Abstract
Pacific salmon are particularly susceptible to copper (Cu)-induced olfactory injuries that can ultimately inhibit neurobehaviors critical to survival. However, the molecular mechanisms underlying Cu-mediated olfactory impairment remain poorly understood. In the present study, we conducted a short-term Cu exposure at levels relevant to urban runoff, and investigated the roles of impaired olfactory signal transduction and induced apoptosis as underlying mechanisms of olfactory injury. Increased cell death in the olfactory epithelium was evident in coho receiving 4 h exposures to 25 and 50 ppb Cu. Expression of olfactory marker protein (omp), a marker of mature olfactory sensory neurons, also decreased at 50 ppb Cu. Immunohistochemical analysis of coho olfactory epithelium demonstrated a loss of type 3 adenylate cyclase (ACIII) in the apical olfactory epithelium cilia at all levels of Cu exposure, suggesting an inhibitory effect of Cu in olfactory signaling. Accompanying the loss of ACIII in Cu-exposed coho were reduced intracellular cyclic guanosine monophosphate (cGMP) levels in the olfactory rosettes. Collectively, these results support a linkage among the initial steps of olfactory signaling in Cu-induced salmon olfactory injury, and suggesting that monitoring olfactory cGMP levels may aid in the assessment of salmon olfactory injury.
Keywords: copper, coho salmon, olfactory injury, apoptosis, olfactory signal transduction
1. Introduction
The decline of salmon populations in the Western United States has been linked to the deterioration of coastal habitats and the contamination of surface waters and riverine sediments (Lackey, 2003; Scholz et al., 2011). Key components of the ecological risk of chemical exposures to salmon are sublethal neurological injury and, in particular, injury to the peripheral nervous system. Peripheral neurotoxicity is often associated with impaired olfactory function, leading to the loss of critical behaviors such as predator avoidance, prey capture, mate selection, and migration (Scott et al., 2003; McIntyre et al., 2012). Accordingly, understanding the mechanisms of olfactory injury can lead to the development of sensitive biomarkers to better evaluate the effects of pollution on sublethal injury in salmonids.
The peripheral component of the fish olfactory system includes a pair of olfactory rosettes lodged in the olfactory pits. These organs are covered by a sensory epithelium containing olfactory receptor neurons (ORNs) that are highly vulnerable to the toxic effects of dissolved contaminants (Tierney et al., 2010). The initiation of an olfactory response occurs in the ORNs and involves the sequential activation of: G-protein coupled receptors via binding with odorant molecules and stimulation of an enzymatic cascade, leading to the generation of second messengers, the opening of cyclic nucleotide-gated (CNG) channels, and the elicitation of a generator current which depolarizes the cell (Hara, 1994; Schild and Restrepo, 1998). Olfactory neuron CNG channels also respond to both cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), underlying the key role of second messengers in olfactory signaling (Nakamura and Gold, 1987). Chemicals that interfere with any component of the signaling process can thus impair key behavioral responses by blocking proper olfactory function.
Copper (Cu) is a ubiquitously distributed olfactory toxicant and a pervasive contaminant in urban runoff at concentrations that vary from 3 to 64 ppb (Soller et al., 2005). Short-term exposure to Cu concentrations within this range of urban watersheds can inhibit the physiological responsiveness of olfactory receptor neurons in a concentration-dependent manner (Sandahl et al., 2004; Sandahl et al., 2007). Using zebrafish as a laboratory model, we previously demonstrated that Cu alters the expression of olfactory signaling genes including odorant receptors, G-proteins, and ion transport proteins (Tilton et al., 2008). The aforementioned study identified multiple olfactory signal transduction (OST) targets and complex biochemical responses to toxicants possibly occurring within the olfactory cilia. Despite these advances, the underlying mechanisms of Cu-mediated olfactory injury are still poorly understood. The objective of the current study was to assess whether Cu exposure affects the initial steps of olfactory signaling in juvenile salmon. Our goal was to define the linkages among cellular injury and secondary messenger cascades in order to potentially generate novel biomarkers of metal-induced olfactory injury in salmon.
