (Time Cen OF) measures in the open field. In (d), memory/learning ([L3 − L0]/L0) in the step-down passive avoidance test. Values are means ± SEM. For each treatment group, 20–36 animals were examined, divided into males and females. EPM measures: %Time OA, time spent in open arms divided by time spent in open + closed arms; %Entries OA, number of entries in open arms divided by number of entries in open + closed arms; Time Cen, time spent in the center; Entries CA, number of closed arms entries. Open field measures: Ambulation OF, traveled distance; Time Cen OF, time spent in the center. Passive avoidance: (L3 − L0)/L0, memory/learning index based on the latency (L) to descend from the platform on the first (L0) and second (L3) sessions. *p < 0.05 versus respective control group.
In the forced swimming test, the ANOVA demonstrated that early methamidophos exposure elicited an increase in immobility time (Treatment: F 1,44 = 5.05, p = 0.02), suggestive of depressive-like behavior (Fig. 4a). Methamidophos failed to elicit significant alterations on all anxiety-related measures: There were no differences between groups in %Time OA and %Entries OA in the EPM (Fig. 4b), and these results were corroborated by the lack of alteration in the time spent in the center of the OF (Time Cen OF) (Fig. 4c). Despite the lack of effects in most measures obtained in the EPM, methamidophos elicited a decrease in the time spent in the center of the maze (Time Cen—Treatment: F 1,63 = 5.5, p = 0.02), which indicates less time spent in the cognitive task of choosing which arm (open or closed) to go into (Fig. 4b). Locomotor activity was also not affected by methamidophos exposure both in the EPM (Entries CA, Fig. 4b) and in the OF (Ambulation OF, Fig. 4c). There were no significant alterations on memory/learning ([L3 − L0]/L0) as assessed in the passive avoidance test (Fig. 4d).
Discussion
In this study, methamidophos exposure during the “brain growth spurt” was effective in producing immediate and late-emergent alterations in cholinergic and serotonergic synaptic markers and in eliciting behavioral effects suggestive of depressive-like behavior and impaired decision making in adult mice.
Methamidophos elicited time-dependent and region-selective effects, which may be associated to the fact that distinct regions have specific timetables of maturation. Distinct populations originate from different brain areas that undergo differentiation processes with widely varying schedules (Rodier, 1988). As an example, each component of the cholinergic system follows a characteristic and region-specific developmental plan (Abreu-Villaça et al., 2011); therefore, it is expected that methamidophos elicits effects that are specific to each component and brain region. Moreover, dissimilar immediate and late-emergent effects reinforce the idea that developmental exposure to OPs can act either producing immediate effects or programing changes that may appear later during development or at adulthood (Ahlbom et al., 1995; Aldridge et al., 2005a; Dam et al., 2000; Levin et al., 2010; Slotkin et al., 2001) and suggest that distinct mechanisms of action are responsible for the immediate and delayed effects. Finally, the selective effects were evident with similar AChE inhibition in both brain regions and even after full recovery of AChE activity at adulthood, which gives support to previous evidence of noncholinesterase effects of OPs (Ahlbom et al., 1995; Aldridge et al., 2005b; Levin et al., 2010; Slotkin and Seidler, 2007, 2008; Slotkin et al., 2006b, 2008c).
Our results were also dependent on the sex. Descriptions of sex differences are common when it comes to the effects of OP exposure on developing animals (Aldridge et al., 2005b; Johnson et al., 2009; Slotkin et al., 2008c). These results, together with ours, reinforce the multiplicity of effects of OPs during development and their capacity to affect several parameters of the cholinergic and serotonergic systems with sex selectivity. The time course of the GABAergic system during development may, in part, explain our sex-dependent effects. GABA per se has a trophic role in neuronal maturation, affecting neuronal migration and synapse formation; in addition, it was shown to modulate the expression of neurotrophic factors (for review: Represa and Ben-Ari, 2005). During the early postnatal development, there is a shift in GABAA receptor–mediated responses from excitatory to inhibitory, which, in turn, could alter the expression of neurotrophic factors (Berninger et al., 1995). This shift, which is dependent on cholinergic activation (Liu et al., 2006), occurs earlier in females compared with males (Nuñez and McCarthy, 2007). Therefore, it is possible that the increased cholinergic stimulation elicited by OPs, including methamidophos, differentially affects males and females. It is also possible that sex-selective actions of OPs are associated with their potential actions as endocrine disruptors. In this regard, chlorpyrifos exposure in the early postnatal period was shown to reduce aromatase activity, an enzyme responsible for a key step in the biosynthesis of estrogens (Buratti et al., 2011). To our knowledge, there are no studies that investigate methamidophos effects on pituitary and sex hormones during the perinatal period; however, there is evidence of significant reduction in testosterone levels (Maia et al., 2011) and increase of ACTH (Spassova et al., 2000) in rodents exposed to methamidophos at adulthood (Maia et al., 2011).
