Abstract
Human pluripotent stem cells hold promising potential in many therapeutics applications including regenerative medicine and drug discovery. Over the past three decades, embryonic stem cell research has illustrated that embryonic stem cells possess two important and distinct properties: the ability to continuously self-renew and the ability to differentiate into all specialized cell types. In this article, we will discuss the continuing evolution of human pluripotent stem cell culture by examining requirements needed for the maintenance of self-renewal in vitro. We will also elaborate on the future direction of the field toward generating a robust and completely defined culture system, which has brought forth collaborations amongst biologists and engineers. As human pluripotent stem cell research progresses towards identifying solutions for debilitating diseases, it will be critical to establish a defined, reproducible and scalable culture system to meet the requirements of these clinical applications.
Keywords: defined culture, feedback system control, feeder-free and single cell culture, human embryonic stem cells, human induced pluripotent stem cells, regenerative medicine, small molecules, suspension culture, synthetic surfaces
Murine embryonic stem cells
An important breakthrough in the field of developmental biology transpired in 1981 when two independent groups, led by Martin Evans and Matthew Kaufman, as well as Gail R Martin, derived embryonic stem cells (ESCs) from early mouse embryos [1,2]. The in vitro maintained cells, termed ESCs, were shown to have unlimited proliferative potential with the remarkable property of differentiating into various specialized cell types, including those of germ cell, hematopoietic and neural lineages [1–7]. To provide undisputable evidence of their ability to give rise to all cells of the embryo, mouse ESCs were introduced into a tetraploid blastocyst to obtain an embryo completely derived from the mouse ESC; a method termed tetraploid complementation (Table 1) [8,9]. These advancements have laid the groundwork for various developmental studies including the generation of mouse chimeras as well as germlines with genetic alterations, leading to important discoveries in fields such as cancer, metabolism and neurobiology [10–12].
Table 1.
Definition of stem cell potency.
| Category | Differentiation potential | Examples |
|---|---|---|
| Totipotent | Ability to give rise to all cell types of the embryonic and extra-embryonic lineages | Blastomeres |
| Pluripotent | Ability to give rise to the embryo proper | Human embryonic stem cells and human induced pluripotent stem cells |
| Multipotent | Ability to give rise to multiple cell types | Adult stem cells including hematopoietic and neural |
| Oligopotent | Ability to give rise to few cell types | Myeloid stem cells |
| Unipotent | Ability to give rise to a single cell type | Hepatocytes |
The culture of mouse ESCs was accomplished by using a feeder layer combined with medium containing fetal calf serum and other undefined supplements [1]. Although feeder cells and serum served as critical additives to the culture of mouse ESCs, they introduce significant variability and elicit undefined biological signals. To decipher the key components that can replace feeder cells, Austin Smith and colleagues identified leukemia inhibitory factor and bone morphogenic protein 4 as the two cytokines both necessary and sufficient for supporting the continuous culture of mouse ESCs [13–17]. These studies have led to the routine culture of mouse ESCs in completely defined culture void of feeder cells and animal-derived medium supplements [4].
The derivation of human ESCs & induced pluripotent stem cells
The concept of having an unlimited supply of a cell population with the potential to differentiate into any cell type in the mouse not only raised significant interest in the scientific community, but also began the race to derive human ESCs for more medically relevant applications, including cell therapy and drug discovery. And so, in 1998, James Thomson and colleagues generated the first human ESCs by harvesting the inner cell mass of a human embryo, cultured on irradiated murine-derived feeder cells with bovine serum and other supplements [18]. Although the newly derived human ESCs could not be subjected to the battery of pluripotent tests, such as germline transmission and tetraploid complementation, as conducted with mouse ESCs, they were shown to propagate indefinitely, express high levels of telomerase, differentiate into various cell types and retain normal karyotype [18]. Interestingly, leukemia inhibitory factor did not appear to support the undifferentiated state of human ESCs, suggesting differences of the self-renewal network amongst various species and impeding the direct application of some of the tissue culture advancements made with mouse ESC system [18–21]. After the initial derivation of human ESCs by Thomson and colleagues, other institutions from several countries quickly followed, deriving additional human ESCs that in the most part had similar morphologies, parallel expression of genes associated with the pluripotent circuitry and analogous ability to differentiate into numerous cell types, albeit with some differences in methods of derivation and culture [22].
More recently, the generation of human induced pluripotent stem (iPS) cells by defined transcription factors has facilitated the derivation of patient-specific pluripotent stem cells (PSCs) for regenerative medicine while eliminating the technical and ethical barriers of human ESCs [23–25]. Many studies have continued to demonstrate the utility of human iPS cells in disease correction and cell replacement therapy [26–29]. With extremely similar properties including morphology, gene expression profiles and pluripotent potential, it is quite feasible to perceive how culture platforms of human iPS cells have mirrored those of human ESCs [30]. Although this article will not focus specifically on human iPS cells, as it is well reviewed elsewhere [31,32], we will draw parallel comparisons in regards to relevant tissue culture platforms and advancements. While the derivations of both human ESCs and iPS cells have marked important breakthroughs, several key hurdles need to be addressed before human PSCs can be utilized in clinical applications [33–39]. Perhaps the most important challenge is to eliminate all animal products from the culture system in an effort to derive a completely defined system. Animal products serve as a source of pathogens and xenogenic contaminants, which can elicit an immune response when introduced to patients. Studies have shown that human ESCs can metabolically incorporate products available in the culture, such as nonhuman sialic acid Neu5Gc [40]. Animal products also introduce variability into the culture system that will hinder the reproducibility and reliability for clinical grade manufacturing [41]. Another challenge lays in the passaging strategy of human PSCs. In the original study by Thomson and colleagues human ESCs were passaged as clumps of cells, a strategy that has been followed with the culture of human iPS cells. This method has been carried forward by most laboratories, as it has been noticed that single cell dissociation of both human ESCs and iPS cells leads to significant cell death and genomic aberrations [42,43]. However, single cell culture is critical for clonal selection, gene targeting and disease correction [44]. Furthermore, both human ESC and iPS cell cultures have a propensity to differentiate and often require laborious effort in separating the differentiated cells from the undifferentiated cell culture [45,46]. These obstacles are the forefront research of many laboratories, and indeed will need to be addressed before the successful transition to therapeutic applications (Table 2). In this article, we will focus on the recent progress on the establishment of culture systems that are chemically defined, efficient and enable single cell passaging.
Table 2.