2. Material and Methods
2.1 Cu exposure and tissue processing
All animal welfare and experimental procedures were carried out in strict accordance with the University of Washington’s Institutional Animal Care and Use Committee (IACUC) guidelines. Juvenile coho salmon were provided by the National Oceanic and Atmospheric Administration (NOAA), Seattle, Washington and raised under natural photoperiod in cylindrical tanks containing recirculated, tempered (10–12 °C) freshwater from Lake Washington. The fish were fed Bio Vita Fry Feed (Bio-Oregon Inc., OR) and water quality conditions were typically 80–120 mg/L total hardness as calcium carbonate, pH 7.4 ± 0.2, and 8.1 mg/L dissolved oxygen content. Exposures were preceded by a 24 h clean water acclimation period during which the fish received no food. For the 4 h exposures, 10–12 coho (body length: 11.86 ± 1.95 cm and body mass: 17.95 ± 7.97 µg) were exposed to the intended concentrations of 0, 5, 25, and 50 ppb Cu (as CuCl2) in 70 µL, individually-aerated aquaria contained within a large, chilled (11 °C), re-circulating water bath. The targeted nominal Cu concentrations closely tracked the measured waterborne Cu concentrations (Table 1). The background concentration of total dissolved copper in the source water was 1 ppb, which is extremely low relative to other studies (Baldwin et al., 2003). Following the Cu exposures, fish were euthanized and the olfactory rosettes were isolated and prepared for molecular and biochemical endpoints as described below.
Table 1.
Nominal and measured copper concentrations.a
| Sample | Nominal (ppb) | Measured (ppb) |
|---|---|---|
| Control | 0 | 1 |
| Cu-L | 5 | 5 |
| Cu-M | 25 | 23 |
| Cu-H | 50 | 44 |
Waterborne Cu concentrations were analyzed by the UW Trace Organics Laboratory using inductively coupled plasma-mass spectrometry (ICP-MS) using U.S. Environmental Protection Agency (EPA) method 6020A (EPA, 2008).
2.2 Analysis of cell death (TUNEL assay)
Cu-induced cell death was measured in olfactory rosette cryosections by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) according to the manufacturer’s protocol (In Situ Cell Death Detection Kit, TMR red; Roche Diagnostics). Slides were counterstained with Hochest 33342 prior to microscopic analysis, and images were collected using a Nikon Labophot 2 microscope equipped with a Nuance Multispectral Imaging System (Caliper Life Science, Hopkinton, MA). TUNEL signals were detected with a red filter set (Nikon G-1B, EX540/10, DM580, BA590), and images were captured with Nuance 3.0.1 software. Quantitative measurement of apoptosis was conducted by counting the number of TUNEL-positive cells per 104 m2 of olfactory tissue.
2.3 RNA Isolation and Quantitative Real-time PCR
Total RNA was isolated from coho olfactory rosettes using the Trizol (Invitrogen, Carlsbad, CA) method. First strand cDNA synthesis and qPCR analysis of olfactory gene expression was conducted as previously described (Wang et al., 2012). Expression of olfactory marker protein (omp) was quantified using real-time PCR (forward primer: 5’-GACCCCTGACCTCACACACT-3’ and reverse primer: 5’-GTACATGACCTTGCGGACCT-3’). Omp gene expression was normalized against the housekeeping gene β-actin, which was unaffected by the exposures.
2.4 Immunohistochemical analysis of type 3adenylate cyclase (ACIII) expression
Cryosections of olfactory tissue were incubated overnight with anti-ACIII (1:200, sc-588 Santa Cruz Biotechnology) in blocking solution (10% normal donkey serum, 0.1% Triton X-100, and 3% BSA). A Cy3-conjugated donkey anti-rabbit secondary antibody (1:500, Jackson Laboratories) was used in the immunohistochemical analysis. As a negative control, the primary antibody was replaced with blocking solution. Images were captured at 610 nm with Nuance 3.0.1 software (Caliper Life Science, Hopkinton, MA). The threshold values obtained from the negative controls were subsequently used when analyzing captured images with Image J software (NIH).