Methodological Issues
Methamidophos was dissolved in DMSO, which may constitute a cause of concern, even though all experimental groups were exposed to this substance, because there are reports of neurotoxic effects of this solvent. Interestingly, we failed to find studies describing an association between DMSO and behavioral or neurochemical alterations during the early postnatal development although, at adulthood, there is little evidence of DMSO-elicited histopathological and behavioral alterations at doses close to the one used in the present study (Authier et al., 2002; Kaye et al., 1983). These findings suggest that it is unlikely that DMSO exposure, in the present study, has affected the results.
Here, we chose two time points to perform the analyses. In the first time point (1 day postexposure), the biochemical findings were identified when AChE inhibition was at 20%, well below the minimum necessary to elicit cholinergic toxicity (Bignami et al., 1975; Slotkin, 2004). In the second time point (at adulthood), both biochemical and behavioral alterations were observed in spite of the fact that AChE activity had already returned to control levels. In spite of the low inhibition levels observed during these time points, during exposure, the IntD used in this study elicited higher levels of AChE inhibition, which is in accordance with previous evidence of rapid recovery of AChE inhibition in pups exposed to OPs, including methamidophos (Moser, 1999). These higher levels could have elicited systemic toxicity; however, the fact that inhibition levels surpassed the minimum necessary to elicit this adverse effect only at one brain region (cortex) and at one time point (4h after the last injection), together with the lack of effects in body weight during exposure, suggest that the IntD of methamidophos was devoid of cholinergic toxicity. This conclusion is consistent with those from a previous report in which rats were exposed to a similar dose of methamidophos at PN17 (Moser, 1999).
Effects on the Cholinergic and Serotonergic Systems During Early Postnatal Development
In this study, methamidophos exposure was able to increase Ch transporter binding in the brainstem of developing females. Considering that the Ch transporter binding is responsive to neuronal activity (Klemm and Kuhar, 1979; Simon et al., 1976), the increase in binding indicates increased cholinergic activity, which is consistent and may be directly related to the inhibition of AChE. The inhibition of AChE activity described here may elicit a significant decrease of choline in the synaptic clefts. Accordingly, the increased binding to the Ch transporter may indicate a compensatory response of the presynaptic cell as an effort to maintain physiological intracellular levels of choline to be used as substrate to ACh synthesis. In this regard, in rat brain synaptosomes, primary cultures from the basal forebrain, and mammalian cell lines transfected with Ch transporter, high levels of extracellular choline were shown to rapidly decrease cell surface Ch transporter expression by accelerating its internalization (Okuda et al., 2011). Accordingly, the inverse response is likely to occur, so that the inhibition of AChE and consequent reduction of choline in the synaptic clefts could be responsible for the increase of Ch transporter at the cholinergic presynaptic terminal. Besides this possibility, it was recently shown, in sympathetic ganglia from mice with a disruption in the α3 nAChR subunit gene and rescued fast synaptic transmission by overexpressing α3 cDNA, that the Ch transporter is induced by retrograde signals downstream of postsynaptic activity (Krishnaswamy and Cooper, 2009). In mice exposed to OPs, high ACh levels in the synaptic cleft may hyperstimulate the postsynaptic cell. Accordingly, we suggest that the increased postsynaptic activity may also retrogradely induce the increase of the Ch transporter. Even though there is evidence that the increase in Ch transporter by the end of methamidophos exposure is directly linked to the inhibition of AChE, it is unlikely that this is the sole mechanism that mediate our results because, despite similar levels of AChE inhibition in the brainstem and cortex, Ch transporter binding results were restricted to the brainstem and to females.
Previous reports have shown that chlorpyrifos, parathion, and diazinon elicit general alterations in the serotonergic system (Aldridge et al., 2003; Slotkin et al., 2006b), which is in accordance with previous findings that this system is especially sensitive to developmental disruption by OP exposure. In contrast, here we found that methamidophos was not able to affect the serotonergic system in the same way: There were no significant alterations in the serotonergic measures by the end of exposure. Despite methodological differences between previous studies and the present one (e.g., rats vs. mice; distinct length of postnatal exposure), differences between the effects of methamidophos compared with other OPs reinforce the conclusion that various OPs diverge in their effects on neurodevelopment.
Effects on the Cholinergic and Serotonergic Systems at Adulthood
At adulthood, long after the end of exposure and after recovery of AChE activity, the binding to the Ch transporter was decreased in the brainstem in both males and females, which is suggestive of a late-emergent decrease in cholinergic activity. Neonatal exposure to parathion and chlorpyrifos was also shown to evoke Ch transporter binding decrements in the brain; however, there were region- and sex-dependent effects distinct from those identified after methamidophos exposure (Slotkin et al., 2001, 2008b). In addition, regarding ChAT activity, despite the lack of significant alterations by the end of methamidophos exposure, ChAT was decreased in the cortex at adulthood. This result suggests that methamidophos programs a reduction in the density of cholinergic terminals and corroborates Slotkin and collaborators’ (2008a, b) findings associated to parathion or diazinon exposures but not to chlorpyrifos (Slotkin et al., 2001) exposure.