Components of conventional culture.
| Factor | Pros | Cons |
|---|---|---|
| Feeder cell | • Supports the maintenance of both human ESCs and iPS cells • Enhances seeding efficiency during both clump and single cell passaging |
• A source of xenogeneic contamination • A source for animal-derived pathogens |
| Serum | • Provides known and unknown factors in supporting the growth and proliferation of human ESCs and iPS cells | • Animal derived • Lot-to-lot variation • Undefined |
| Clump passage | • Maintains viability during passages • Reduces the potential for genomic aberrations |
• Impedes scale-up expansion at manufacturing level • Laborious and time consuming • Prevents single cell cloning strategies such as genetic modifications and disease correction |
ESC: Embryonic stem cell; iPS: Induced pluripotent stem.
Progressing towards a defined & efficient culture system
Significant progress has been made in the ultimate development of a defined human ESC culture system [47–52]. One strategy has been to replace nonhuman products in the culture system (i.e., animal serum and feeder cells) with more defined or human origin substitutes such as human-derived feeder cells and human serum [53–58]. The feeder cell substitutes include allogeneic cells derived from various human tissues as well as autogeneic cells derived by isolating differentiated fibroblast-like cells from human ESCs [57,59]. Other studies have attempted to eliminate the feeder cells completely by introducing either feeder cell- conditioned medium or animal-derived surface coatings such as Matrigel™ (BD Biosciences, Franklin Lakes, NJ, USA) [49,60]. Although both have demonstrated to be quite effective individually or in combination, they are still animal derived and/or undefined. In an attempt to identify the key components of conditioned medium to eliminate the need for feeder cells, Choo and colleagues identified approximately 30 proteins that were secreted by feeder cells derived from human fetal, mouse embryonic fibroblast and human neonates, of which six soluble proteins were shown to support human ESC culture [61]. Suspension cultures have also been utilized to eliminate the need for feeder cells while providing a mode for scaling culture (see ‘Future perspective’) [62–67]. In a different approach, several studies have replaced Matrigel with purified extracellular matrix (ECM) proteins (also see section on ‘Developing defined surfaces’) and have substituted feeder cell-conditioned medium with supplements such as Knockout™ Serum Replacement (Invitrogen, Carlsbad, CA, USA), N2 supplement and B-27® supplement (Gibco®, Invitrogen) with combinations of various cytokines and growth factors [47,48,50–52,68–72]. For example, Thomson and colleagues aimed to identify a defined culture by optimizing the combination of the physicochemical environment, growth factor supplements and matrix components [51]. They identified several factors that had a positive effect on the proliferation of undifferentiated human ESCs: basic FGF (bFGF; high concentration), lithium chloride, GABA, pipecolic acid and TGF-β. In addition, they found that the combination of collagen IV, fibronectin, laminin and vitronectin effectively replaced Matrigel, a major step towards establishing a fully defined culture [51]. Another important step in this study was the derivation of human ESCs in a culture system that did not contain feeder cells [51]. Unfortunately, the derived human ESCs were not able to maintain their genomic stability in long-term culture by gaining trisomy of chromosomes 12 and X, albeit maintaining genomic stability in a feeder-free culture for 7 months is a remarkable accomplishment. As a sign of increased desire for the community to progress towards a defined culture system, several of the above conditions have been made as commercially available products, including StemPro® (Invitrogen) and mTeSR®1 (STEMCELL Technologies, Inc., Vancouver, Canada, TeSR1, Figure 1a).
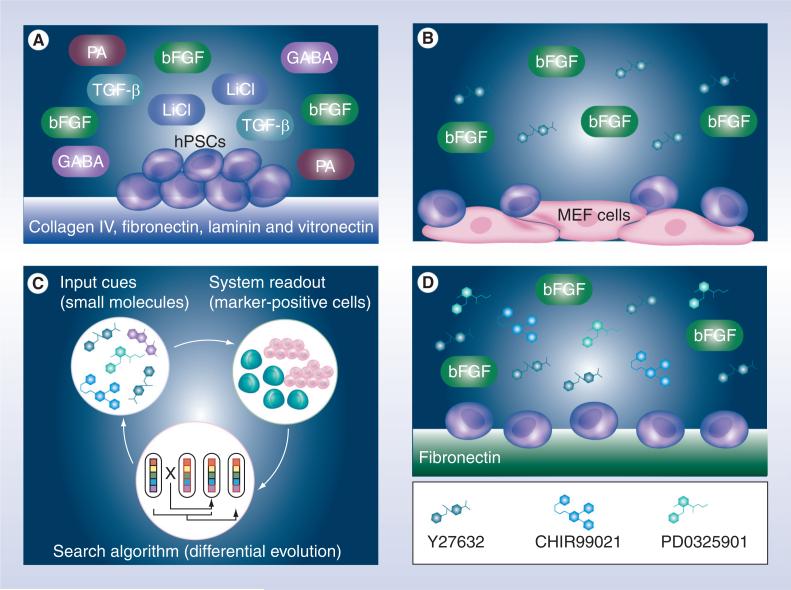
Figure 1. Improving human pluripotent stem cell culture toward chemically defined systems that can support routine single cell passaging.
(A) The first fully defined culture was achieved by combining a set of soluble factors and four human recombinant proteins [51]. (B) Addition of Rho-associated kinase inhibitor significantly improved survival of human embryonic stem cells upon enzymatic dissociation into individual cells, thus enabling routine passaging of single cells on substrate coated with MEF cells or Matrigel™ (BD Biosciences, Franklin Lakes, NJ, USA) [74]. (C & D) An engineering closed-loop optimization of small molecule inhibitors of key signaling pathways was used to quickly identify the optimal combination and concentrations that can enable robust culture of human embryonic stem cells initiating from single cells on fibronectin-coated substrate [89]. bFGF: Basic FGF; hPSC: Human pluripotent stem cell; LiCl: Lithium chloride; MEF: Mouse embryonic fibroblast; PA: Pipecolic acid.
Another development in the defined human PSC culture system has been the prevention of apoptosis after single cell dissociation. As described in the original study and the commonly used practice today, to prevent massive cell death, human ESCs and iPS cells are passaged as clumps by various dissociation techniques including mechanical, enzymatic or both that aim to break large colonies into smaller colonies. These techniques are not only laborious but also require a degree of skill; if the clumps are too big the subsequent culture is prone to differentiation and if the clumps are too small the subsequent culture will exhibit a high degree of cell death [42,73]. Sasai and colleagues found that Y-27632, a small molecule inhibitor of the Rho-associated kinase (ROCK) could enhance the survival of dissociated human ESCs [74]. By simply adding the small molecule to the culture medium, the massive cell death associated with the single cell dissociation of human ESCs during routine passage was significantly reduced, improving cloning efficiencies from approximately 1 to 27% (Figure 1b) [74]. The anti-apoptotic effect of Y-27632 was also demonstrated in feeder-free culture, suggesting that it can be incorporated into defined culture [74]. Although in the original study the exact mechanism of action was not fully understood, many studies have now begun to shed light on the biology behind the anti-apoptotic effect of ROCK inhibition [75–80]. Collectively, studies suggest that single cell dissociation triggers ROCK- dependent myosin hyperactivation and cell death while inhibiting this pathway can prevent apoptosis [78]. In a different strategy, the anti- apoptosis gene BCL2 was genetically introduced to human ESCs to prevent cell death after single cell dissociation [81]. Even though this strategy appears to have several advantages, it is unclear whether this pro-survival strategy will lead to abnormal karyotype or whether it will be therapeutically relevant as genetic modifications may lead to enhanced tumorgenic potential. Nonetheless, with improved recovery after single cell dissociation, studies requiring rare cell selection, such as homologous recombination, are now more feasible [82].