2.5 Analysis of cAMP and cGMP
Pooled coho olfactory rosettes were homogenized on ice with 5 volumes of 5% trichloroacetic acid (TCA) (1 mL of solution/ gram of tissue) and centrifuged at 1500 × g for 10 minutes at 4 °C. The supernatants were extracted (3X) with water-saturated ether to remove the TCA, and the residual ether was removed from the aqueous layer by heating samples to 70 °C for 5 minutes. To prepare the standard curve matrix solution, 20 mL of the 5% TCA preparation was treated similarly by extraction of water-saturated ether and heating. Subsequently, all standards and sample dilutions were prepared according to manufacturer guidelines (Cyclic AMP and Cyclic GMP EIA Kit, Cayman Chemical Company). Samples were acetylated with potassium hydroxide and acetic anhydride, and cAMP and cGMP levels were assayed in triplicate in a 96-well plate format at 405 nm.
2.6 Statistical Analysis
The Mann-Whitney U test was used to assess differences in the number of TUNEL-positive cells among Cu treatments and controls. The effects of Cu on omp, ACIII signal intensity, and intracellular cAMP and cGMP levels were analyzed by one-way ANOVA, followed by the Dunnett’s test. All data were considered statistically significant at p < 0.05. All statistical analyses were conducted using GraphPad Prism Ver 5.0 (Graph Pad Software Inc., San Diego, CA).
3. Results and Discussion
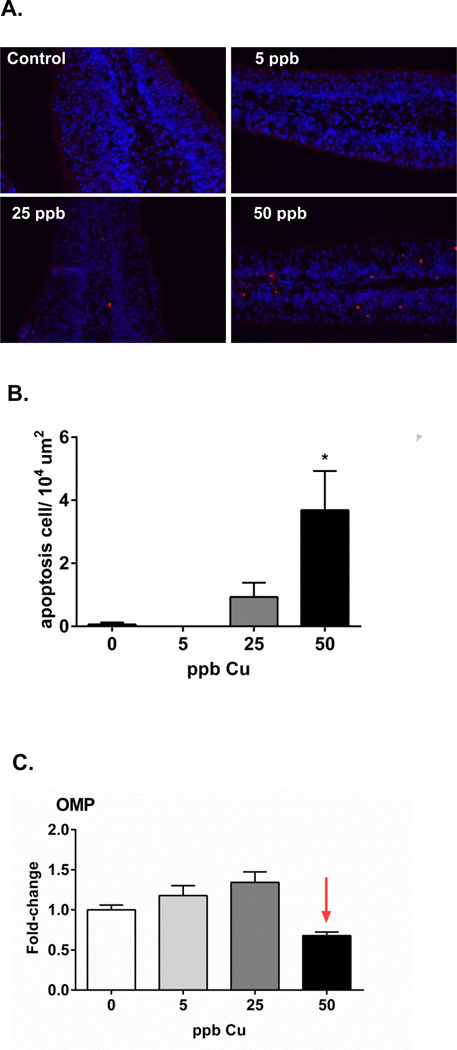
As observed, brief exposures to 25 and 50 ppb Cu cause an increase in the number of cells undergoing apoptosis in coho olfactory epithelium (Figure 1A). Semi-quantitative analyses of TUNEL staining indicated a dramatic increase in apoptotic cells in coho exposed to 50 ppb as compared to controls (Figure 1B). By contrast, no cell death was observed in the control and 5 ppb Cu groups. Hansen et al. reported a similar loss of olfactory cilia and microvillus cells, ruptured cell membranes, and swollen mitochondria in Chinook salmon following similar exposure conditions (50 µg/L Cu, 4 h) (Hansen et al., 1999). Other studies have also reported degenerated ORNs and apoptotic cells in fish olfactory epithelium following chronic low-level Cu exposure (Julliard et al., 1993, 1996). The loss of omp expression (~32%) was concomitant with olfactory apoptosis in coho exposed to 50 ppb Cu (Figure 1C). Our study indicates that the early initiation (as early as 4 h) of Cu-mediated cell death may play an essential role in ORN degeneration. As such, it would be of interest in future studies to determine the linkage between regeneration of ORNs post Cu-exposure and recovery of olfactory function.