Late-emergent effects in the serotonergic system included a decrease in 5HT1A binding in the brainstem of females and an increase in 5HT2 binding in the cortex (males and females) and in the brainstem (males). Despite the fact that the direction of the receptor alterations and the magnitude of the effects may be different among distinct OPs (Aldridge et al., 2004; Slotkin and Seidler, 2008; Slotkin et al., 2009), the increase in 5HT2 binding is consistent with previous findings in postmortem studies of suicide victims (Pandey et al., 2002). Additionally, previous findings of decreased expression of the mRNA encoding the 5HT1A receptor in patients with major depression (López-Figueroa et al., 2004) and of reduced 5HT1A receptor binding in suicide victims (Savitz et al., 2009) present similarities to the late-emergent decrease in 5HT1A binding in mice exposed to methamidophos. However, our findings were identified only in females, which indicates that alterations found in our model do not completely parallel neurochemical alterations described in humans. It should be noted that receptor binding effects may not be the only alterations associated with OP exposure. In this regard, a previous study reported altered expression of genes that encode the enzymes of 5HT synthesis, storage, and degradation in undifferentiated and differentiating PC12 cells exposed to chlorpyrifos or diazinon (Slotkin and Seidler, 2008).
Altogether both cholinergic and serotonergic results indicate that despite restricted similarities, distinct OPs are able to elicit dissimilar effects. These may be associated to OP selective interference with cell functioning. In this regard, targets for OPs such as cell signaling mediated by adenylyl cyclase (Meyer et al., 2003; Song et al., 1997), the m2 muscarinic receptor (Slotkin et al., 2006a), lipases (Casida et al., 2008), and microtubule proteins (Jiang et al., 2010) have been identified.
Behavioral Effects
Cholinergic and serotonergic alterations in methamidophos-exposed mice were accompanied by an increase in immobility time in the forced swimming test, indicative of depressive-like behavior at adulthood. This result shows similarities to those of other OPs (Aldridge et al., 2005a; Roegge et al., 2008); however, methamidophos effects in behavior were specific; there were no anxiety and memory/learning alterations. Considering that there is evidence that serotonergic and cholinergic alterations could underlie disturbances observed in depression (for review: Carr and Lucki, 2011; Dagytė et al., 2011; Graef et al., 2011), the increased immobility time long after the end of methamidophos exposure may, in part, be explained by the neurotransmitter system alterations described here. In this regard, because the depressive-like behavior was significant in both males and females, while several neurochemical findings were sex dependent, it is likely that other effects of methamidophos exposure also play a role in the behavioral findings. Future investigation of serotonergic and other alterations elicited by methamidophos exposure may provide a more complete picture of the consequences of early methamidophos exposure.
Methamidophos was also able to elicit a decrease in the time spent in the center of the EPM. Even though there is no consensus regarding the meaning of this measure (Wall and Messier, 2001), considering that anxiolytics reduce time spent in the center of the maze (Cruz et al., 1994), our results could indicate reduced anxiety levels due to methamidophos exposure. However, this possibility should be considered with caution because it was not supported by the classic and most frequently used measures to evaluate anxiety in this test. There is also evidence that time in the center of the maze reflects decision making, perhaps related to approach/avoid conflict (Rodgers and Johnson, 1995; Rodgers et al., 1997). The choice between two alternatives (in this case the open and closed arms of the maze) was described as a cognitive function based on information collected from the environment, so that a decision is made when a threshold of neuronal activity is reached (for review: Gold and Shadlen, 2007). If this is the case, the reduced time spent in the center of the maze seems to indicate that, in methamidophos-exposed mice, a decision is made without sufficient data from the environment. Interestingly, there is a clear link between altered decision making in humans and neuropsychiatric disorders including major depression (Cella et al., 2010), as well as suicidal behavior (Jollant et al., 2005). In addition, both depression and decision making seem to be modulated by the serotonergic function (Carr and Lucki, 2011; Rogers et al., 2003).
Conclusions
We investigated in mice the effects of methamidophos on the “brain growth spurt” period of brain development. The present results lead to several inferences. The first one is that despite the presence of cholinergic alterations due to early postnatal methamidophos exposure, these changes do not necessarily imply on similar alterations at adulthood. That is, the effects of OP exposure on the cholinergic system during the neonatal period may occur through pathways other than those that culminate on later alterations both in the cholinergic and serotonergic systems. The second one is the reinforcement of evidence that the pattern of effects elicited by an OP is highly dependent on the developmental stage. Interestingly, in a previous study we observed an overall decrease of the 5HT markers analyzed here after a subchronic exposure to methamidophos at adulthood (Lima et al., 2011). These alterations were observed even at doses of exposure that caused only 15% of brain AChE inhibition. Finally, the third inference is that early methamidophos exposure is able to elicit delayed, mood-related effects, suggestive of depressive-like behavior and impaired decision making, a cognitive task, at adulthood. Overall these results indicate that, as previously described for other OPs, methamidophos exposure during the early postnatal period may be deleterious to the developing brain. Finally, neurochemical effects and evidence of mood and cognitive alterations at adulthood further indicate harmful effects throughout life.