Unfortunately, the majority of improvements made towards generating a defined culture system often end up not performing as well or as consistently as conventional human ESC cultures supplemented with serum and feeder cells, with spontaneous differentiation being a common outcome. In addition, the medium compositions and the use of supporting matrices differ so significantly among the defined culture conditions that it questions the importance of various components. Collectively, it has been suggested that the current defined culture systems are far from being optimized and the ground state of human ESC has yet to be identified [83]. However, work from the laboratory of Austin Smith once again revolutionized the way we look at ESC culture. Traditionally it has been suggested that ESC self-renewal is elicited with empirical combinations of inducers including cytokines, feeder cells, fetal bovine serum and growth factors, with the removal of these key signaling cues leading to differentiation [84]. However, through the detailed studies of the Smith laboratory, it is now commonly accepted that self-renewal is the default pathway in mouse ESCs and by simply blocking cues for differentiation, ESC self-renewal can be maintained [84,85]. To this end, it has been demonstrated that by blocking differentiation propagating pathways (i.e., ERK/MAPK, FGF4 and glycogen synthase kinase 3), the self-renewal of mouse ESCs is maintained [84]. Through this powerful perspective the difficulty of deriving rat ESCs has been overcome, leading to the generation of rat chimeras and germlines for disease modeling [86–88]. Similar strategies have also been applied to human ESCs [89,90]. By surveying multiple small molecules, Jaenisch and colleagues were able to identify a unique combination of small molecules that, in combination with transgene expression of OCT4 and KLF2 and -4, were able to not only support human ESC culture, but also appeared to convert them to a more ‘naive’ state, with similar properties of mouse ESCs [90]. These properties included high clonality and repressed Xist expression. Although the study demonstrated the importance of key pathway inhibition, the majority of the long-term studies were accomplished with the introduction of transgene expression, which will need to be removed prior to clinical applications.
We aimed to identify a fully defined culture system to support the maintenance of human ESCs while eliminating the need for undefined surfaces and transgene expression by using an optimal concentration and combination of key small molecules [89]. However, it is a formidable task to screen through a large number of combinations, even with high- throughput approaches. Thus, we developed a feedback system control (FSC) scheme, which can save three to four orders of magnitude of efforts to search for the optimal combination of molecule and concentrations [91,92]. In our FSC, combinations of five small molecules were iteratively optimized by a mathematical optimization algorithm so that number of undifferentiated human ESCs, experimentally measured using alkaline phosphatase and OCT4 expressions, increased after every iteration (Figure 1C). To this end, early iterations identified a combination of three small molecules, capable of inhibiting MEK/ERK, ROCK and glycogen synthase kinase 3, that are important for the survival and proliferation of various human ESC lines on fibronectin- coated surface [89]. Continued iterations further improved this unique combination with defined concentrations that supported a nearly pure population of OCT4–GFP-positive human ESCs. However, when the culture was continuously passaged during routine single cell culture, the undifferentiated state of the human ESCs was compromised after several passages. With the addition of bFGF, we observed long-term maintenance of undifferentiated human ESCs on defined surfaces and single cell culture (Figure 1D). The long-term cultured human ESCs in defined culture demonstrated their pluripotent potential by giving rise to all three germ layers when introduced subcutaneously into severely immune compromised mice and genomic stability as seen in their normal karyotype [89]. However, other biological features associated with human ESCs, such as the status of X chromosome reactivation, was not fully explored. Further studies will also be necessary to determine whether bFGF is a bona fide requirement or simply a necessity of the studied human ESCs as bFGF was used in their derivation.
Developing defined surfaces
Most chemically defined media, including commercial products mTeSR1, StemPro and Nutristem™ (Biological Industries Ltd, Kibbutz Beit Haemek, Israel), were designed to support growth of undifferentiated human PSCs on Matrigel or other proprietary mixtures of ECM proteins. Therefore, many of the latest developments in defined ESC culture focused on surface substrates that can mimic the efficacy of Matrigel and ECM proteins using chemically defined components.
Several groups studied integrin expressions on human ESCs and/or iPS cells and identified subtypes of ECM proteins including human vitronectin [93] and human laminin [94,95]. Laminin is a major component of Matrigel, and purified laminin from human placenta was previously shown to support long-term maintenance of human ESCs in a defined medium [69]. Studies of integrins expressed on cell surface revealed distinct isoforms of laminin, laminin-111, -332 and -511, which supported undifferentiated growth of human ESCs in combination with a feeder-conditioned medium [94]. More recently, human recombinant laminin-511 was shown to support both human ESCs and iPS cells under a completely defined medium based on mTeSR1 [95]. Other studies of integrin protein expressions identified human recombinant vitronectin as a defined substitute for Matrigel that can support human ESCs [93] and human iPS cells [96] in mTeSR1 medium.
Whilst the above recombinant vitronectin and laminin targeted integrin-mediated cell–ECM signaling, a study by Duncan and colleagues used a recombinant E-cadherin substratum to mimic cell–cell signaling and demonstrated maintenance of human PSCs using mTeSR1 medium [97]. This demonstration is consistent with the discovery by Ding and colleagues that E-cadherin signaling can support survival and growth of human PSCs while minimizing the requirement for integrin signaling [80].
Although recombinant proteins are chemically defined, their mechanism of action could be complex due to multiple binding domains that can interact with various surface receptors. To further dissect key substrate- mediated signaling that are essential for human PSC self-renewal, Brandenberger and colleagues synthesized acrylate substrates conjugated with short peptide sequences of active domains of ECM proteins and studied the efficacy of peptides to support human ESCs [98]. Among tested peptides, including bone sialoprotein (Ac-KGGNGEPRGDTYRAY), vitronectin (Ac-KGGPQVTRGDVFTMP), fibronectin (GRGDSPK and Ac-KGGAVTGRGDSPASS) and laminin (KYGAASIKVAVSADR), only bone sialoprotein- and vitronectin-derived peptides were able to support attachment and growth of human ESCs under a defined condition. Interestingly, other peptides containing the integrin-binding motif RGD could not support human ESC attachment, indicating that the RGD sequence alone is not sufficient as a functional substitute for Matrigel or full ECM proteins. More recently, through the screening of 18 bioactive peptides Kiessling and colleagues identified a vitronectin-derived heparin-binding peptide (GKKQRFRHRNRKG) that can support long-term maintenance of human PSCs when combined with ROCK inhibitor or a short cyclic RGD peptide [99]. These observations indicate that a combination of a glycosaminoglycan-binding peptide and integrin-binding peptide can functionally replace Matrigel.