Figure 1.
(A) TUNEL staining of coho olfactory rosette cyrosections. Apoptotic cells stained red, whereas Hoechst 33342 was used for blue nuclear counterstaining (scale bar=50 µm). (B) Graph shows the quantification of the positive TUNEL signal per 104 m2 (n=4 individuals). Asterisks indicate statistically significant difference compared to control animals not receiving Cu (ANOVA, * p < 0.05). (C) Graphs are presented as fold-change of omp mRNA expression relative to the corresponding controls, with the 5, 25, and 50 ppb Cu datasets represented by light green, light grey, and dark bars, respectively. Data represent the mean ± SEM of n=6 individuals.
Although the underlying mechanisms of olfactory cell injury was not a focus of the present study, others have suggested a similar injury is associated with an increase in reactive oxygen species (ROS) (Olivari et al., 2008). Previous studies from our laboratory have demonstrated that short-term exposure to cadmium, another common trace metal pollutant and olfactory toxicant, induces the expression of antioxidant genes in the olfactory system of salmon (Espinoza et al., 2012), and zebrafish (Wang and Gallagher, 2013). In the zebrafish study, loss of sensory neurons under conditions of cadmium exposure coincided with impaired olfactory function, and was further exacerbated in fish with a compromised antioxidant defense system (Wang and Gallagher, 2013).
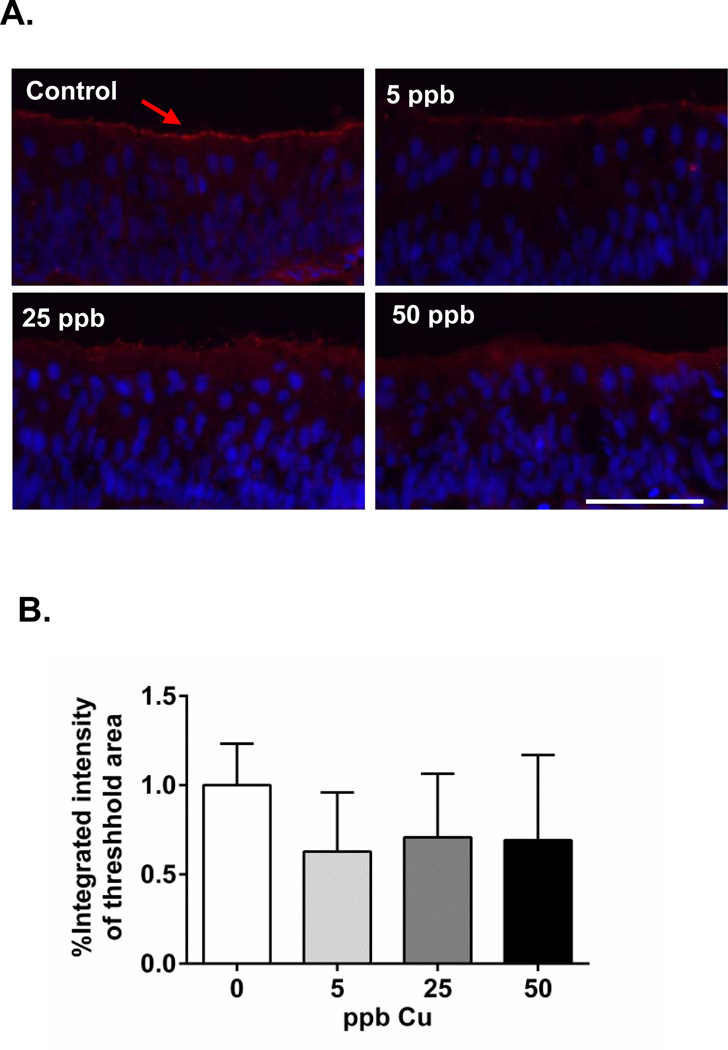
To investigate the effect of Cu on olfactory signaling, we focused on three key molecular components of olfactory signal transduction pathways: ACIII, cAMP, and cGMP. ACIII is highly enriched in olfactory cilia, where it exhibits a regulatory role in coupling cAMP signaling and ion channel regulation (Bakalyar and Reed, 1990; Ronnett and Moon, 2002). We examined the alteration of basal ACIII expression in the absence of odorant stimulation in olfactory epithelium (Figure 2A), and observed decreased ACIII expression in all three groups of Cu-exposed coho relative to control animals (37, 30, and 31% loss of expression in 5, 25, and 50 ppb Cu, respectively; Figure 2B). The reduced ACIII expression may have been due to a loss of olfactory cilia and disruption of regulatory networks. In mammals, adenylyl cyclases display varied regulatory properties (Xia and Storm, 1996). For example, ACIII is activated by G protein-coupled receptors and inhibited by intracellular Ca2+ via calmodulin-dependent protein kinase II (Wayman et al., 1995; Wei et al., 1996). Odorant stimulation elevates intracellular Ca2+ and inhibits ACIII activity, potentially contributing to the transient cAMP production.