Funding
Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro—FAPERJ (E26/111.160/2011, E26/103.029/2008 to Y.A.-V., C.C.F., and A.C.M.); and fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq and FAPERJ (C.S.L.); Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior—CAPES (F.N., A.L.N.-F.). Y.A.-V. and A.M. are Irving J. Selikoff International Scholars of the Mount Sinai School of Medicine. Their work was supported in part by an Award Number D43TW000640 from the Fogarty International Center.
Acknowledgments
The authors are thankful to Ulisses Risso for animal care. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or the National Institutes of Health. C.S.L., A.C.D.-T., F.N., A.L.N.-F., A.R.-C., C.C.F., A.C.M., A.M., and Y.A.-V. report no potential conflicts of interest related to this report.
References
- Abreu-Villaça Y., Filgueiras C. C., Manhães A. C. (2011). Developmental aspects of the cholinergic system. Behav. Brain Res. 221, 367–378 [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y., Graça A. C. C., Ribeiro-Carvalho A., Naiff V. F., Manhães A. C., Filgueiras C. C. (2013). Combined exposure to tobacco smoke and ethanol in adolescent mice elicits memory and learning deficits both during exposure and withdrawal. Nic. Tob. Res. In press. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y., Nunes F., do E Queiroz-Gomes F., Manhães A. C., Filgueiras C. C. (2008). Combined exposure to nicotine and ethanol in adolescent mice differentially affects anxiety levels during exposure, short-term, and long-term withdrawal. Neuropsychopharmacology. 33, 599–610 [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y., Seidler F. J., Qiao D., Tate C. A., Cousins M. M., Thillai I., Slotkin T. A. (2003). Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: Critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 28, 1935–1949 [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y., Seidler F. J., Tate C. A., Cousins M. M., Slotkin T. A. (2004). Prenatal nicotine exposure alters the response to nicotine administration in adolescence: Effects on cholinergic systems during exposure and withdrawal. Neuropsychopharmacology. 29, 879–890 [DOI] [PubMed] [Google Scholar]
- Ahlbom J., Fredriksson A., Eriksson P. (1995). Exposure to an organophosphate (DFP) during a defined period in neonatal life induces permanent changes in brain muscarinic receptors and behaviour in adult mice. Brain Res. 677, 13–19 [DOI] [PubMed] [Google Scholar]
- Aldridge J. E., Levin E. D., Seidler F. J., Slotkin T. A. (2005a). Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ. Health Perspect. 113, 527–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge J. E., Meyer A., Seidler F. J., Slotkin T. A. (2005b). Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ. Health Perspect. 113, 1027–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge J. E., Seidler F. J., Meyer A., Thillai I., Slotkin T. A. (2003). Serotonergic systems targeted by developmental exposure to chlorpyrifos: Effects during different critical periods. Environ. Health Perspect. 111, 1736–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge J. E., Seidler F. J., Slotkin T. A. (2004). Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: Critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ. Health Perspect. 112, 148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agência Nacional de Vigilância Sanitária (ANVISA) (2004). Suspensos inseticidas que trazem risco à saúde. ANVISA, Brasília, Brazil: [Google Scholar]
- Agência Nacional de Vigilância Sanitária, Diário Oficial da União (ANVISA) (2011). Resolução- RDC Nº1. ANVISA, Brasília, Brazil: [Google Scholar]
- Arango V., Underwood M. D., Boldrini M., Tamir H., Kassir S. A., Hsiung S., Chen J. J., Mann J. J. (2001). Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 25, 892–903 [DOI] [PubMed] [Google Scholar]
- Aubert I., Cécyre D., Gauthier S., Quirion R. (1996). Comparative ontogenic profile of cholinergic markers, including nicotinic and muscarinic receptors, in the rat brain. J. Comp. Neurol. 369, 31–55 [DOI] [PubMed] [Google Scholar]
- Authier N., Dupuis E., Kwasiborski A., Eschalier A., Coudoré F. (2002). Behavioural assessment of dimethylsulfoxide neurotoxicity in rats. Toxicol. Lett. 132, 117–121 [DOI] [PubMed] [Google Scholar]
- Azmitia E. C. (2001). Modern views on an ancient chemical: Serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res. Bull. 56, 413–424 [DOI] [PubMed] [Google Scholar]
- Bandeira F., Lent R., Herculano-Houzel S. (2009). Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc. Natl. Acad. Sci. U. S. A. 106, 14108–14113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer S. A., Altman J., Russo R. J., Zhang X. (1993). Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 14, 83–144 [PubMed] [Google Scholar]