Even more defined substrates are the ones based on fully synthetic plastic coatings. Such plastic substrates can be manufactured at a much larger scale and at a much lower cost than either recombinant proteins or peptide- based substrates. Recently, a fully synthetic polymer coating, poly[2-(methacryloyloxy) ethyl dimethyl-(3-sulfopropyl)ammonium hydroxide], was reported to support attachment and growth of undifferentiated human ESCs using a feeder-conditioned medium [100]. However, this substrate supported only one of two tested human ESC lines under StemPro medium but not under mTeSR1 medium, implying it might be missing some essential signaling cues. Another group performed a high-throughput screening of 450 polymer surfaces (90 distinct polymers at five concentrations each) and found that poly(methyl vinyl ether-alt-maleic anhydride) supported the attachment and growth of three undifferentiated human PSCs (two human ESC lines and one human iPS cell line) for five passages under a chemically defined StemPro medium [101]. Poly(methyl vinyl ether-alt-maleic anhydride) not only supported initial cell attachment, but also promoted endogenous expression of both integrins and ECM proteins of human PSCs, presumably helping these cells create their own microenvironments needed for the long-term growth and maintenance [101].
In a recent study by Anderson and colleagues, screening of 496 distinct synthetic polymer coatings prepared from 22 acrylate monomers identified several surfaces that supported clonal growth of human PSCs [102]. These substrates did not directly present surface receptor-binding ligands, but rather optimized absorption of vitronectin from fetal bovine serum or human serum precoating. Interestingly, colony-formation frequency, a measure of clonal growth, was strongly affected by the substrate wettability, roughness and elastic modulus of the polymer coating [102]. Notably, mechanical properties of substrates have previously been found to have strong impacts on self-renewal of mouse ESCs [103] and differentiation of mesenchymal stem cells [104]. It is anticipated that optimizing mechanical properties of synthetic polymer coatings can lead to further improved fully synthetic substrates for human PSCs.
Future perspective
The development of a fully defined human PSC culture platform is critical to the clinical production of specialized cells for regenerative medicine. Currently, there are several strategies to tackle this challenge. Most recently, it has been shown that small molecules inhibiting pathways of differentiation and cell death may be the best option in developing a defined system that is reproducible while promoting a homogeneous population of undifferentiated cells. Although small molecules in combination with a single ECM protein has been demonstrated [89], the next challenge will be to combine small molecule cocktails with completely synthetic surfaces. Of course many challenges lay in this path. For example, genomic instability is seen to readily generate in systems where enzymatic dissociation and feeder-free culture create a harsh environment, permitting the proliferation of human PSCs that have gained a selective advantage thorough chromosomal aberrations, especially those pertaining to chromosomes 8, 12, 17, 20 and X [105,106]. Furthermore, large libraries of small molecules should be tested; however, unlike previous methodologies, the experiments should be conducted in a combinational manner. To further optimize this search, better understanding of all regulatory pathways associated with human PSC survival and self-renewal will be required to create ‘smart’ libraries of relevant small molecules. Lastly, a FSC scheme should be implemented to facilitate the search for optimal concentrations and combinations (Figure 2).
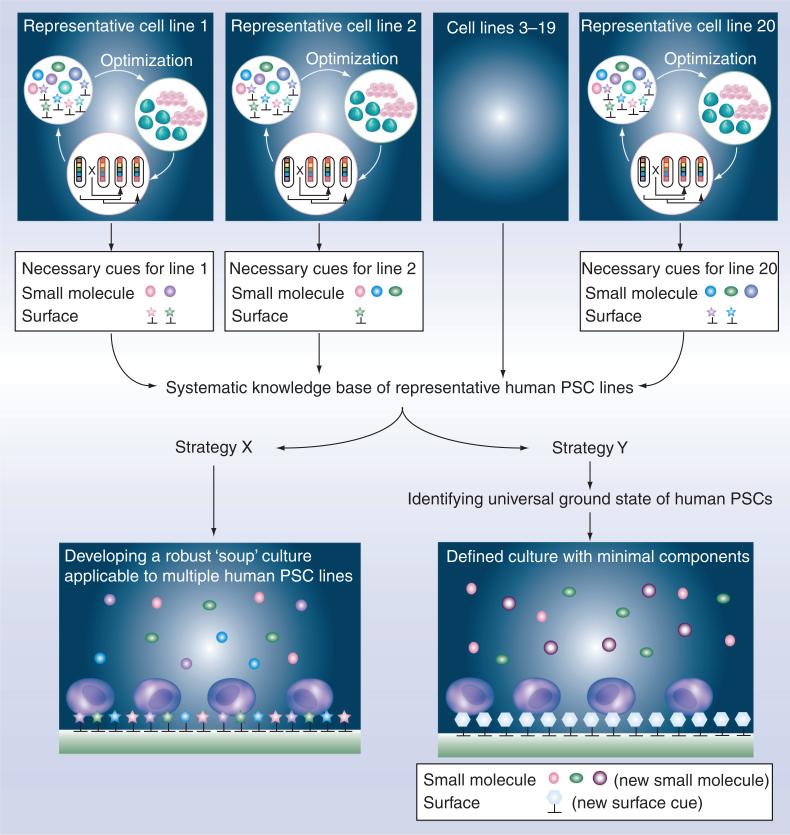
Figure 2. Strategies to develop universal ‘ground state’ defined culture that can support many different human pluripotent stem cell lines under an identical formula.
Optimization of a combinatorial small molecule library and surface cues using a human PSC line will lead to a set of necessary cues to maintain undifferentiated status of that specific line. Repeating the same process on a number of representative cell lines (e.g., 20 human PSC lines from various sources) will lead to the same number of cell line-specific customized culture systems and a systematic knowledge base on necessary signaling cues of various lines. Such a knowledge base could be used to develop a robust defined culture system by summing up small molecules and surface cues required by various human PSC lines, providing a wide range of signaling, that is, strategy X. On the other hand, detailed investigations of individually customized culture could lead to elucidation of molecular mechanisms governing the universal ‘ground state’ of human PSC lines. Such a discovery will then lead to identification of novel small molecules and surface cues that, when combined together, can be used to develop a robust defined culture with minimal number of components, that is, strategy Y.