Figure 2.
(A) Immunohistochemical analysis of ACIII expression on coho olfactory rosette cryosections (arrow, red; scale bar=50 µm). Hoechst 33342 was used as a nuclear counterstain (blue). (B) Graph shows the percentage integrated intensity of threshold area relative to controls (n=4 individuals). The mean fluorescence intensity at, or above, the threshold value was measured for each image, and the calculated mean intensities for each treated group were converted to a percentage of control values.
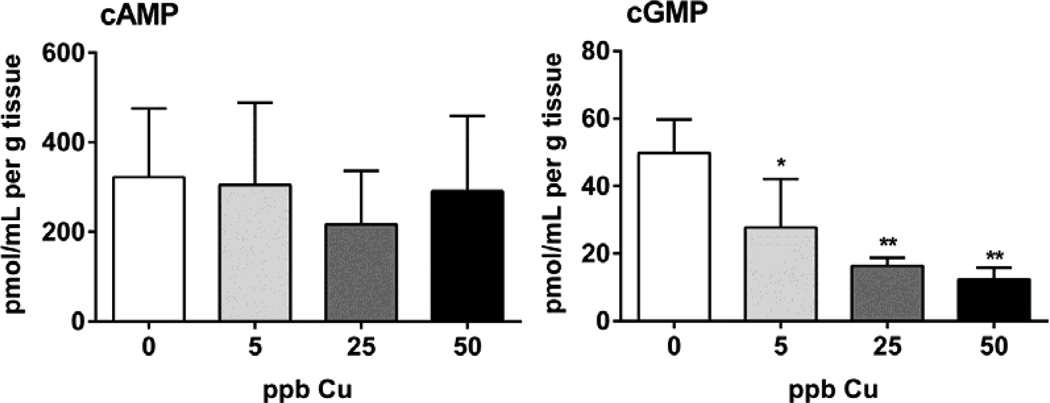
Similar to our analysis of Cu-induced modulation of ACIII, we observed a significant reduction in olfactory cGMP in all animals receiving Cu exposures (44, 67, and 75% deceases in 5, 25, and 50 ppb Cu groups, respectively; Figure 3). Interestingly, however, no statistically significant differences were observed among Cu-exposed and control fish with respect to cAMP expression. In fish, the cAMP and cGMP pathways play key roles in olfactory signal transduction (Restrepo et al., 1993; Vielma et al., 2008), with the olfactory CNG channels displaying similar sensitivities to cAMP and cGMP (Nakamura, 2000). Nonetheless, others have reported that relative to cAMP, cGMP exhibited a slower, yet sustained increase upon odor activation, suggesting that cGMP may be involved in long-term cellular events rather than initial signaling events (Zufall and Leinders-Zufall, 1998). For example, juvenile salmon imprinted to a specific odorant, -phenylethyl alcohol (PEA), demonstrated increased guanylyl cyclase activity in response to PEA exposure during the homing migration period (Dittman et al., 1997), indicating an important role for cGMP in salmon olfactory imprinting. These data suggest that olfactory imprinting involves sensitization of the peripheral olfactory system to specific home stream odorants during a single, critical window of development that coincides with changes in the reproductive hormone profile (e.g. parr-smolt transformation) (Hasler et al., 1983). The fact that we observed a sensitivity of cGMP modulation in juvenile coho exposed to Cu suggests that a key mechanism of heavy metal toxicity to salmon olfactory function may involve disruption of cyclic nucleotides during the critical olfactory imprinting stages.