- Berninger B., Marty S., Zafra F., da Penha Berzaghi M., Thoenen H., Lindholm D. (1995). GABAergic stimulation switches from enhancing to repressing BDNF expression in rat hippocampal neurons during maturation in vitro. Development. 121, 2327–2335 [DOI] [PubMed] [Google Scholar]
- Bignami G., Rosı′c N., Michałek H., Milosˇevic′ M., Gatti G. L. (1975). Behavioral toxicity of anticholinesterase agents: Methodological, neurochemical, and neuropsychological aspects. In Behavioral Toxicology. (Weiss B., Laties V. G, Eds.), pp. 155–216 Plenum Press, New York, NY: [Google Scholar]
- Buratti F. M., De Angelis G., Ricceri L., Venerosi A., Calamandrei G., Testai E. (2011). Foetal and neonatal exposure to chlorpyrifos: Biochemical and metabolic alterations in the mouse liver at different developmental stages. Toxicology. 280, 98–108 [DOI] [PubMed] [Google Scholar]
- Carr G. V., Lucki I. (2011). The role of serotonin receptor subtypes in treating depression: A review of animal studies. Psychopharmacology (Berl). 213, 265–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida J. E., Nomura D. K., Vose S. C., Fujioka K. (2008). Organophosphate-sensitive lipases modulate brain lysophospholipids, ether lipids and endocannabinoids. Chem. Biol. Interact. 175, 355–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Dymond S., Cooper A. (2010). Impaired flexible decision-making in Major Depressive Disorder. J. Affect. Disord. 124, 207–210 [DOI] [PubMed] [Google Scholar]
- Clancy B., Finlay B. L., Darlington R. B., Anand K. J. (2007). Extrapolating brain development from experimental species to humans. Neurotoxicology. 28, 931–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A. P., Frei F., Graeff F. G. (1994). Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol. Biochem. Behav. 49, 171–176 [DOI] [PubMed] [Google Scholar]
- Dagytė G., Den Boer J. A., Trentani A. (2011). The cholinergic system and depression. Behav. Brain Res. 221, 574–582 [DOI] [PubMed] [Google Scholar]
- Dam K., Seidler F. J., Slotkin T. A. (2000). Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Brain Res. Dev. Brain Res. 121, 179–187 [DOI] [PubMed] [Google Scholar]
- Dobbing J., Sands J. (1979). Comparative aspects of the brain growth spurt. Early Hum. Dev. 3, 79–83 [DOI] [PubMed] [Google Scholar]
- Dribben W. H., Creeley C. E., Farber N. (2011). Low-level lead exposure triggers neuronal apoptosis in the developing mouse brain. Neurotoxicol. Teratol. 33, 473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J. B., Broide R. S., Leslie F. M. (2008). Nicotine and brain development. Birth Defects Res. C. Embryo Today. 84, 30–44 [DOI] [PubMed] [Google Scholar]
- Ellman G. L., Courtney K. D., Andres V., Jr, Feather-Stone R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7, 88–95 [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAOSTAT) (2010). Available at: http://faostat3.fao.org/home/index.html#DOWNLOAD Accessed March 15, 2013
- Filgueiras C. C., Abreu-Villaça Y., Krahe T. E., Manhães A. C. (2006). Unilateral hemispherectomy at adulthood asymmetrically affects immobile behavior of male Swiss mice. Behav. Brain Res. 172, 33–38 [DOI] [PubMed] [Google Scholar]
- Filgueiras C. C., Ribeiro-Carvalho A., Nunes F., Abreu-Villaça Y., Manhães A. C. (2009). Early ethanol exposure in mice increases laterality of rotational side preference in the free-swimming test. Pharmacol. Biochem. Behav. 93, 148–154 [DOI] [PubMed] [Google Scholar]
- Flaskos J. (2012). The developmental neurotoxicity of organophosphorus insecticides: A direct role for the oxon metabolites. Toxicol. Lett. 209, 86–93 [DOI] [PubMed] [Google Scholar]
- Fraga M. C., Moura E. G., Silva J. O., Bonomo I. T., Filgueiras C. C., Abreu-Villaça Y., Passos M. C., Lisboa P. C., Manhães A. C. (2011). Maternal prolactin inhibition at the end of lactation affects learning/memory and anxiety-like behaviors but not novelty-seeking in adult rat progeny. Pharmacol. Biochem. Behav. 100, 165–173 [DOI] [PubMed] [Google Scholar]
- Fujita M., Charney D. S., Innis R. B. (2000). Imaging serotonergic neurotransmission in depression: Hippocampal pathophysiology may mirror global brain alterations. Biol. Psychiatry. 48, 801–812 [DOI] [PubMed] [Google Scholar]
- Gold J. I., Shadlen M. N. (2007). The neural basis of decision making. Annu. Rev. Neurosci. 30, 535–574 [DOI] [PubMed] [Google Scholar]