PSC: Pluripotent stem cell.
Although optimization of combinatorial soluble and surface cues will elucidate a set of key signaling steps required for self-renewal by the specific human PSC line used during individual tests, such an optimized culture condition may not necessarily support the survival and growth of the other human PSC lines. Indeed, a recent study by the International Stem Cell Initiative Consortium tested eight serum-free and feeder-free culture conditions for their ability to support ten distinct human ESC lines, and only two culture conditions based on the two commercial media, mTeSR1 and StemPro, were able to support healthy growth of the most cell lines for ten continuous passages [107]. These two commercial formulations, in combination with Matrigel or Geltrex™ (Invitrogen; as discussed earlier, both are poorly defined extracts from mouse tumor cells containing multiple ECM proteins and growth factors), effectively covered multiple cell lines by presumably providing complex growth factor combinations and ECM proteins. Given the current diversity in protocols for human ESC derivation and human iPS cell reprogramming strategies combined with various genetic backgrounds of the starting cell lines, developing a single fully defined system that can robustly support tens or even hundreds of human PSC lines will be extremely challenging. However, it is likely that such a robust system could be realized by intentional redundancy of soluble and surface cues. In other words, series of culture optimizations using tens of representative human PSC lines will lead to a knowledge base, from which we can formulate a redundant but robust culture system for variety of human PSC lines (strategy X, Figure 2). Alternatively, by elucidating the molecular mechanisms governing universal ‘ground state’, a concise culture platform can be derived to support the majority of human PSC lines (strategy Y, Figure 2). Indeed, both strategies have their advantages and disadvantages. For example, in strategy X many of the current cultured lines can be readily adapted to and carried forward in such a medium formulation that encompasses many factors to support maintenance and growth. However, such a culture system will be rather expensive as it may contain a number of cytokines, small molecules and ECM proteins. Aided by a few small molecules that target the key pathways, strategy Y will be an inexpensive formulation. However, currently cultured lines may not readily adapt to a culture system that favors cells in a ground state, as it is believed that many of today's human ESCs and iPS cells are in a prime state culture and will differentiate if transitioned to a medium that supports the ground state. No matter which model provides the most promise and usefulness, for future studies to be most comparable, a commonly used culture platform would be most advantageous.
Human PSC lines intended for clinical applications should be derived and propagated under a defined and unified culture system in order to circumvent problems associated with the existing lines. To this end, a recent study has systematically re-examined each component of human PSC culture to generate a minimal medium formulation in which all factors are deemed necessary and chemically defined, termed E8 [108]. This systematic study demonstrated that many components are not critical, including serum albumin [108]. In fact, albumin only appears to be important when β-mercaptoethanol was added to the formulation and removal of β-mercaptoethanol appears to negate the value of albumin [108]. Interestingly, the combination of E8 with a vitronectin-coated surface yielded improved derivation efficiencies of human iPS cells, providing further evidence that systematic methods and FSC strategies can greatly improve current culture systems. Indeed, it will be interesting to determine whether the addition of small molecule inhibitors of differentiation to E8 will further enhance this defined culture system.
This article mainly focuses on the recent development of a 2D adherent culture for expanding undifferentiated human PSCs. Although such a development has continuously improved the quality and reproducibility of culture, the issue of scalability, a key technical hurdle against large-scale production of clinically relevant PSCs, had not been addressed until very recently. With the emergence of commercially available defined media such as mTeSR1 and supplements of ROCK inhibitors allowing robust handling of single cells, several groups are studying various forms of suspension cultures that are more scalable compared with their 2D adherent counterparts. For instance, microcarriers provide a surface to which PSCs can attach and grow into colonies while increasing the density of cultured cells in a culture vessel [109–113]. Although a general consensus has been that PSCs require attachment to a surface in order to efficiently maintain their undifferentiated status and that spherical aggregation induces differentiation as is the case for embryoid body-mediated differentiations, recent studies showed that efficient expansion of undifferentiated PSCs in suspended aggregates without microcarriers is possible [65–67]. Among them, Reubinoff and colleagues demonstrated the derivation, propagation and controlled differentiation of human ESCs all in suspension culture systems [67]. Interestingly, their culture medium contains a rather empirically selected combination of neurotrophic factors, growth factors and ECM protein components in an attempt to provide a wide range of supposedly important signaling cues. Further refinement of such a suspension culture again presents extensive combinatorial possibilities, where the optimization approach suggested above could play an important role in identifying essential signaling cues required for a robust suspension culture of human PSCs.
Executive summary.
Human pluripotent stem cells
■ With distinct abilities for both unlimited self-renewal and differentiation potential into all specialized cell types, human embryonic stem cells and induced pluripotent stem cells offer great promise in developmental and therapeutic applications including pharmaceutical drug screening, disease modeling and regenerative medicine.
Conventional culture of human pluripotent stem cells
■ Traditional and most commonly used culture methods of human pluripotent stem cells (PSCs) consist of feeder cells, serum and clump passaging.
■ Feeder cells and serum are possible sources of variability, xenogeneic contaminations and animal-derived pathogens, which impede the translation of human PSCs towards clinical applications. However, culture systems absent of these factors often result in spontaneous cell death and cell differentiation.
■ Clump passaging is an inefficient and laborious strategy for manufacturing scale expansion. However, single cell culture leads to significant cell death and chromosomal aberrations.
Progressing towards a defined & scalable culture
■ Media formulations have incorporated cytokines and growth factors to replace serum in culture.
■ Feeder cells have been substituted by more defined extracellular components or synthetic surfaces.
■ Single cell passaging has been made more efficient by targeting the Rho kinase pathway.
■ Small molecules targeting pathways of differentiation hold great promise in the development of a defined culture platform amenable to human PSCs.
■ Suspension cultures in various bioreactor formats are important key steps toward industrial scale manufacturing of clinical grade human PSCs.