Figure 3.
Analysis of intracellular cAMP and cGMP levels in Cu-exposed and control olfactory tissue. Data are expressed as the mean ± SEM (n=3 pools, ANOVA, * p < 0.05).
Although we did not measure electro-olfactory-gram (EOG) responses in the current study, others have shown a reduced EOG response to amino acids within 30 minutes of exposure to 5 ppb Cu in juvenile salmon (Sandahl et al., 2004), that were further reduced over 3 h (Sandahl et al., 2007). Furthermore, the reduction in EOG responses was more dramatic at higher Cu concentrations (10–20 ppb), and was accompanied by the inhibition of olfactory-driven behaviors (Sandahl et al., 2007). The fact that we observed significant cell death in the olfactory epithelium of coho exposed to 50 ppb Cu, as well as a loss of omp, suggest that these events are contributing factors to the dramatic loss of EOG signal observed in other studies. By contrast, the decreased EOG signal reported by Sandahl et. al at low Cu concentrations (5 ppb) is likely associated with the disrupted secondary messenger cascade involving ACIII and intracellular cyclic nucleotide levels. Continuing studies in our laboratory will examine kinetic changes in olfactory second messengers under conditions of odorant stimulation in olfactory neurons, an approach that will better define initiating molecular events and adverse olfactory function outcomes.
4. Conclusions
In conclusion, we have demonstrated that brief exposure to Cu under environmentally-relevant scenarios alters cellular and biochemical markers of sensory neurons and second messengers in the salmon olfactory system. Our data provides a causal linkage among the initial molecular events following metal exposures and subsequent alterations to olfactory signaling in salmon, and suggests that biomarkers of olfactory neurons and second messengers may aid in the assessment of olfactory injury in salmonids.
Highlights.
Short-term Cu exposure increased cell death in salmon olfactory epithelium.
Decreased omp expression was associated with increased cell death.
ACIII expression decreased in apical olfactory epithelium at all Cu levels.
Intracellular cGMP was significantly reduced at all Cu doses.
Acknowledgements
This work was supported in part by the National Institute of Environmental Health Sciences Superfund Basic Sciences Grant [NIEHS P42ES004696]. The authors appreciate the assistance of Dr. Brian Beckman and Abby Tillotson at NOAA fisheries, Seattle, WA, who provided the juvenile coho salmon for these experiments. We also thank Ke’ale Louie for technical assistance with the exposures.