- Graef S., Schönknecht P., Sabri O., Hegerl U. (2011). Cholinergic receptor subtypes and their role in cognition, emotion, and vigilance control: An overview of preclinical and clinical findings. Psychopharmacology (Berl). 215, 205–229 [DOI] [PubMed] [Google Scholar]
- Gupta R. C. (2004). Brain regional heterogeneity and toxicological mechanisms of organophosphates and carbamates. Toxicol. Mech. Methods. 14, 103–143 [DOI] [PubMed] [Google Scholar]
- Happe H. K., Murrin L. C. (1992). High-affinity choline transport regulation by drug administration during postnatal development. J. Neurochem. 58, 2053–2059 [DOI] [PubMed] [Google Scholar]
- Jiang W., Duysen E. G., Hansen H., Shlyakhtenko L., Schopfer L. M., Lockridge O. (2010). Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Toxicol. Sci. 115, 183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F. O., Chambers J. E., Nail C. A., Givaruangsawat S., Carr R. L. (2009). Developmental chlorpyrifos and methyl parathion exposure alters radial-arm maze performance in juvenile and adult rats. Toxicol. Sci. 109, 132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollant F., Bellivier F., Leboyer M., Astruc B., Torres S., Verdier R., Castelnau D., Malafosse A., Courtet P. (2005). Impaired decision making in suicide attempters. Am. J. Psychiatry. 162, 304–310 [DOI] [PubMed] [Google Scholar]
- Kaye T. S., Egorin M. J., Riggs C. E., Jr, Olman E. A., Chou F. T., Salcman M. (1983). The plasma pharmacokinetics and tissue distribution of dimethyl sulfoxide in mice. Life Sci. 33, 1223–1230 [DOI] [PubMed] [Google Scholar]
- Klemm N., Kuhar M. J. (1979). Post-mortem changes in high affinity choline uptake. J. Neurochem. 32, 1487–1494 [DOI] [PubMed] [Google Scholar]
- Krishnaswamy A., Cooper E. (2009). An activity-dependent retrograde signal induces the expression of the high-affinity choline transporter in cholinergic neurons. Neuron. 61, 272–286 [DOI] [PubMed] [Google Scholar]
- Levin E. D., Timofeeva O. A., Yang L., Petro A., Ryde I. T., Wrench N., Seidler F. J., Slotkin T. A. (2010). Early postnatal parathion exposure in rats causes sex-selective cognitive impairment and neurotransmitter defects which emerge in aging. Behav. Brain Res. 208, 319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima C. S., Nunes-Freitas A. L., Ribeiro-Carvalho A., Filgueiras C. C., Manhães A. C., Meyer A., Abreu-Villaça Y. (2011). Exposure to methamidophos at adulthood adversely affects serotonergic biomarkers in the mouse brain. Neurotoxicology. 32, 718–724 [DOI] [PubMed] [Google Scholar]
- Lima C. S., Ribeiro-Carvalho A., Filgueiras C. C., Manhães A. C., Meyer A., Abreu-Villaça Y. (2009). Exposure to methamidophos at adulthood elicits depressive-like behavior in mice. Neurotoxicology. 30, 471–478 [DOI] [PubMed] [Google Scholar]
- Liu Z., Neff R. A., Berg D. K. (2006). Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 314, 1610–1613 [DOI] [PubMed] [Google Scholar]
- López-Figueroa A. L., Norton C. S., López-Figueroa M. O., Armellini-Dodel D., Burke S., Akil H., López J. F., Watson S. J. (2004). Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol. Psychiatry. 55, 225–233 [DOI] [PubMed] [Google Scholar]
- Maes M., Meltzer H. (1995). The serotonin hypothesis of major depression. In Psychopharmacology: The Fourth Generation of Progress. (Bloom F. E., Kupfer D. J., Bunney B. S., Ciaranello R. D., Davis K. L., Koob G. F., Meltzer H. Y., Schuster C. R., Shader R. I., Watson S. J, Eds.), pp. 933–944 Raven Press, New York, NY: [Google Scholar]
- Maia L. O., Júnior W. D., Carvalho L. S., Jesus L. R., Paiva G. D., Araujo P., Costa M. F., Andersen M. L., Tufik S., Mazaro-Costa R. (2011). Association of methamidophos and sleep loss on reproductive toxicity of male mice. Environ. Toxicol. Pharmacol. 32, 155–161 [DOI] [PubMed] [Google Scholar]
- Meyer A., Seidler F. J., Cousins M. M., Slotkin T. A. (2003). Developmental neurotoxicity elicited by gestational exposure to chlorpyrifos: When is adenylyl cyclase a target? Environ. Health Perspect. 111, 1871–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileson B. E., Chambers J. E., Chen W. L., Dettbarn W., Ehrich M., Eldefrawi A. T., Gaylor D. W., Hamernik K., Hodgson E., Karczmar A. G., et al. (1998). Common mechanism of toxicity: A case study of organophosphorus pesticides. Toxicol. Sci. 41, 8–20 [DOI] [PubMed] [Google Scholar]
- Moser V. C. (1999). Comparison of aldicarb and methamidophos neurotoxicity at different ages in the rat: Behavioral and biochemical parameters. Toxicol. Appl. Pharmacol. 157, 94–106 [DOI] [PubMed] [Google Scholar]
- Nemeroff C. B. (1998). The neurobiology of depression. Sci. Am. 278, 42–49 [DOI] [PubMed] [Google Scholar]