Acknowledgments
The authors appreciate the support from the Center for Cell Control (PN2 EY018228) through NIH Roadmap for Nanomedicine and the UCLA Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1■■.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [First demonstration of the derivation of mouse embryonic stem cells (ESCs).] [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suda Y, Suzuki M, Ikawa Y, Aizawa S. Mouse embryonic stem cells exhibit indefinite proliferative potential. J. Cell. Physiol. 1987;133:197–201. doi: 10.1002/jcp.1041330127. [DOI] [PubMed] [Google Scholar]
- 4.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 5.Smith AG. Embryo-derived stem cells: of mice and men. Annu. Rev. Cell Dev. Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 6.Beddington RS, Robertson EJ. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 1989;105:733–737. doi: 10.1242/dev.105.4.733. [DOI] [PubMed] [Google Scholar]
- 7.Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- 8.Eggan K, Akutsu H, Loring J, et al. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc. Natl Acad. Sci. USA. 2001;98:6209–6214. doi: 10.1073/pnas.101118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubiak JZ, Tarkowski AK. Electrofusion of mouse blastomeres. Exp. Cell Res. 1985;157:561–566. doi: 10.1016/0014-4827(85)90143-0. [DOI] [PubMed] [Google Scholar]
- 10.Jaenisch R. Transgenic animals. Science. 1988;240:1468–1474. doi: 10.1126/science.3287623. [DOI] [PubMed] [Google Scholar]
- 11.Carver BS, Pandolfi PP. Mouse modeling in oncologic preclinical and translational research. Clin. Cancer Res. 2006;12:5305–5311. doi: 10.1158/1078-0432.CCR-06-0482. [DOI] [PubMed] [Google Scholar]
- 12.Hofker MH. Introduction: strategies for developing genetically modified mice. Methods Mol. Biol. 2011;693:1–10. doi: 10.1007/978-1-60761-974-1_1. [DOI] [PubMed] [Google Scholar]
- 13.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14■■.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [Demonstrates that LIF and BMP4 are necessary and sufficient for the culture of mouse ESCs.] [DOI] [PubMed] [Google Scholar]
- 15.Smith AG, Heath JK, Donaldson DD, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 16.Williams RL, Hilton DJ, Pease S, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 17.Smith AG, Hooper ML. Buffalo rat liver cells produce a diffusible activity which inhibits the differentiation of murine embryonal carcinoma and embryonic stem cells. Dev. Biol. 1987;121:1–9. doi: 10.1016/0012-1606(87)90132-1. [DOI] [PubMed] [Google Scholar]
- 18■■.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [First reported derivation of human ESCs.] [DOI] [PubMed] [Google Scholar]
- 19.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 20.Burdon T, Chambers I, Stracey C, Niwa H, Smith A. Signaling mechanisms regulating self-renewal and differentiation of pluripotent embryonic stem cells. Cells Tissues Organs. 1999;165:131–143. doi: 10.1159/000016693. [DOI] [PubMed] [Google Scholar]
- 21.Pera MF. Human pluripotent stem cells: a progress report. Curr. Opin. Genet. Dev. 2001;11:595–599. doi: 10.1016/s0959-437x(00)00238-0. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman LM, Carpenter MK. Characterization and culture of human embryonic stem cells. Nat. Biotechnol. 2005;23:699–708. doi: 10.1038/nbt1102. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 26.Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 27.Wernig M, Zhao JP, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc. Natl Acad. Sci. USA. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida Y, Yamanaka S. iPS cells: a source of cardiac regeneration. J. Mol. Cell. Cardiol. 2011;50:327–332. doi: 10.1016/j.yjmcc.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Nelson TJ, Martinez-Fernandez A, Terzic A. Induced pluripotent stem cells: developmental biology to regenerative medicine. Nat. Rev. Cardiol. 2010;7:700–710. doi: 10.1038/nrcardio.2010.159. [DOI] [PubMed] [Google Scholar]
- 30.Plath K, Lowry WE. Progress in understanding reprogramming to the induced pluripotent state. Nat. Rev. Genet. 2011;12:253–265. doi: 10.1038/nrg2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saha K, Jaenisch R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell. 2009;5:584–595. doi: 10.1016/j.stem.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida Y, Yamanaka S. Recent stem cell advances: induced pluripotent stem cells for disease modeling and stem cell-based regeneration. Circulation. 2010;122:80–87. doi: 10.1161/CIRCULATIONAHA.109.881433. [DOI] [PubMed] [Google Scholar]
- 33.McDevitt TC, Palecek SP. Innovation in the culture and derivation of pluripotent human stem cells. Curr. Opin. Biotechnol. 2008;19:527–533. doi: 10.1016/j.copbio.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skottman H, Narkilahti S, Hovatta O. Challenges and approaches to the culture of pluripotent human embryonic stem cells. Regen. Med. 2007;2(3):265–273. doi: 10.2217/17460751.2.3.265. [DOI] [PubMed] [Google Scholar]
- 35.Hewitt ZA, Amps KJ, Moore HD. Derivation of GMP raw materials for use in regenerative medicine: hESC-based therapies, progress toward clinical application. Clin. Pharmacol. Ther. 2007;82:448–452. doi: 10.1038/sj.clpt.6100321. [DOI] [PubMed] [Google Scholar]
- 36.Hodges H, Pollock K, Stroemer P, et al. Making stem cell lines suitable for transplantation. Cell Transplant. 2007;16:101–115. [PubMed] [Google Scholar]
- 37.Lei T, Jacob S, Ajil-Zaraa I, et al. Xeno-free derivation and culture of human embryonic stem cells: current status, problems and challenges. Cell Res. 2007;17:682–688. doi: 10.1038/cr.2007.61. [DOI] [PubMed] [Google Scholar]
- 38.Skottman H, Dilber MS, Hovatta O. The derivation of clinical-grade human embryonic stem cell lines. FEBS Lett. 2006;580:2875–2878. doi: 10.1016/j.febslet.2006.03.083. [DOI] [PubMed] [Google Scholar]
- 39.Unger C, Skottman H, Blomberg P, Dilber MS, Hovatta O. Good manufacturing practice and clinical-grade human embryonic stem cell lines. Hum. Mol. Genet. 2008;17:R48–R53. doi: 10.1093/hmg/ddn079. [DOI] [PubMed] [Google Scholar]
- 40.Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat. Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 41.Ilic D. Culture of human embryonic stem cells and the extracellular matrix microenvironment. Regen. Med. 2006;1:95–101. doi: 10.2217/17460751.1.1.95. [DOI] [PubMed] [Google Scholar]
- 42.Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 43.Eiges R, Schuldiner M, Drukker M, et al. Establishment of human embryonic stem cell-transfected clones carrying a marker for undifferentiated cells. Curr. Biol. 2001;11:514–518. doi: 10.1016/s0960-9822(01)00144-0. [DOI] [PubMed] [Google Scholar]
- 44.Tenzen T, Zembowicz F, Cowan CA. Genome modification in human embryonic stem cells. J. Cell. Physiol. 2010;222:278–281. doi: 10.1002/jcp.21948. [DOI] [PubMed] [Google Scholar]
- 45.Sathananthan AH, Trounson A. Human embryonic stem cells and their spontaneous differentiation. Ital. J. Anat. Embryol. 2005;110:151–157. [PubMed] [Google Scholar]