Abbreviations
- ORN
olfactory receptor neuron
- OST
olfactory signal transduction
- ACIII
type III adenylate cyclase
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- CNG channel
cyclic nucleotide-gated channel
- omp
olfactory marker protein
- EOG
electroolfactorygram
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakalyar HA, Reed RR. Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science. 1990;250:1403–1406. doi: 10.1126/science.2255909. [DOI] [PubMed] [Google Scholar]
- Baldwin DH, Sandahl JF, Labenia JS, Scholz NL. Sublethal effects of copper on coho salmon: impacts on nonoverlapping receptor pathways in the peripheral olfactory nervous system. Environ Toxicol Chem. 2003;22:2266–2274. doi: 10.1897/02-428. [DOI] [PubMed] [Google Scholar]
- Dittman AH, Quinn TP, Nevitt GA, Hacker B, Storm DR. Sensitization of olfactory guanylyl cyclase to a specific imprinted odorant in coho salmon. Neuron. 1997;19:381–389. doi: 10.1016/s0896-6273(00)80947-2. [DOI] [PubMed] [Google Scholar]
- EPA. Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, Method 6020A, Inductively Coupled Plasma-Mass Spectrometry. SW-846. Washington, DC: U.S. Environmental Protection Agency; 2008. [Google Scholar]
- Espinoza HM, Williams CR, Gallagher EP. Effect of cadmium on glutathione S-transferase and metallothionein gene expression in coho salmon liver, gill and olfactory tissues. Aquat Toxicol. 2012;110–111:37–44. doi: 10.1016/j.aquatox.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JA, Rose JD, Jenkins RA, Gerow KG, Bergman HL. Chinook salmon (Oncorhynchus tshawytscha) and rainbow trout (Oncorhynchus mykiss) exposed to copper: Neurophysiological and histological effects on the olfactory system. Environmental Toxicology and Chemistry. 1999;18:1979–1991. [Google Scholar]
- Hara TJ. Olfaction and gustation in fish: an overview. Acta Physiol Scand. 1994;152:207–217. doi: 10.1111/j.1748-1716.1994.tb09800.x. [DOI] [PubMed] [Google Scholar]
- Hasler AD, Scholz AT, Goy RW. Olfactory imprinting and homing in salmon: investigations into the mechanism of the imprinting process. Berlin; New York: Springer-Verlag; 1983. [Google Scholar]
- Julliard AK, Saucier D, Astic L. Effects of chronic low-level copper exposure on ultrastructure of the olfactory system in rainbow trout (Oncorhynchus mykiss) Histol Histopathol. 1993;8:655–672. [PubMed] [Google Scholar]
- Julliard AK, Saucier D, Astic L. Time-course of apoptosis in the olfactory epithelium of rainbow trout exposed to a low copper level. Tissue & cell. 1996;28:367–377. doi: 10.1016/s0040-8166(96)80023-1. [DOI] [PubMed] [Google Scholar]
- Lackey RT. Pacific Northwest Salmon: Forecasting Their Status in 2100. Reviews in Fisheries Science. 2003;11:35–88. [Google Scholar]
- McIntyre JK, Baldwin DH, Beauchamp DA, Scholz NL. Low-level copper exposures increase visibility and vulnerability of juvenile coho salmon to cutthroat trout predators. Ecological applications: a publication of the Ecological Society of America. 2012;22:1460–1471. doi: 10.1890/11-2001.1. [DOI] [PubMed] [Google Scholar]
- Nakamura T. Cellular and molecular constituents of olfactory sensation in vertebrates. Comp Biochem Physiol A Mol Integr Physiol. 2000;126:17–32. doi: 10.1016/s1095-6433(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Gold GH. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature. 1987;325:442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Olivari FA, Hernandez PP, Allende ML. Acute copper exposure induces oxidative stress and cell death in lateral line hair cells of zebrafish larvae. Brain Res. 2008;1244:1–12. doi: 10.1016/j.brainres.2008.09.050. [DOI] [PubMed] [Google Scholar]
- Restrepo D, Boekhoff I, Breer H. Rapid kinetic measurements of second messenger formation in olfactory cilia from channel catfish. Am J Physiol. 1993;264:C906–C911. doi: 10.1152/ajpcell.1993.264.4.C906. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Moon C. G proteins and olfactory signal transduction. Annu Rev Physiol. 2002;64:189–222. doi: 10.1146/annurev.physiol.64.082701.102219. [DOI] [PubMed] [Google Scholar]