- Nunes F., Ferreira-Rosa K., Pereira Mdos. S., Kubrusly R. C., Manhães A. C., Abreu-Villaça Y., Filgueiras C. C. (2011). Acute administration of vinpocetine, a phosphodiesterase type 1 inhibitor, ameliorates hyperactivity in a mice model of fetal alcohol spectrum disorder. Drug Alcohol Depend. 119, 81–87 [DOI] [PubMed] [Google Scholar]
- Nunes-Freitas A. L., Ribeiro-Carvalho A., Lima C. S., Dutra-Tavares A. C., Manhães A. C., Lisboa P. C., Oliveira E., Gaspar de Moura E., Filgueiras C. C., Abreu-Villaça Y. (2011). Nicotine exposure during the third trimester equivalent of human gestation: Time course of effects on the central cholinergic system of rats. Toxicol. Sci. 123, 144–154 [DOI] [PubMed] [Google Scholar]
- Nuñez J. L., McCarthy M. M. (2007). Evidence for an extended duration of GABA-mediated excitation in the developing male versus female hippocampus. Dev. Neurobiol. 67, 1879–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D. J. (2002). The neuropharmacology of serotonin and noradrenaline in depression. Int. Clin. Psychopharmacol. 17 Suppl 1, S1–12 [DOI] [PubMed] [Google Scholar]
- Okuda T., Konishi A., Misawa H., Haga T. (2011). Substrate-induced internalization of the high-affinity choline transporter. J. Neurosci. 31, 14989–14997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey G. N., Dwivedi Y., Rizavi H. S., Ren X., Pandey S. C., Pesold C., Roberts R. C., Conley R. R., Tamminga C. A. (2002). Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am. J. Psychiatry. 159, 419–429 [DOI] [PubMed] [Google Scholar]
- Pohl-Guimaraes F., Krahe T. E., Medina A. E. (2011). Early valproic acid exposure alters functional organization in the primary visual cortex. Exp. Neurol. 228, 138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C. N. (1999). Organophosphorus pesticides: Do they all have the same mechanism of toxicity? J. Toxicol. Environ. Health. B. Crit. Rev. 2, 161–181 [DOI] [PubMed] [Google Scholar]
- Prut L., Belzung C. (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 463, 3–33 [DOI] [PubMed] [Google Scholar]
- Quinn R. (2005). Comparing rat’s to human’s age: How old is my rat in people years? Nutrition. 21, 775–777 [DOI] [PubMed] [Google Scholar]
- Represa A., Ben-Ari Y. (2005). Trophic actions of GABA on neuronal development. Trends Neurosci. 28, 278–283 [DOI] [PubMed] [Google Scholar]
- Ribeiro F. M., Black S. A., Prado V. F., Rylett R. J., Ferguson S. S., Prado M. A. (2006). The “ins” and “outs” of the high-affinity choline transporter CHT1. J. Neurochem. 97, 1–12 [DOI] [PubMed] [Google Scholar]
- Ribeiro-Carvalho A., Lima C. S., Filgueiras C. C., Manhães A. C., Abreu-Villaça Y. (2008). Nicotine and ethanol interact during adolescence: Effects on the central cholinergic systems. Brain Res. 1232, 48–60 [DOI] [PubMed] [Google Scholar]
- Ribeiro-Carvalho A., Lima C. S., Medeiros A. H., Siqueira N. R., Filgueiras C. C., Manhães A. C., Abreu-Villaça Y. (2009). Combined exposure to nicotine and ethanol in adolescent mice: Effects on the central cholinergic systems during short and long term withdrawal. Neuroscience. 162, 1174–1186 [DOI] [PubMed] [Google Scholar]
- Rodier P. M. (1988). Structural–functional relationships in experimentally induced brain damage. Prog. Brain Res. 73, 335–348 [DOI] [PubMed] [Google Scholar]
- Rodgers R. J., Cao B. J., Dalvi A., Holmes A. (1997). Animal models of anxiety: An ethological perspective. Braz. J. Med. Biol. Res. 30, 289–304 [DOI] [PubMed] [Google Scholar]
- Rodgers R. J., Dalvi A. (1997). Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 21, 801–810 [DOI] [PubMed] [Google Scholar]
- Rodgers R. J., Johnson N. J. (1995). Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol. Biochem. Behav. 52, 297–303 [DOI] [PubMed] [Google Scholar]
- Roegge C. S., Timofeeva O. A., Seidler F. J., Slotkin T. A., Levin E. D. (2008). Developmental diazinon neurotoxicity in rats: Later effects on emotional response. Brain Res. Bull. 75, 166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R. D., Tunbridge E. M., Bhagwagar Z., Drevets W. C., Sahakian B. J., Carter C. S. (2003). Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology. 28, 153–162 [DOI] [PubMed] [Google Scholar]
- Rotterdam Convention (2010a). Database of Notifications of Final Regulatory Action Available at: http://www.pic.int/Procedures/NotificationsofFinal RegulatoryActions/Database/tabid/1368/language/es-CO/Default.aspx Accessed December 14, 2012
- Rotterdam Convention (2010b). Productos Químicos del Anexo III Available at: http://www.pic.int/ElConvenio/ProductosQu%C3%ADmicos/AnexoIII/tabid/2031/language/es-CO/Default.aspx Accessed January 23, 2013