- 46.Pera MF, Trounson AO. Human embryonic stem cells: prospects for development. Development. 2004;131:5515–5525. doi: 10.1242/dev.01451. [DOI] [PubMed] [Google Scholar]
- 47.Yao S, Chen S, Clark J, et al. Long-term self- renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc. Natl Acad. Sci. USA. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furue MK, Na J, Jackson JP, et al. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc. Natl Acad. Sci. USA. 2008;105:13409–13414. doi: 10.1073/pnas.0806136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montes R, Ligero G, Sanchez L, et al. Feeder-free maintenance of hESCs in mesenchymal stem cell-conditioned media: distinct requirements for TGF-β and IGF-II. Cell Res. 2009;19:698–709. doi: 10.1038/cr.2009.35. [DOI] [PubMed] [Google Scholar]
- 50.Lu J, Hou R, Booth CJ, Yang SH, Snyder M. Defined culture conditions of human embryonic stem cells. Proc. Natl Acad. Sci. USA. 2006;103:5688–5693. doi: 10.1073/pnas.0601383103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51■.Ludwig TE, Levenstein ME, Jones JM, et al. Derivation of human embryonic stem cells in defined conditions. Nat. Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [Gives a clear demonstration that a combination of cytokines and extracellular matrix (ECM) proteins can support feeder-free culture and derivation of human ESCs.] [DOI] [PubMed] [Google Scholar]
- 52.Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nat. Biotechnol. 2006;24:344–350. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- 53.Ellerstrom C, Strehl R, Moya K, et al. Derivation of a xeno-free human embryonic stem cell line. Stem Cells. 2006;24:2170–2176. doi: 10.1634/stemcells.2006-0130. [DOI] [PubMed] [Google Scholar]
- 54.Hovatta O, Mikkola M, Gertow K, et al. A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Hum. Reprod. 2003;18:1404–1409. doi: 10.1093/humrep/deg290. [DOI] [PubMed] [Google Scholar]
- 55.Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 56.Richards M, Tan S, Fong CY, et al. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21:546–556. doi: 10.1634/stemcells.21-5-546. [DOI] [PubMed] [Google Scholar]
- 57.Stojkovic P, Lako M, Stewart R, et al. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells. 2005;23:306–314. doi: 10.1634/stemcells.2004-0137. [DOI] [PubMed] [Google Scholar]
- 58.Wang Q, Fang ZF, Jin F, et al. Derivation and growing human embryonic stem cells on feeders derived from themselves. Stem Cells. 2005;23:1221–1227. doi: 10.1634/stemcells.2004-0347. [DOI] [PubMed] [Google Scholar]
- 59.Stacey GN, Cobo F, Nieto A, et al. The development of ‘feeder’ cells for the preparation of clinical grade hES cell lines: challenges and solutions. J. Biotechnol. 2006;125:583–588. doi: 10.1016/j.jbiotec.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 60.Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 61.Chin AC, Fong WJ, Goh LT, et al. Identification of proteins from feeder conditioned medium that support human embryonic stem cells. J. Biotechnol. 2007;130:320–328. doi: 10.1016/j.jbiotec.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 62.Cormier JT, zur Nieden NI, Rancourt DE, Kallos MS. Expansion of undifferentiated murine embryonic stem cells as aggregates in suspension culture bioreactors. Tissue Eng. 2006;12:3233–3245. doi: 10.1089/ten.2006.12.3233. [DOI] [PubMed] [Google Scholar]
- 63.Amit M, Laevsky I, Miropolsky Y, et al. Dynamic suspension culture for scalable expansion of undifferentiated human pluripotent stem cells. Nat. Protoc. 2011;6:572–579. doi: 10.1038/nprot.2011.325. [DOI] [PubMed] [Google Scholar]
- 64.Zweigerdt R, Olmer R, Singh H, Haverich A, Martin U. Scalable expansion of human pluripotent stem cells in suspension culture. Nat. Protoc. 2011;6:689–700. doi: 10.1038/nprot.2011.318. [DOI] [PubMed] [Google Scholar]
- 65.Olmer R, Haase A, Merkert S, et al. Long term expansion of undifferentiated human iPS and ES cells in suspension culture using a defined medium. Stem Cell Res. 2010;5:51–64. doi: 10.1016/j.scr.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Singh H, Mok P, Balakrishnan T, Rahmat SNB, Zweigerdt R. Up-scaling single cell-inoculated suspension culture of human embryonic stem cells. Stem Cell Res. 2010;4:165–179. doi: 10.1016/j.scr.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Steiner D, Khaner H, Cohen M, et al. Derivation, propagation and controlled differentiation of human embryonic stem cells in suspension. Nat. Biotechnol. 2010;28:361–364. doi: 10.1038/nbt.1616. [DOI] [PubMed] [Google Scholar]
- 68.Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol. Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- 69.Beattie GM, Lopez AD, Bucay N, et al. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- 70.Li Y, Powell S, Brunette E, Lebkowski J, Mandalam R. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol. Bioeng. 2005;91:688–698. doi: 10.1002/bit.20536. [DOI] [PubMed] [Google Scholar]
- 71.Liu Y, Song Z, Zhao Y, et al. A novel chemical-defined medium with bFGF and N2B27 supplements supports undifferentiated growth in human embryonic stem cells. Biochem. Biophys. Res. Commun. 2006;346:131–139. doi: 10.1016/j.bbrc.2006.05.086. [DOI] [PubMed] [Google Scholar]
- 72.Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells. 2006;24:1476–1486. doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- 73.Hasegawa K, Fujioka T, Nakamura Y, Nakatsuji N, Suemori H. A method for the selection of human embryonic stem cell sublines with high replating efficiency after single-cell dissociation. Stem Cells. 2006;24:2649–2660. doi: 10.1634/stemcells.2005-0657. [DOI] [PubMed] [Google Scholar]
- 74■.Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [First demonstration of improved survival of human ESCs upon single cell dissociation using ROCK inhibition.] [DOI] [PubMed] [Google Scholar]
- 75.Chen G, Hou Z, Gulbranson DR, Thomson JA. Actin–myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell. 2010;7:240–248. doi: 10.1016/j.stem.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Damoiseaux R, Sherman SP, Alva JA, Peterson C, Pyle AD. Integrated chemical genomics reveals modifiers of survival in human embryonic stem cells. Stem Cells. 2009;27:533–542. doi: 10.1634/stemcells.2008-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ohgushi M, Matsumura M, Eiraku M, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225–239. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 78.Ohgushi M, Sasai Y. Lonely death dance of human pluripotent stem cells: ROCKing between metastable cell states. Trends Cell Biol. 2011;21(5):274–282. doi: 10.1016/j.tcb.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 79.Walker A, Su H, Conti MA, et al. Non-muscle myosin II regulates survival threshold of pluripotent stem cells. Nat. Commun. 2010;1:71. doi: 10.1038/ncomms1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu Y, Zhu X, Hahm HS, et al. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc. Natl Acad. Sci. USA. 2010;107:8129–8134. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ardehali R, Inlay MA, Ali SR, et al. Overexpression of BCL2 enhances survival of human embryonic stem cells during stress and obviates the requirement for serum factors. Proc. Natl Acad. Sci. USA. 2011;108:3282–3287. doi: 10.1073/pnas.1019047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakayama M. Homologous recombination in human iPS and ES cells for use in gene correction therapy. Drug Discov. Today. 2010;15:198–202. doi: 10.1016/j.drudis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 83.Stewart MH, Bendall SC, Bhatia M. Deconstructing human embryonic stem cell cultures: niche regulation of self-renewal and pluripotency. J. Mol. Med. 2008;86:875–886. doi: 10.1007/s00109-008-0356-9. [DOI] [PubMed] [Google Scholar]