- Sandahl JF, Baldwin DH, Jenkins JJ, Scholz NL. Odor-evoked field potentials as indicators of sublethal neurotoxicity in juvenile coho salmon (Oncorhynchus kisutch) exposed to copper, chlorpyrifos, or esfenvalerate. Can J Fish Aquat. 2004;61:404–413. [Google Scholar]
- Sandahl JF, Baldwin DH, Jenkins JJ, Scholz NL. A sensory system at the interface between urban stormwater runoff and salmon survival. Environ Sci Technol. 2007;41:2998–3004. doi: 10.1021/es062287r. [DOI] [PubMed] [Google Scholar]
- Schild D, Restrepo D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol Rev. 1998;78:429–466. doi: 10.1152/physrev.1998.78.2.429. [DOI] [PubMed] [Google Scholar]
- Scholz NL, Myers MS, McCarthy SG, Labenia JS, McIntyre JK, Ylitalo GM, Rhodes LD, Laetz CA, Stehr CM, French BL, McMillan B, Wilson D, Reed L, Lynch KD, Damm S, Davis JW, Collier TK. Recurrent die-offs of adult coho salmon returning to spawn in Puget Sound lowland urban streams. PLoS One. 2011;6:e28013. doi: 10.1371/journal.pone.0028013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GR, Sloman KA, Rouleau C, Wood CM. Cadmium disrupts behavioural and physiological responses to alarm substance in juvenile rainbow trout (Oncorhynchus mykiss) J Exp Biol. 2003;206:1779–1790. doi: 10.1242/jeb.00353. [DOI] [PubMed] [Google Scholar]
- Soller J, Stephenson J, Olivieri K, Downing J, Olivieri AW. Evaluation of seasonal scale first flush pollutant loading and implications for urban runoff management. Journal of environmental management. 2005;76:309–318. doi: 10.1016/j.jenvman.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Tierney KB, Baldwin DH, Hara TJ, Ross PS, Scholz NL, Kennedy CJ. Olfactory toxicity in fishes. Aquat Toxicol. 2010;96:2–26. doi: 10.1016/j.aquatox.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Tilton F, Tilton SC, Bammler TK, Beyer R, Farin F, Stapleton PL, Gallagher EP. Transcriptional biomarkers and mechanisms of copper-induced olfactory injury in zebrafish. Environ Sci Technol. 2008;42:9404–9411. doi: 10.1021/es801636v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielma A, Ardiles A, Delgado L, Schmachtenberg O. The elusive crypt olfactory receptor neuron: evidence for its stimulation by amino acids and cAMP pathway agonists. J Exp Biol. 2008;211:2417–2422. doi: 10.1242/jeb.018796. [DOI] [PubMed] [Google Scholar]
- Wang L, Gallagher EP. Role of Nrf2 antioxidant defense in mitigating cadmium-induced oxidative stress in the olfactory system of zebrafish. Toxicology and applied pharmacology. 2013;266:177–186. doi: 10.1016/j.taap.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Wang L, Harris SM, Espinoza HM, McClain V, Gallagher EP. Characterization of phospholipid hydroperoxide glutathione metabolizing peroxidase (gpx4) isoforms in Coho salmon olfactory and liver tissues and their modulation by cadmium. Aquat Toxicol. 2012;114–115:134–141. doi: 10.1016/j.aquatox.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Storm DR. Ca2+ inhibition of type III adenylyl cyclase in vivo. J Biol Chem. 1995;270:21480–21486. doi: 10.1074/jbc.270.37.21480. [DOI] [PubMed] [Google Scholar]
- Wei J, Wayman G, Storm DR. Phosphorylation and inhibition of type III adenylyl cyclase by calmodulin-dependent protein kinase II in vivo. J Biol Chem. 1996;271:24231–24235. doi: 10.1074/jbc.271.39.24231. [DOI] [PubMed] [Google Scholar]
- Xia Z, Storm DR. Regulatory properties of the mammalian adenylyl cyclases. New York: R.G. Landes Company; Chapman & Hall, Austin Tex.; 1996. [Google Scholar]
- Zufall F, Leinders-Zufall T. Role of cyclic GMP in olfactory transduction and adaptation. Annals of the New York Academy of Sciences. 1998;855:199–204. doi: 10.1111/j.1749-6632.1998.tb10566.x. [DOI] [PubMed] [Google Scholar]