- Savitz J., Lucki I., Drevets W. C. (2009). 5-HT(1A) receptor function in major depressive disorder. Prog. Neurobiol. 88, 17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. R., Atweh S., Kuhar M. J. (1976). Sodium-dependent high affinity choline uptake: A regulatory step in the synthesis of acetylcholine. J. Neurochem. 26, 909–922 [DOI] [PubMed] [Google Scholar]
- Slotkin T. A. (2004). Cholinergic systems in brain development and disruption by neurotoxicants: Nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol. 198, 132–151 [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Bodwell B. E., Levin E. D., Seidler F. J. (2008a). Neonatal exposure to low doses of diazinon: Long-term effects on neural cell development and acetylcholine systems. Environ. Health Perspect. 116, 340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A., Bodwell B. E., Ryde I. T., Levin E. D., Seidler F. J. (2008b). Exposure of neonatal rats to parathion elicits sex-selective impairment of acetylcholine systems in brain regions during adolescence and adulthood. Environ. Health Perspect. 116, 1308–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A., Cousins M. M., Tate C. A., Seidler F. J. (2001). Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure. Brain Res. 902, 229–243 [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Levin E. D., Seidler F. J. (2006a). Comparative developmental neurotoxicity of organophosphate insecticides: Effects on brain development are separable from systemic toxicity. Environ. Health Perspect. 114, 746–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A., Levin E. D., Seidler F. J. (2009). Developmental neurotoxicity of parathion: Progressive effects on serotonergic systems in adolescence and adulthood. Neurotoxicol. Teratol. 31, 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A., Ryde I. T., Levin E. D., Seidler F. J. (2008c). Developmental neurotoxicity of low dose diazinon exposure of neonatal rats: Effects on serotonin systems in adolescence and adulthood. Brain Res. Bull. 75, 640–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A., Seidler F. J. (2007). Comparative developmental neurotoxicity of organophosphates in vivo: Transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res. Bull. 72, 232–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A., Seidler F. J. (2008). Developmental neurotoxicants target neurodifferentiation into the serotonin phenotype: Chlorpyrifos, diazinon, dieldrin and divalent nickel. Toxicol. Appl. Pharmacol. 233, 211–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. A., Tate C. A., Ryde I. T., Levin E. D., Seidler F. J. (2006b). Organophosphate insecticides target the serotonergic system in developing rat brain regions: Disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ. Health Perspect. 114, 1542–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor G. W., Cochran W. G. (1967). Statistical Methods. 6th ed Iowa State University Press, Ames, IA: [Google Scholar]
- Song X., Seidler F. J., Saleh J. L., Zhang J., Padilla S., Slotkin T. A. (1997). Cellular mechanisms for developmental toxicity of chlorpyrifos: Targeting the adenylyl cyclase signaling cascade. Toxicol. Appl. Pharmacol. 145, 158–174 [DOI] [PubMed] [Google Scholar]
- Spassova D., White T., Singh A. K. (2000). Acute effects of acephate and methamidophos on acetylcholinesterase activity, endocrine system and amino acid concentrations in rats. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 126, 79–89 [DOI] [PubMed] [Google Scholar]
- Terry A. V., Jr (2012). Functional consequences of repeated organophosphate exposure: Potential non-cholinergic mechanisms. Pharmacol. Ther. 134, 355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva O. A., Sanders D., Seemann K., Yang L., Hermanson D., Regenbogen S., Agoos S., Kallepalli A., Rastogi A., Braddy D., et al. (2008). Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Res. Bull. 77, 404–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (2002). Chlorpyrifos: End-Use Products Cancellation Order. Fed Reg 67:3698–3700 Available at: http://www.epa.gov/fedrgstr/EPA-PEST/2002/January/Day-25/p1764.htm Accessed January 23, 2013
- Wall P. M., Messier C. (2001). Methodological and conceptual issues in the use of the elevated plus-maze as a psychological measurement instrument of animal anxiety-like behavior. Neurosci. Biobehav. Rev. 25, 275–286 [DOI] [PubMed] [Google Scholar]
- Zahalka E. A., Seidler F. J., Lappi S. E., McCook E. C., Yanai J., Slotkin T. A. (1992). Deficits in development of central cholinergic pathways caused by fetal nicotine exposure: Differential effects on choline acetyltransferase activity and [3H]hemicholinium-3 binding. Neurotoxicol. Teratol. 14, 375–382 [DOI] [PubMed] [Google Scholar]