- 84■■.Ying QL, Wray J, Nichols J, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [Introduces the concept of the ground state of ESCs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Silva J, Smith A. Capturing pluripotency. Cell. 2008;132:532–536. doi: 10.1016/j.cell.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buehr M, Meek S, Blair K, et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 87.Li P, Tong C, Mehrian-Shai R, et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88■.Kawamata M, Ochiya T. Generation of genetically modified rats from embryonic stem cells. Proc. Natl Acad. Sci. USA. 2010;107:14223–14228. doi: 10.1073/pnas.1009582107. [Demonstrates that human ESCs behave similarly to mouse ESCs when cultured in a unique small molecule cocktail.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89■.Tsutsui H, Valamehr B, Hindoyan A, et al. An optimized small molecule inhibitor cocktail supports long-term maintenance of human embryonic stem cells. Nat. Commun. 2011;2:167. doi: 10.1038/ncomms1165. [Utilized a feedback system control to develop a human ESC culture system consisting of only small molecules and a single ECM protein surface.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hanna J, Cheng AW, Saha K, et al. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl Acad. Sci. USA. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun CP, Usui T, Yu F, et al. Integrative systems control approach for reactivating Kaposi's sarcoma-associated herpesvirus (KSHV) with combinatory drugs. Integr. Biol. 2009;1:123–130. doi: 10.1039/b815225j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wong PK, Yu F, Shahangian A, et al. Closed-loop control of cellular functions using combinatory drugs guided by a stochastic search algorithm. Proc. Natl Acad. Sci. USA. 2008;105:5105–5110. doi: 10.1073/pnas.0800823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Braam SR, Zeinstra L, Litjens S, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via aVb5 integrin. Stem Cells. 2008;26:2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 94.Miyazaki T, Futaki S, Hasegawa K, et al. Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2008;375:27–32. doi: 10.1016/j.bbrc.2008.07.111. [DOI] [PubMed] [Google Scholar]
- 95.Rodin S, Domogatskaya A, Strom S, et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 2010;28:611–615. doi: 10.1038/nbt.1620. [DOI] [PubMed] [Google Scholar]
- 96.Rowland TJ, Miller LM, Blaschke AJ, et al. Roles of integrins in human induced pluripotent stem cell growth on Matrigel and vitronectin. Stem Cells Dev. 2010;19:1231–1240. doi: 10.1089/scd.2009.0328. [DOI] [PubMed] [Google Scholar]
- 97.Nagaoka M, Si-Tayeb K, Akaike T, Duncan SA. Culture of human pluripotent stem cells using completely defined conditions on a recombinant E-cadherin substratum. BMC Dev. Biol. 2010;10:60. doi: 10.1186/1471-213X-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Melkoumian Z, Weber JL, Weber DM, et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat. Biotechnol. 2010;28:606–610. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- 99.Klim JR, Li LY, Wrighton PJ, Piekarczyk MS, Kiessling LL. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat. Methods. 2010;7:989–994. doi: 10.1038/nmeth.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Villa-Diaz LG, Nandivada H, Ding J, et al. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat. Biotechnol. 2010;28:581–583. doi: 10.1038/nbt.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101■.Brafman DA, Chang CW, Fernandez A, et al. Long-term human pluripotent stem cell self-renewal on synthetic polymer surfaces. Biomaterials. 2010;31:9135–9144. doi: 10.1016/j.biomaterials.2010.08.007. [Reports a synthetic substrate that enhances endogenous ECM proteins production by human pluripotent stem cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102■.Mei Y, Saha K, Bogatyrev SR, et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat. Mater. 2010;9:768–778. doi: 10.1038/nmat2812. [Demonstrates that physicochemical properties of polymer surfaces influence growth of human pluripotent stem cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chowdhury F, Li YZ, Poh YC, et al. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS One. 2010;5:e15655. doi: 10.1371/journal.pone.0015655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 105.Taapken SM, Nisler BS, Newton MA, et al. Karotypic abnormalities in human induced pluripotent stem cells and embryonic stem cells. Nat. Biotechnol. 2011;29:313–314. doi: 10.1038/nbt.1835. [DOI] [PubMed] [Google Scholar]
- 106.Mitalipova MM, Rao RR, Hoyer DM, et al. Preserving the genetic integrity of human embryonic stem cells. Nat. Biotechnol. 2005;23:19–20. doi: 10.1038/nbt0105-19. [DOI] [PubMed] [Google Scholar]
- 107.Akopian V, Andrews PW, Beil S, et al. Comparison of defined culture systems for feeder cell free propagation of human embryonic stem cells. In Vitro Cell. Dev. Biol. Anim. 2010;46:247–258. doi: 10.1007/s11626-010-9297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen G, Gulbranson DR, Hou Z, et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lock LT, Tzanakakis ES. Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng. Part A. 2009;15:2051–2063. doi: 10.1089/ten.tea.2008.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nie Y, Bergendahl V, Hei DJ, Jones JM, Palecek SP. Scalable culture and cryopreservation of human embryonic stem cells on microcarriers. Biotechnol. Prog. 2009;25:20–31. doi: 10.1002/btpr.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oh SKW, Chen AK, Mok Y, et al. Long-term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Res. 2009;2:219–230. doi: 10.1016/j.scr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 112.Phillips BW, Horne R, Lay TS, et al. Attachment and growth of human embryonic stem cells on microcarriers. J. Biotechnol. 2008;138:24–32. doi: 10.1016/j.jbiotec.2008.07.1997. [DOI] [PubMed] [Google Scholar]
- 113.Serra M, Brito C, Sousa MFQ, et al. Improving expansion of pluripotent human embryonic stem cells in perfused bioreactors through oxygen control. J. Biotechnol. 2010;148:208–215. doi: 10.1016/j.jbiotec.2010.06.015. [DOI] [PubMed] [Google Scholar]